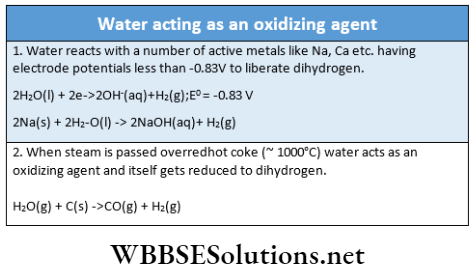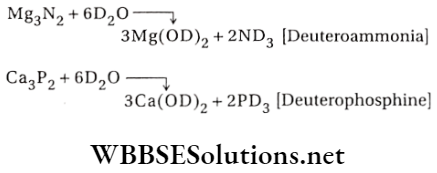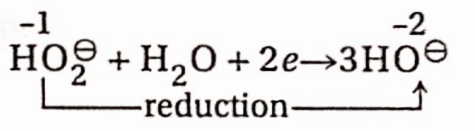Hydrogen Introduction
WBBSE Class 11 Hydrogen Notes Summary
- Symbol: H
- Molecular formula: H2
- Atomic mass: 1.008
- Electronic configuration: S1
- Atomic number:1
- Position in the periodic table: group -1 (IA) or group-17 (VII A), first period
- Oxidation number: +1, -1
Position of hydrogen in the periodic table: Hydrogen is the first element in the periodic table to have atomic number 1, i.e., its electronic configuration is Is1. Because of hydrogen’s resemblance with alkali metals as well as with halogens, it can either be placed with the alkali metals in group-1 (IA) or with the halogens in group-17 (VII A) in the periodic table. The dual behavior of hydrogen is due to its electronic configuration.
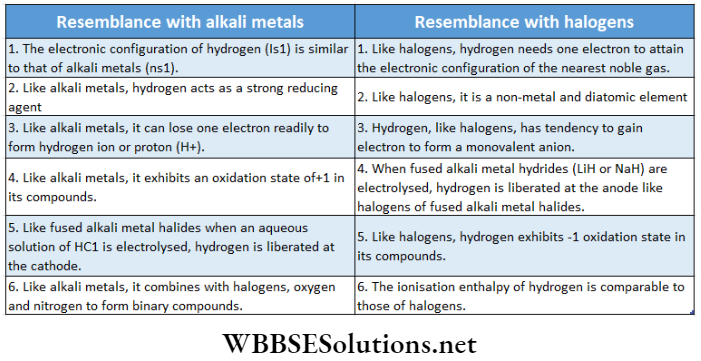
From the above discussion, it is clear that hydrogen is unique in its behavior and it is not justified to place it either with the alkali metals of group-1 or with halogens of group-17. Thus, the position of hydrogen in the periodic table is anomalous (sometimes it is referred to as a ‘rogue element’) and it may be best placed separately in the periodic table. In the modern periodic table (IUPAC), hydrogen has been placed separately [a position in between group -l(IA) and 17(VHA)].
Read and Learn More WBCHSE Class 11 Chemistry
Occurrence Of Hydrogen
Hydrogen is the most abundant element in the universe (70% of the total mass of the universe). Hydrogen is not found in the atmosphere due to its lightweight. It is the third most abundant element on the earth’s surface. In a free state, it occurs in traces in volcanic gases and in the outer atmosphere of the sun and stars of the universe. In combined state, it exists mainly as water, natural gas, and petroleum. It is also an important constituent of organic matter in plants and animal tissues, carbohydrates, proteins, etc.
The extremely high temperature 4He + 0 energy of the sun is due to the nuclear helium positron fusion of hydrogen atoms liberating a large amount of energy. this energy. this energy is the main source of solar energy.
Isotopes Of Hydrogen
Hydrogen has three isotopes. These are protium or hydrogen, deuterium or heavy hydrogen, and tritium.
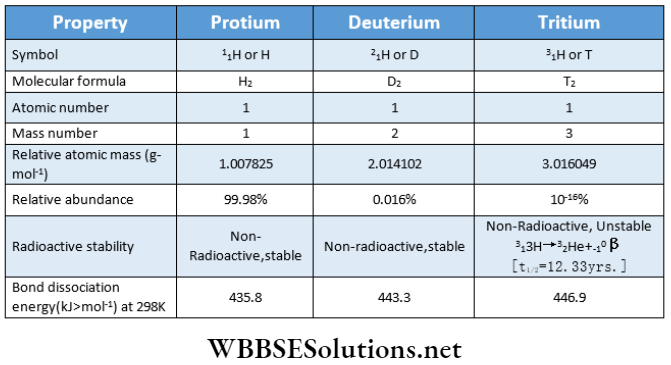
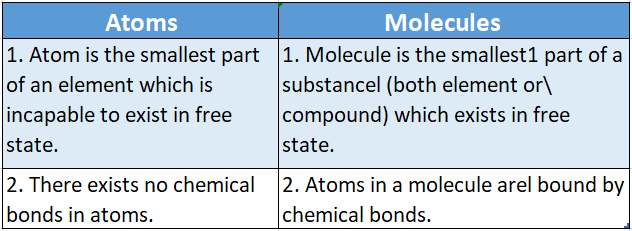
- The three isotopes of hydrogen have the same chemical properties since they have the same electronic configuration (1s1), but differ from one another only in the number of neutrons in the nucleus.
- However, because of different bond dissociation enthalpies, they have different rates of chemical reactions. Due to many differences in their atomic masses, they differ considerably in their physical properties.
- The difference in properties arising due to differences in atomic masses is called the isotopic effect. Diatomic molecules containing only a protium atom (H2), only a deuterium atom (D2), and only tritium atoms (T2) are called diprotium, deuterium, and tritium respectively.
- The term dihydrogen is used for the mixture of H2, D2, and HD concerning their natural abun¬ dance. The term hydron is used for the mixture of proton (H+) and deuteron (D+) concerning their natural abundance.
- Tritium can be artificially synthesized by bombarding the isotope of lithium \(\left({ }_3^6 \mathrm{Li}\right)\)or nitrogen by neutron.
- Deuterium is widely used as a tracer in determining the mechanism of organic reactions.
- Tritium gas is stored by converting it into a UT3 complex. When UT3 is heated at 673K it releases T2. It is used in the research on nuclear fusion reactions.
Water
Water is an important hydride of oxygen. In 1781, Cavendish first prepared water by exploding a mixture of 2 volumes of hydrogen and 1 volume of oxygen and proved that water is a compound that consists of the elements hydrogen and oxygen. Although water is the most abundant in the world, it is not always available in pure form. Hence, water in its natural state is not always fit for consumption and needs to be purified for drinking and laboratory use.
Structure Of Water Molecule
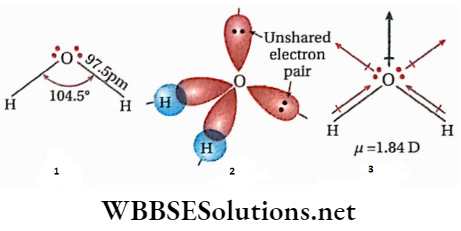
- In water molecules, the two H-atoms are bonded to the O-atom by two covalent bonds. The oxygen atoms in water are sp3 -sp3-hybridized. Each of the covalent O —H bonds is formed by the axial overlap of the Is orbital of the H-atom and the sp3 -hybrid orbital of the O-atom.
- The two bond pairs and the two lone pairs of electrons around the oxygen atom assume a tetrahedral arrangement. As a consequence, the H20 molecule has a bent structure.
- Since the lone pair-lone pair and the lone pair-bond pair repulsions are greater than the bond pair-bond pair repulsive interaction, the H —O —H bond angle decreases from 109o28′ to 104.5°.
- A molecule of water has a bent shape and hence the resultant of the two O —H bond moments adds to the moments produced by the lone pairs.
- As a result, water molecules possess dipole moment (μ= 1.84D), i.e., it is a polar molecule. The bent structure of water, the orbital overlap picture of water, and the water molecule dipole are shown respectively.
Water has higher melting and boiling points:
- Since the electronegativity of the smaller oxygen atom is much higher, the O—H bond is considerably polar. This causes water molecules to remain associated through intermolecular hydrogen bonding.
- Because of intermolecular H-bonding, water is liquid at room temperature and its melting and boiling points are relatively much higher than those of the hydrides of the other elements of group 16 having higher molecular mass.

The density of water is the highest at 4°C:
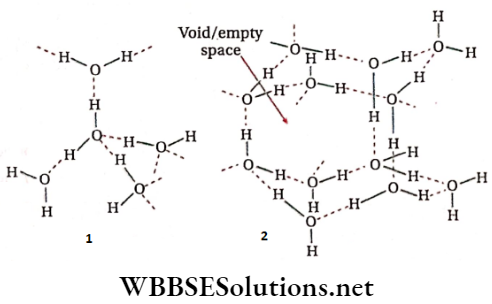
As the temperature is increased beyond 0°C, the open cage-like structure starts breaking due to cleavage of some H -bonds and ice starts melting. This causes water molecules to move into the holes or vacant spaces and to come closer to each other resulting in a decrease in volume and thereby increase in density. This continues till 4°C, when the density becomes maximum (1.00g . cm-3). Beyond this temperature, more H-bonds cleave due to the increased kinetic energy of the molecules. As a result, the expansion of water starts, and its density starts decreasing. At 100°C, most of the H -bonds break and water starts boiling.
Structure of ice: In a hexagonal crystal of ice each O-atom is tetrahedrally surrounded by four neighboring O-atoms. This gives rise to a three-dimensional structure having a large vacant space similar to an open cage. Each O-atom in the crystal is connected to four H-atoms — out of which two H-atoms are covalently bonded while the other two H atoms are bonded by weak hydrogen bonds. The density of ice is less than water because of the vacant space in its crystal structure. So, ice floats on water. Actually, 11 cm3 of water solidifies to form 12 cm3 ice.
Properties Of Water
Physical properties
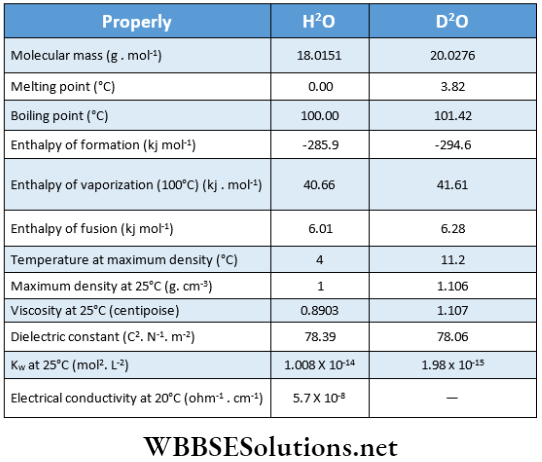
Pure water is a tasteless, odorless, and colorless liquid. Its physical properties are given below along with the physical properties of heavy water.
Chemical Properties
1. Nature: Water is a neutral oxide and neutral to litmus.
2. Solvent property: Water is an excellent solvent.
- Being a highly polar compound (μ = 1.84D), water can stabilize ions by ion-dipole interactions. Again, its dielectric constant is much higher (∈ = 78.39) so, its ability to decrease the forces of attraction between oppositely charged ions is much higher.
- For these reasons, water can dissolve many ionic or electrovalent compounds. All sodium, potassium, and ammonium salts [exception potassium perchlorate (KClO4), ammonium perchlorate (NH4ClO4), and all metal nitrates [exception: bismuth subnitrate, Bi(OH)2NO3] are soluble in water.
- Water can dissolve many covalent compounds such as alcohols, amines, sugars, etc., by forming H-bonds with them. A molecule of water is capable of both accepting and donating protons.
- So, water can dissolve some polar covalent compounds (For example HCl, NH3, etc.) by acid-base reactions. Because of such versatile solvent properties, water is called a ‘universal solvent.
3. Stability: Water is a very stable compound because it has a higher negative value of enthalpy of formation (A/7y° = -285.9kJ. mol-1). It does not dissociate even at much higher temperatures. Only 0.02% of it dissociates at 1200°C.
\(\mathrm{H}_2 \mathrm{O}(l) \rightarrow \mathrm{H}_2(g)+\frac{1}{2} \mathrm{O}_2(g)\)
4. Acid-base character: Water is an amphoteric compound because it acts both as an acid and a base. According to the Bronsted-Lowry concept, it acts as an acid with NH3 by donating a proton and as a base with HCl by accepting a proton. Since water acts as a proton donor as well as a proton acceptor, it is called an amphoteric amphiprotic solvent.
⇒ \(\begin{aligned} & \mathrm{H}_2 \mathrm{O}(l)+\mathrm{NH}_3(a q) \rightleftharpoons \mathrm{NH}_4^{+}(a q)+\mathrm{OH}^{-}(a q) \\ & \text { acid-1 base-2 } \quad \text { acid-2 } \quad \text { base- } 1 \\ & \end{aligned}\)
⇒ \(\underset{\text { base-1 }}{\mathrm{H}_2 \mathrm{O}(l)}+\underset{\text { acid-2 }}{\mathrm{HCl}}(a q) \rightleftharpoons \underset{\text { acid-1 }}{\mathrm{H}_3 \mathrm{O}^{+}(a q)}+\underset{\mathrm{Cl}^{-}(a q)}{\text { base-2 }}\)
Usually, water acts as a base in the presence of an acid stronger than it and acts as an acid in the presence of a base stronger than it. Because of such amphoteric character, water undergoes self-ionisation or autoprotolysis as follows:
⇒ \(\begin{aligned} & \mathrm{H}_2 \mathrm{O}(l)+\mathrm{H}_2 \mathrm{O}(l) \rightleftharpoons \mathrm{H}_3 \mathrm{O}^{+}(a q)+\mathrm{OH}^{-}(a q) \\ & \text { acid-1 base-2 acid-2 base-1 } \\ & \text { (Acid) (Base) (Conjugate acid) (Conjugate base) } \\ & \end{aligned}\)
As the degree of self-ionization of water is much lower, the electrical conductivity of pure water is very low.
5. Oxidation-reduction reaction: Besides the acid-base reaction, water can undergo redox reactions.
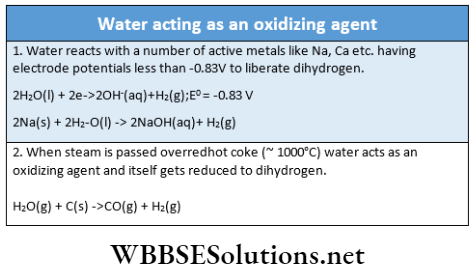
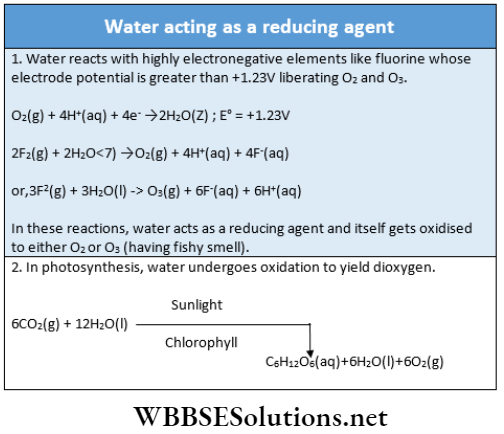
6. Hydrolytic reactions: Water can hydrolyze many metallic and non-metallic oxides, hydrides, nitrides, carbides, phosphides, and some other salts.
⇒ \(\mathrm{CO}_2(g)+\mathrm{H}_2 \mathrm{O}(l) \rightarrow \mathrm{H}_2 \mathrm{CO}_3(a q)\)
⇒ \(\mathrm{SO}_2(g)+\mathrm{H}_2 \mathrm{O}(l) \rightarrow \mathrm{H}_2 \mathrm{SO}_3(a q)\)
⇒ \(\mathrm{P}_4 \mathrm{O}_{10}(s)+6 \mathrm{H}_2 \mathrm{O}(l) \rightarrow 4 \mathrm{H}_3 \mathrm{PO}_4(a q)\)
⇒ \(\mathrm{Na}_2 \mathrm{O}(s)+\mathrm{H}_2 \mathrm{O}(l) \rightarrow 2 \mathrm{NaOH}(a q)\)
⇒ \(\mathrm{CaH}_2(s)+2 \mathrm{H}_2 \mathrm{O}(l) \rightarrow \mathrm{Ca}(\mathrm{OH})_2(a q)+2 \mathrm{H}_2(g)\)
⇒ \( \mathrm{Ca}_3 \mathrm{P}_2+6 \mathrm{H}_2 \mathrm{O}(l) \rightarrow 3 \mathrm{Ca}(\mathrm{OH})_2(a q)+2 \mathrm{PH}_3(g)\)
[Phosphine]
⇒ \( \mathrm{CaC}_2(s)+2 \mathrm{H}_2 \mathrm{O}(l) \rightarrow \mathrm{Ca}(\mathrm{OH})_2(a q)+\mathrm{C}_2 \mathrm{H}_2(g)\)
[Acetylene]
⇒ \( \mathrm{Al}_4 \mathrm{C}_3(s)+12 \mathrm{H}_2 \mathrm{O}(l) \rightarrow 4 \mathrm{Al}(\mathrm{OH})_3(a q)+3 \mathrm{CH}_4(g)\)
[Methane]
⇒ \(\mathrm{Mg}_3 \mathrm{~N}_2(s)+6 \mathrm{H}_2 \mathrm{O}(l) \rightarrow 3 \mathrm{Mg}(\mathrm{OH})_2(s)+2 \mathrm{NH}_3(g)\)
Physical Properties of Hydrogen for Class 11
7. Hydration reaction: Water is able to combine with some metal salts to form compounds known as hydrates.
There are three types of hydrates:
- Water molecules may combine with metal ions through coordinate bonds to form complexions.
For example:
⇒ \(\left[\mathrm{Ni}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}\left(\mathrm{NO}_3^{-}\right)_2 ;\left[\mathrm{Al}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right] \mathrm{Cl}_3 ;\left[\mathrm{Cr}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right] \mathrm{Cl}_3\)
- Water molecules may remain hydrogen-bonded to certain oxygen-containing anions. For example, in CuSO4-5H2O, the four water molecules are coordinated to the central Cu2+ ion while the fifth water molecule is hydrogen bonded to the sulfate group. Thus, CuSO4 . 5H2O be represented as [C(H2O)4]SO4.H2O.
- Water molecules may occupy the interstitial sites in the may crystal lattice. In barium chloride dihydrate, (BaCl2-2H2O), for example, the two H2O molecules occupy the voids of the crystal lattice.
8. Water absorbents: Many substances like concentrated H2SO4, P2O5, fused CaCl2, CaO, magnesium perchlorate [Mg(CO4)2, anhydrous], dehydrated silica gel (SiO2 . xH2O); anhydrous Na2SO4, etc., has the capacity to absorb a certain amount of water. These are known as dehydrating or desiccating agents. Although such absorption of water by the desiccating agents is often a physical process, in some cases chemical changes may also occur. For example, P2O5 and CaO absorb water to form H3PO4 and Ca(OH)2 respectively.
Desiccating agents are usually employed to dry moist substances and also to remove water from the sphere of the ‘ reaction.
- Concentrated sulphuric acid is used as a dehydrating agent in the esterification of an organic acid with 1 alcohol, where it absorbs the water formed in the reaction and thus helps to increase the yield of the ester.
- A moist gas or a moist liquid may be dried with the help of a desiccating agent. But, in such cases, the desiccating agent should be properly chosen so that it does not react chemically with the substance to be dried. Thus, moist NH3 gas cannot be dried by P2O5 or concentrated H2SO4 because they react with NH3 to form ammonium phosphate and ammonium sulfate respectively. Also, it cannot be dried by fused CaCl2 1 because it forms an additional compound CaCl2 • 8NH3.
- Similarly, moist H2S cannot be dried by the cone. H2SO4 or CaO and this is because cone. H2SO4 oxidizes H2S to sulfur and CaO reacts with acidic H2S to form calcium sulphide.
- Organic liquids are generally dried by anhydrous Na2SO4, fused CaCl2, or anhydrous K2CO3.
Unusual Properties Of Water
- The three states of water (solid, liquid, and gas) can easily be interconverted.
- Despite having low molecular mass water is a liquid at room temperature because its molecules remain associated through hitermolcciilar hydrogen bonding.
- Water is an excellent solvent for many Ionic as well as covalent compounds because of its high dielectric constant, dipole moment, and ability to form 11 bonds.
- The density of water is the highest in cm-3 at 4C, Ice has a larger volume for a given mass of water (11 cm3 of water freezes to yield 12cm, of ice), Thus, the density of ice is less than that of water and it floats over water.
- Conversion of ice into water and vaporization of water involves cleavage of numerous H-bonds and because of this, the melting point of ice, the latent heat of fusion of ice, the specific heat of water, the boiling point of water, the latent heat of vaporization of water, etc., have remarkably high values. Due to much higher values of the specific heat of the water and the latent heat of its vaporization, water plays a significant role in controlling the atmospheric and body temperatures.
- Water is a very stable compound and its dissociation temperature is extremely high. So the production of superheated steam by application of heat under pressure has been feasible and with its help, the generation of electricity through a turbine has been a very common commercially available process.
- Pure water is a conductor of heat and electricity.
Identification Of Water
- When a drop of water is added to anhydrous copper sulfate (CuSO4), its color changes from white to blue due to its conversion into hydrated copper sulfate (CUSO4-5H2O).
- Blue-colored silica gel (SiO2-xH2O) containing Co (2) salt becomes reddish-pink in the presence of water.
- Pure water can be identified from its melting point (0°C) and boiling point (100°C) at atmospheric pressure.
Heavy Water Or Deuterium Oxide (D2O)
Chemical Properties of Hydrogen: Class 11 Notes
Chemically, heavy water is deuterium oxide (D2O). It is called heavy water because it is obtained when oxygen combines with deuterium \(\left({ }_1^2 \mathrm{H}\right)\), the heavy isotope of hydrogen. It was discovered by Harold C. Urey, an American chemist in 1932. He showed that 6000 parts of ordinary water contain 1 part of heavy water.
Preparation
The main source of heavy water is ordinary water from which it is prepared by
The following methods:
By prolonged electrolysis of ordinary water:
The electrolysis of H2O occurs at a faster rate as compared to D2O and as the electrolysis continues, the concentration of heavy water in ordinary water gradually increases. When the amount of the liquid reduces to a small volume, almost pure D2O is obtained.
Electrolyte: Alkaline solution of water [-0.5 (N) NaOH].
Anode: Cylindrical sheet of nickel.
Cathode: Cylindrical steel cell.
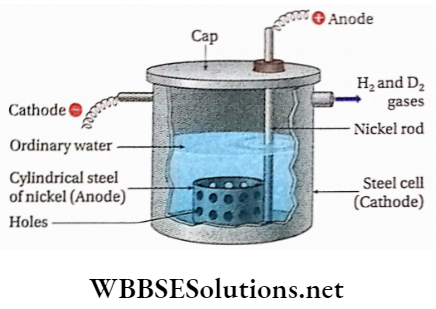
Procedure: In this method, electrolysis of ordinary water containing NaOH [nearly 0.5(N) NaOH solution] is carried out in a cylindrical steel cell. The cell itself acts as a cathode. A perforated cylindrical sheet of nickel acts as an anode. The electrolysis is carried out in different stages. The concentration of D2O in the residual liquid obtained after the 7th stage is about 99%. Almost 29000L of ordinary water is to be electrolyzed to obtain 1L of 99% pure D2O.
When ordinary water is electrolyzed, diprotium (H2) is liberated much more rapidly than deuterium (D2) because:
- Relatively smaller H+ ions have greater mobility than that of D+ ions.
- H+ ions having lower discharge potential are discharged at the cathode more easily than D+ ions.
- H-atoms combine more rapidly to form H, than D-; atoms to form D2.
- The O —H bond is weaker than the O—D bond.
By fractional distillation of ordinary water: The boiling points of ordinary water (H2O) and heavy water (D2O) are 100°C and 101.42°C respectively. Because of the small difference in boiling points, they cannot be separated by ordinary distillation but they can be separated by fractional distillation.
The fractional distillation of ordinary water is carried out in a very long (about 13m) fractionating column and the process is repeated several times. The lighter fraction (H2O) is distilled first while the heavier fraction (D2O) is left behind in the vessel. This residual liquid becomes rich in D2O.
Properties Of Heavy Water
Physical properties: Like ordinary water, heavy water is a colorless, tasteless, and odorless liquid. Because of higher molecular mass, all the physical constants of heavy water are higher than the corresponding values of ordinary water.
Chemical properties: Although heavy water is chemically similar to ordinary water, its reactions are slower than those of ordinary water and this is because the O —D bond is stronger than the O —H bond.
Some of the important reactions are given below:
1. Reaction with alkali and alkaline earth metals:
2Na+2D2O→2NaOD+D2; Ca+2D2O→Ca(OD)2+D2
2. Reaction with metal oxides:
Na2O+D2O→2NaOD [Sodium deuteroxide]
CaO+D2O→Ca(OD)2 [Calcium deuteroxide]
3. Reaction with non-metallic oxides:
N2O5+D2O→2DNO3 [Deuteronitric acid]
SO3+D2O→D2SO4 [Deuterosulphuric acid]
CO2+D2O→D2CO3 [Deuterocarbonic acid]
P2O5+3D2O→2D3PO4 [Deuterophosphoric acid]
4. Reactions with metallic carbides, nitrides, phosphides and arsenides:
CaC2+2D2O→Ca(OD)2+C2D2 [Deuteroacetylene]
Al4C3+12D2O→4A1(OD)3+3CD4 [Deuteromethane]
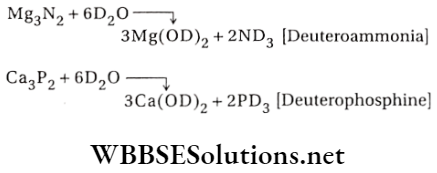
Na3As+3D2O→3NaOD+AsD3 [Deuteroarsine]
5. Electrolysis: 2D2O→2D2 [at cathode]+O2 [at anode]
4. Exchange reactions: When compounds having active hydrogen are treated with D2O, hydrogen is exchanged by deuterium partially or completely. For example:
⇒ \(\mathrm{NH}_4 \mathrm{Cl}+4 \mathrm{D}_2 \mathrm{O} \rightleftharpoons \mathrm{ND}_4 \mathrm{Cl}+4 \mathrm{HDO}\)
⇒ \(\mathrm{NaOH}+\mathrm{D}_2 \mathrm{O}\rightleftharpoons\mathrm{NaOD}+\mathrm{HDO}\)
⇒ \(\mathrm{HCl}+\mathrm{D}_2 \mathrm{O} \rightleftharpoons \mathrm{DCl}+\mathrm{HDO}\)
⇒ \(\mathrm{CHCl}_3+\mathrm{D}_2 \mathrm{O} \rightleftharpoons \mathrm{CDCl}_3+\mathrm{HDO}\)
Formation of deuterates: Like ordinary water heavy water combines with many salts as heavy water of crystallization. The heavy hydrates thus obtained are called deuterates. Some examples are Na2SO4-10D2, CuSO4-5D2O, MgSO4-7D2O etc.
Physiological effect: Heavy water (D2O) is injurious to men, animals, and plants because it slows down the reactions occurring in them. It has also been established that heavy water has germicide and bactericide properties.
Uses Of Heavy Water
- Heavy water is extensively used as a moderator (the substance used for slowing down the speed of neutrons) in 4 nuclear reactions.
- It is used as a tracer compound for studying various mechanisms or organic reactions and various physiological processes occurring in the body.
- It is used for the preparation of deuterium (D2).
- It is used for the preparation of various deuterium-containing compounds.
- It is used as a solvent in NMR spectroscopic studies.
Soft Water And Hard Water
Water may be classified into two categories depending on its behavior towards soap.
These are as follows:
Soft water: Water that readily forms lather with soap is called soft water. Some examples of soft water are distilled water, demineralized water, and rainwater.
Hard water: Water that does not form lather with soap readily is called hard water. Hard water forms insoluble scum with soap. Some examples of hard water are river water, seawater, spring water, lake water, well water, and tap water.
Causes Of Hardness Of Water
- The hardness of natural water is due to the presence of soluble salts like bicarbonates, chlorides, and sulfates of calcium and magnesium. Water gets contaminated by these salts when it passes through the soil, mountains, and rocks.
- Ordinary soap is sodium or potassium salt of certain higher fatty acids such as stearic acid, palmitic acid, oleic acid, etc. These salts are soluble in water and dissolve in water to form a lather.
- But the calcium and magnesium salts of these acids, being insoluble in water, do not produce lather. If the water contains calcium or magnesium salts, then they react with soap to form scum or curdy white precipitates of calcium or magnesium salts of the higher fatty acids.
⇒ \(\begin{aligned} & 2 \mathrm{C}_{17} \mathrm{H}_{35} \mathrm{COONa}+\mathrm{M}^{2+} \rightarrow\left(\mathrm{C}_{17} \mathrm{H}_{35} \mathrm{COO}\right)_2 \mathrm{M} \downarrow+2 \mathrm{Na}^{+} \\ & \text {Sodium stearate } \quad \text { (from } \quad \text { Metal stearate } \quad(\mathrm{M}=\mathrm{Ca}, \mathrm{Mg}) \\ & \text { (soap) hard water) (white precipitate) } \end{aligned}\)
As the soap water now contains no sodium salt or fatty acids, lather is not produced. After the complete removal of Ca2+ and Mg2+ ions as precipitate by using a sufficient amount of soap, lather is again produced. For these reasons, the use of soap in hard water leads to the wastage of soap. Hard water is, therefore, not suitable for washing purposes.
- With the knowledge of the cause of the hardness of water, it becomes quite clear that the presence of Na and K-salts in water does not make it hard. But, if some soluble salts of heavy metals like Zn, Al, Ag, Pb, etc. whose stearates, palmitates, and oleates are insoluble in water, are added to a sample of soft water (For example distilled water), will behave as hard water.
- If some acid (For example HCl, H2SO4, etc.) which may react with soap to precipitate the fatty acids, is added to soft water, it will also behave as hard water
For example:
⇒ \(\begin{aligned} & \mathrm{C}_{17} \mathrm{H}_{35} \mathrm{COONa}+\mathrm{HCl} \rightarrow \mathrm{C}_{17} \mathrm{H}_{35} \mathrm{COOH} \downarrow+\mathrm{NaCl} \\ & \text { Sodium stearate } \quad \text { Stearic acid } \\ & \end{aligned}\)
Types Of Hardness Of Water
Depending on the nature of the salt present, the hardness of water may be divided into two types Temporary hardness and Permanent hardness.
Temporary hardness: The hardness of water which is caused by the presence of bicarbonates of calcium and magnesium and can easily be removed by simply boiling the water is known as temporary hardness and water possessing such hardness is called temporary hard water. It is also termed as carbonate hardness.
Rainwater dissolves small quantities of atmospheric carbon dioxide forming a very dilute solution of carbonic acid. This water reacts with the calcium and magnesium carbonates present mainly in mountains and rocks over which it flows and as a result, soluble bicarbonates are formed. Thus soft rain water becomes hard.
⇒ \(\begin{aligned} & \mathrm{MCO}_3+\mathrm{CO}_2+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{M}\left(\mathrm{HCO}_3\right)_2[\mathrm{M}=\mathrm{Ca} \text { or } \mathrm{Mg}] \\ & \text { [insoluble] } \quad \text { [soluble] } \\ & \end{aligned}\)
Permanent hardness: The hardness of water which is caused by the presence of chlorides and sulfates of calcium and magnesium and cannot be removed by simply boiling is known as permanent hardness and water possessing such hardness is called permanent hard water. It is also called non-carbonate hardness.
Disadvantages of using hard water
In domestic use: Hard water is not suitable for cooking because pulses and vegetables are not cooked well in it. Moreover, water with excessive hardness is not suitable for drinking and is harmful to health.
In laundry use: Hard water is not suitable for laundry purposes because its use results in considerable wastage of soap. Yellow stains may appear on clothsifiron salts are present in hard water.
This disadvantage can be overcome If detergent is used In hard water instead of soap. Calcium and magnesium salts of higher fatty acids are insoluble in water while calcium or magnesium salts of detergent are soluble in water. So, the use of hard water does not involve any wastage of detergent. Moreover, it gives lather more easily than soap.
In boiler use: Hard water cannot be used to produce steam in boilers.
The reasons are as follows:
- Hard water containing Mg(HCO3)2 and Ca(HCO3)2, on boiling, forms a hard heat-insulating thick layer or scale of MgCO3 and CaCO3 on the inner surface of the boiler. As a result of this, much heat is required to raise the temperature of the boiler, and thus, fuel economy is adversely affected.
Again at much higher temperatures, the boiler scales and the metal of the boiler expand unequally. Due to such uneven expansion, cracks are formed on the scales.
Through these cracks, when hot water comes in contact with the hot metal surface of the boiler, it is suddenly converted into steam. Due to the high pressure thus developed, the boiler may burst leading to serious accidents.
- MgCl2, MgSO4, CaCl2, etc., likely to be present in hard water, may undergo hydrolysis at high temperatures, producing strong acids like HCl or H2SO4. These acids slowly corrode the inner surface of the boiler and thus, reduce the longevity of the boiler.
⇒ \(\mathrm{MgCl}_2+2 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Mg}(\mathrm{OH})_2+2 \mathrm{HCl}\)
⇒ \(\mathrm{MgSO}_4+2 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Mg}(\mathrm{OH})_2+\mathrm{H}_2 \mathrm{SO}_4\)
4. In industrial use: Hard water cannot be used in industry for cooling. This is because if it is used, the inner surface of the cooling coil may be coated with a layer or scale having poor thermal conductivity. Thus, cooling efficiency is affected by the consequent wastage of energy.
Removal Of Hardness Of Water Or Softening Of Hard Water
The process of removal of Ca2+ and Mg2+ ions responsible for the hardness of water is known as softening. water. Depending upon the nature of dissolved salts, many methods are available to soften hard water.
Removal Of Temporary Hardness
The temporary hardness of water can be removed by the following methods:
Boiling process: When temporary hard water is boiled, the bicarbonate salts of calcium and magnesium decompose to form Insoluble calcium and magnesium carbonates respectively which is filtration.
⇒ \(\mathrm{Ca}\left(\mathrm{HCO}_3\right)_2 \rightarrow \mathrm{CaCO}_3 \downarrow+\mathrm{CO}_2 \uparrow+\mathrm{H}_2 \mathrm{O}\)
⇒ \(\mathrm{Mg}_{\left(\mathrm{HCO}_3\right)_2} \rightarrow \mathrm{MgCO}_3 \downarrow+\mathrm{CO}_2 \uparrow+\mathrm{H}_2 \mathrm{O}\)
As MgCO3 has significant solubility in water, the temporary hardness caused by Mg(HCO3)2 cannot be removed completely.
Clark’s process: In this process, a calculated amount of slaked lime, Ca(OH)2, is added to the temporary hard water. The soluble bicarbonates are converted into insoluble carbonates and get precipitated. The precipitate is removed by filtration.
⇒ \(\mathrm{Ca}\left(\mathrm{HCO}_3\right)_2+\mathrm{Ca}(\mathrm{OH})_2 \rightarrow 2 \mathrm{CaCO}_3 \downarrow+2 \mathrm{H}_2 \mathrm{O}\)
Because of the appreciable solubility of MgCO3, it further reacts with Ca(OH)2 to give a precipitate of Mg(OH)2.
⇒ \(\mathrm{MgCO}_3+\mathrm{Ca}(\mathrm{OH})_2 \rightarrow \mathrm{Mg}(\mathrm{OH})_2 \downarrow+\mathrm{CaCO}_3 \downarrow\)
If the quantity of Ca(OH)2 is less than the requisite amount, hardness due to magnesium still persists in water and this is because 1 mol of Mg(HCO3)2 requires 2 mol of Ca(OH)2.
Again, if the quantity of Ca(OH)2 is more than required, artificial hardness is created due to the absorption of atmospheric CO2 by Ca(OH)2 leading to the formation of Ca(HCO3)2. Therefore, in this process, the calculated quantity of slaked lime should be used.
Removal Of Permanent Hardness
The permanent hardness of water can be removed by the following methods:
Washing soda process: in this process, hard water is treated with a calculated amount of washing soda (Na2CO3.10H2O) when Ca2+ and’ Mg2+ ions are precipitated as their insoluble carbonates which can be easily filtered off.
⇒ \(\mathrm{MCl}_2+\mathrm{Na}_2 \mathrm{CO}_3 \rightarrow \mathrm{MCO}_3 \downarrow+2 \mathrm{NaCl} ;\)
⇒ \(\mathrm{MSO}_4+\mathrm{Na}_2 \mathrm{CO}_3 \rightarrow \mathrm{MCO}_3 \downarrow+\mathrm{Na}_2 \mathrm{SO}_4[\mathrm{M}=\mathrm{Mg} \text { or Ca}]\)
Calgon process: In the Ihls method, Ca2+ and Mg2+ Ions are rendered Ineffective (masked) by the addition of sodium Na2[Na4(PO3)6] commercially called ‘Calgon’ (meaning calcium gone) which forms soluble complexes with soap and so, facilitates the formation of lather.
⇒ \(\underset{\text { Calgon }}{2 \mathrm{Ca}^{2+}+\mathrm{Na}_2\left[\mathrm{Na}_4\left(\mathrm{PO}_3\right)_6\right]} \underset{\text { Soluble complex }}{\mathrm{Na}_2\left[\mathrm{Ca}_2\left(\mathrm{PO}_3\right)_6\right]+4 \mathrm{Na}^{+}}\)
⇒ \(\begin{array}{cc} 2 \mathrm{Mg}^{2+}+\mathrm{Na}_2\left[\mathrm{Na}_4\left(\mathrm{PO}_3\right)_6\right] & \mathrm{Na}_2\left[\mathrm{Mg}_2\left(\mathrm{PO}_3\right)_6\right]+4 \mathrm{Na}^{+} \\ \text {Calgon } & \text { Soluble complex } \end{array}\)
Ion Exchange Process: This is the most modern method for softening hard water. In this method, Ca2+ and Mg2+ ions present in hard water are exchanged by those present in ion, exchangers (complex inorganic and organic compounds) which are mainly of two types:
Inorganic cation exchangers (Pcrmutit): These are complex inorganic salts like hydrated sodium-aluminum silicates represented by the general formula Na2Z, where Z = Al2Si2O8. xH2O, which possess interestingproperty of exchanging Ca2+ and Mg2+ ions presentin hard water with their Na+ ions. These complex salts are known as zeolites (naturally occurring) or permit (synthetic).
⇒ \(\underset{\substack{\text { Sodium } \\ \text { zeolite }}}{\mathrm{Na}_2 \mathrm{Z}}+\underset{\text { from hard }}{\mathrm{MCl}_2} \rightarrow \underset{\substack{\text { Calcium or } \\ \text { Magnesium } \\ \text { zeolite }}}{\mathrm{MZ}}+2 \mathrm{NaCl}[\mathrm{M}=\mathrm{Ca} \text { or } \mathrm{Mg}]\)
Regeneration of permit: As the process continues, the whole of the permit gets exhausted because of its conversion into calcium and magnesium zeolite. The exhausted resin can, however, be regenerated by passing a 10% solution of NaCl (called brine) through it.
⇒ \(\underset{\substack{\text { Exhausted } \\ \text { resin }}}{\mathrm{MZ}+2 \mathrm{NaCl}} \underset{\substack{\text { Regenerated } \\ \text { resin }}}{\mathrm{Na}_2 \mathrm{Z}}+\mathrm{MCl}_2[\mathrm{M}=\mathrm{Ca} \text { or } \mathrm{Mg}]\)
Advantages of the permit process: It is an efficient and cheap process (only NaCl is consumed) that can be used to remove both the temporary and permanent hardness of water completely.
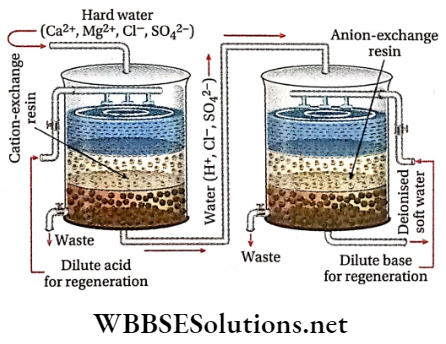
Organic ion exchangers (Resins): These synthetic ion exchangers (also called ion exchange resins) are superior to zeolites because these can remove all types of cations [Na+, Ca2+, Mg2+, etc.] and anions [Cl-, SO4, HCO3, etc.] presentin’ hard water. Thus, water obtained by this method is free from all types of cations and anions and is as good as distilled water. This is called deionized or demineralized water. Ion exchange resins (giant organic molecules of high molecular mass) are of two types.
Cation exchange resins: These are complex organic molecules consisting of a giant hydrocarbon framework attached to acidic groups such as —COOH (carboxyl) or —SO3H (sulphonic acid) groups and are represented by the general formula R —COOH or R —SO3H.
Since these resins are capable of exchanging the H+ ions of their acidic groups with cations such as Ca2+, Mg2+, etc. present in hard water, these are called cation exchange resins or simply cation exchangers.
Anion exchange resins: These are also complex organic molecules consisting of giant hydrocarbon frameworks attached to basic groups, such as OH– ions derived from amines usually in the form of substituted ammonium hydroxide.
These may be represented by the general formula \(\mathrm{R}-\stackrel{\oplus}{\mathrm{N}} \mathrm{H}_3 \stackrel{\ominus}{\mathrm{O}} \mathrm{H}\). Since these resins are capable of exchanging their OH– ions with anions such as Cl–, \(\mathrm{SO}_4^{2-}\), \(\mathrm{HCO}_3^{-}\) etc. present in hard water, these are called anion exchange resins or simply anion exchangers.
Function of resin: Hard water is first passed through cation exchange resins when all the cations (Ca2+, Mg2+, Na+, etc.) present in water are exchanged with H+ ions of the resin.
⇒ \(\mathrm{M}^{2+}+2 \mathrm{RSO}_3 \mathrm{H} \rightarrow \mathrm{M}\left(\mathrm{RSO}_3\right)_2+2 \mathrm{H}^{+}[\mathrm{M}=\mathrm{Ca} \text { or } \mathrm{Mg}]\)
From hard Cation exchange Exhausted water resin resin
Due to the release of the proton, the resulting water becomes acidic. This water is then passed through anion exchange resin when all the anions (Cl-, \(\mathrm{SO}_4^{2-}\), etc.) present in water are exchanged with OH- ions of the resin.
⇒ \(\underset{\substack{\text { From hard } \\ \text { water }}}{\mathrm{CI}^{-}}+\underset{\substack{\text { Anion exchange } \\ \text { resin }}}{\stackrel{+}{\mathrm{NH}_3 \mathrm{OH}^{-}}} \rightarrow \underset{\substack{\text { Exhausted } \\ \text { resin }}}{\stackrel{+}{\mathrm{NH}_3 \mathrm{Cl}^{-}}+\mathrm{OH}^{-}}\)
⇒ \(\underset{\substack{\text { From hard } \\ \text { water }}}{\mathrm{SO}_4^{2-}}+\underset{\substack{\text { Anion exchange } \\ \text { resin }}}{2 \mathrm{RN}_3^{+} \mathrm{OH}^{-}} \rightarrow \underset{\substack{\text { Exhausted } \\ \text { resin }}}{\left(\mathrm{RN}_3\right)_2 \mathrm{SO}_4^{2-}}+2 \mathrm{OH}^{-}\)
The liberated OH– ions neutralize the H+ ions set free in cation exchange resin (H++OH–→H2O). Therefore, the collected water from anion exchange resin is free from all types of cations as well as anions. This is what is called deionized or demineralized water.
This is as pure as distilled water and can be used instead of distilled water in industry and in laboratories. However, this water may contain some organic impurities, some dissolved gases, or pyrogen. Deionized water is prepared by a machine known as a deioniser.
Regeneration of resins: The exhausted cation exchange resin is regenerated by treating it with moderately concentrated HCl or H2SO4 and the exhausted anion exchange resin is regenerated by treating it with moderately concentrated NaOH solution.
⇒ \(\begin{array}{lc} \mathrm{M}\left(\mathrm{RSO}_3\right)_2+2 \mathrm{HCl} \rightarrow \mathrm{MCl}_2 & +2 \mathrm{RSO}_3 \mathrm{H} \\ \quad \text { Exhausted } & \text { Regenerated } \\ \text { resin } & \text { resin } \end{array}\)
⇒ \(\underset{\substack{\text { Exhausted } \\ \text { resin }}}{\stackrel{+}{\mathrm{N}} \mathrm{H}_3 \mathrm{Cl}^{-}}+\mathrm{NaOH} \rightarrow \underset{\substack{\text { Regenerated } \\ \text { resin }}}{\stackrel{+}{\mathrm{N}} \mathrm{H}_3 \mathrm{OH}^{-}}+\mathrm{NaCl}\)
The ion exchange resins are not capable of removing non¬ electrolytes like sugar, urea, etc. from water. Therefore, the deionized water obtained by this process may contain these types of impurities. Distilled water does not contain similar impurities.
Manufacturing Of Drinking Water
Drinking water should necessarily be free from suspended impurities, organic matter, and germs. Water from rivers or lakes, properly purified, is supplied in towns and cities. Sometimes underground water, lifted by a pump, is also supplied for domestic use because it is not generally contaminated with microbes.
Drinking water is prepared from the river or, Jake water through the steps as follows:
- Precipitation of colloidal particles and some bacteria by the process of coagulation using alum [K2SO4-Al2(SO4)3-24H2O],
- Removal of these impurities by filtration,
- Removal of different ions causing hardness by ion exchange method and
- Sterilization by passing Cl2 or O3 gas or by exposing it to UV rays.

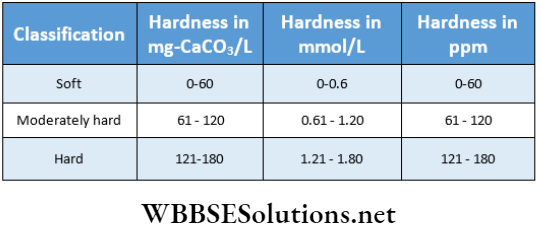
Degree Of Hardness Of Water
Degree Of Hardness Of Water Definition: The degree of hardness of water is defined as the number of parts by mass of CaCO3 (calcium carbonate) equivalent to various calcium and magnesium salts present in one million parts by mass of water.
Unit: It is expressed in ppm (parts per million).
Example: A million parts by mass of a sample of water contain salts causing its hardness which are equivalent to x parts by mass of calcium carbonate, then the hardness of this sample of water is x ppm.
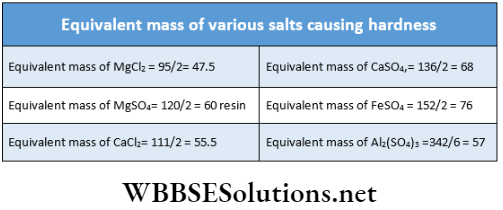
Now, the equivalent mass of CaCO3 \(=\frac{100}{2}=50\)
Therefore, 1 gram-equivalent or 1 /2 mol or 50 g of CaCO3 = 1
Gram-equivalent of any salt causing hardness =\(\equiv \frac{1}{2} \mathrm{~mol} \mathrm{or} 47.5 \mathrm{~g}\) \(\equiv \frac{1}{2} \mathrm{~mol} \text { or } 55.5 \mathrm{~g}\) of \(\mathrm{CaCl}_2 \equiv \frac{1}{2} \mathrm{~mol} \text { or } 60 \mathrm{~g} \text { of }\) \(\mathrm{MgSO}_4 \equiv \frac{1}{6} \mathrm{~mol} \text { or } 57.9 \mathrm{~g} \text { of } \mathrm{Al}_2\left(\mathrm{SO}_4\right)_3\) etc.
Numerical Examples
Question 1. Calculate the degree of hardness of a sample of hard water that is found to contain 72mg of MgSO4 per kg of water.
[Here our objective is to find out the mass of CaCO3 equivalent of MgSO4 present in one million parts of water.]
Answer: Now, 1 kg or 103 g of water contains 72 mg MgSO4
106 g of water contains 72 X 103 mg = 72g MgSO4
Now, 60 g MgSO4 = 50g CaCO3
or, 72g MgSO4 \(\equiv \frac{50 \times 72}{60} \mathrm{~g}\) CaCO3 = 60 g CaCO3
Thus, the degree of hardness of that sample = 60 ppm.
Question 2. Estimate the hardness of a sample of water 1L which contains 0.001 mol of dissolved MgCl2.
Answer: We know, 1 mol MgCl2= 1 mol CaCO3
or, 0.001 mol MgCl2 = 0.001 mol CaCO3
Now, 0.001 mol CaCO3 = 100 x 0.001 for O.lgof CaCO3
So, in 1L 1000 g, or 103g water, the amount of CaCO3 equivalent to 0.001 mol MgCl2 is 0.1 g.
In 106 g water, the amount of CaCO3 is\(\frac{0.1 \times 10^6}{10^3} \mathrm{~g}=100\)
Hence, the degree of hardness of that sample of water = 100 ppm
Question 3. IL of river water contains 6 mg Mg2+ and 20 mg Ca2+ ions as chloride salts. Determine the degree of hardness of that sample of river water.
Answer:
Given
IL of river water contains 6 mg Mg2+ and 20 mg Ca2+ ions as chloride salts.
6mg Mg2+ = 0.006 g Mg2+
and20mg Ca2+ = 0.02 g Ca2+ ion.
Now, 24 g Mg2+ = 95 g MgCl2 = 100 g CaCO3.
[∴ atomic mass of. Mg = 24 and molecular mass of MgCl2 = 95 and CaCO3 = 40]
∴ 0.006 g Mg2+\(=\frac{100 \times 0.006}{24} \mathrm{~g}=0.025 \mathrm{~g} \mathrm{CaCO}_3 .\)
Again, 40 g Ca2+ = 111 g CaCl2 = 100 g CaCO3
[∴ atomic mass of Ca = 40 & molecular mass of CaCl2 = 111]
∴ \(0.02 \mathrm{~g} \mathrm{Ca}^{2+}=\frac{100 \times 0.02}{40} \mathrm{~g}=0.05 \mathrm{~g} \mathrm{CaCO}_3\)
So, the quantity of CaCO3 equivalent to MgCl2 and CaCl2 presentin 1Lor 1 O3 g water = (0.025 + 0.05) g =0.075 g.
∴ \(10^6 \mathrm{~g} \text { of water contains } \frac{0.075 \times 10^6}{10^3}=75 \mathrm{~g}^3 \mathrm{CaCO}_3 .\)
Hence, the degree of hardness of the sample is 75 ppm.
Question 4. The degree of hardness of a sample of water is 40 ppm. If the hardness is only due to the presence of MgSO4, then determine the amount of MgSO4 in 1 kg of that water.
Answer:
Given
The degree of hardness of a sample of water is 40 ppm. If the hardness is only due to the presence of MgSO4,
The hardness of the sample of water is 40 ppm.
Therefore, 106 g of that sample contains 40 g CaCO3.
\(1 \mathrm{~kg} \text { or } 10^3 \mathrm{~g} \text { of water contains } \frac{40 \times 10^3}{10^6}=0.04 \mathrm{~g} \mathrm{CaCO}_3\)
Now,1 mol CaCO3 = 1 mol MgSO4 or, 100 g CaCO3= 120 g MgSO4
[molecular mass of CaCO3 = 100 and MgSO4 = 120]
\(\text { or, } \quad 0.04 \mathrm{~g} \mathrm{CaCO}_3 \equiv \frac{120 \times 0.04}{100} \mathrm{~g} \quad \text { or, } 0.048 \mathrm{~g} \mathrm{MgSO}_4\)
Hence, the amount of MgSO4 present per kg of that water is 0.048 g or 48 mg.
Hydrogen Bonding Explained for Class 11
Question 5. 10 mL of 0.01 (N) HCl is required for titrating 100mL of a sample of cold water usingmethyl orange as an indicator. Determine the temporary hardness of that sample of water.
Answer:
Given
10 mL of 0.01 (N) HCl is required for titrating 100mL of a sample of cold water usingmethyl orange as an indicator.
10mL O.Ol(N) HCl solution =1 mL 0.1(N) HCl
1 g equivalentHCI=1 g equivalent CaCO3
\(1000 \mathrm{~mL} 1(\mathrm{~N}) \mathrm{HCl} \equiv \frac{100}{2} \mathrm{~g} \mathrm{CaCO}_3\)
\(1 \mathrm{~mol} 0.1(\mathrm{~N}) \mathrm{HCl} \equiv 50 \times \frac{1}{1000} \times 0.1 \mathrm{~g} \mathrm{CaCO}_3\)
= 0.005g CaCO3
Therefore, in lOOmL or lOOg of that sample of water contains some hardness-producing substance which is equivalent to 0.005g CaCO3
106g of water contains the hardness-producing substance equivalent to \(\frac{0.005 \times 10^6}{10^2}=50 \mathrm{~g} \mathrm{CaCO}_3\)
Hence, the hardness of that sample of water is = 50 ppm.
Question 6. Determine the weight of CaO required to remove the hardness of a sample of 105L water, 1L of which contains 1.62gof Ca(HCO3)2.
Answer: When CaO is added to a sample of hard water containing Ca(HCO3)2, dissolved bicarbonate is precipitated as CaC3 and as a result, the hardness is removed.
\(\begin{gathered} \mathrm{CaO}+\mathrm{Ca}_{\left(\mathrm{HCO}_3\right)_2}=2 \mathrm{CaCO}_3 \downarrow+\mathrm{H}_2 \mathrm{O} \\ \text { molecular mass }=56 \text { molecular mass }=162 \end{gathered}\)
I L of water contains 1.62 g of Ca(HCO3)2
∴ 105L of water contains 1.62 x 105 g of Ca(HCO3)2.
According to the above equation,
For removing 162 g Ca(HCO3)2, 56 g CaO is required
∴ \(\text { Amount of } \mathrm{CaO} \text { required }=\frac{56 \times 1.62 \times 10^5}{162} \mathrm{~g}=56 \times 10^3 \mathrm{~g}\)
Structure Of H2O2 Molecule
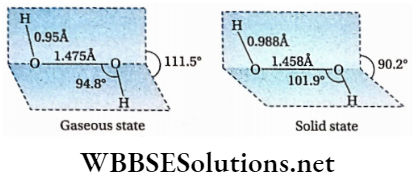
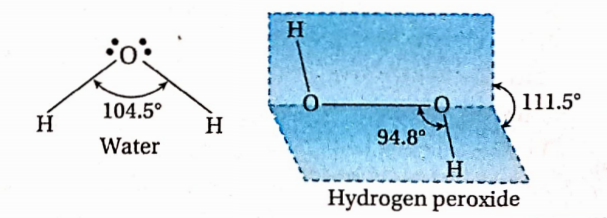
The structure of the hydrogen peroxide molecule is H—O—O—H. There is a peroxide linkage (—0—0— ) in the molecule. The molecule is non-planar and it has an open-book-like shape. The two 0 —H bonds lie in different places and this is due to repulsion between the —OH groups. In the gas phase, the dihedral angle and the H —O —O bond angle are 111.5° and 94.8° respectively. However, in the crystalline state, these are 90.2° and 101.9° due to intermolecular hydrogen bonding, Because of such shape, H2O2 is polar.
Properties Of Hydrogen Peroxide
Physical properties
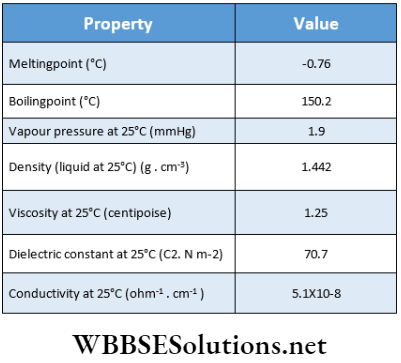
- Pure hydrogen peroxide is an almost colorless syrupy liquid (with a tinge of pale blue) that has a bitter taste and smell similar to HNO3.
- It is denser (1.44 g-cm-3) and more viscous than water because the molecules of H2O2 (with two different —OH groups) are even more highly associated through intermolecular H-bonding than H20 molecules. Also due to this reason, its boiling point is higher than that of water.
- It is soluble in water, ether, and alcohol in all proportions.
- It has both polar and non-polar bonds.
⇒ \(\begin{array}{r} \text { polar bond } \\ \mathrm{H}-\mathrm{O}-\mathrm{O} \downarrow-\mathrm{H} \\ \text { non-polar bond } \end{array}\)
Chemical Properties
Decomposition: Hydrogen peroxide is an unstable liquid that decomposes into water and oxygen on long-standing or heating.
⇒ \(2 \mathrm{H}_2^{-1} \mathrm{O}_2 \rightarrow 2 \mathrm{H}_2^{-2} \mathrm{O}+\stackrel{0}{\mathrm{O}_2} ; \Delta H=-196.0 \mathrm{~kJ}\)
It is an example of a disproportionation reaction because H2O2 undergoes both oxidation and reduction. Since it is a very unstable compound and decomposes readily on heating, it is impossible to determine its boiling point at atmospheric pressure. However, it can be determined by the extrapolation method.
- The presence composition of metal of powders H2O2is[e.g,furtherCo, Au, accelerated, Pt, etc.), by some metal ions [Example Fe2+, Co2+, Ni2+, etc.), metal oxides (for example MnO2), charcoal, basic substances, dust particles, rough surfaces or even sunlight Because of such properties, the solution of H2O2 must be handled with care as uncontrolled rapid decomposition may resultin an explosion.
- Its decomposition may, however, be suppressed by the addition of glycerol, acetanilide, phosphoric acid, or urea (all acting as negative catalysts).
- H2O2, because of its very unstable nature, is preserved with a small amount of stabilizers like H3PO4, glycerol, or acetanilide in an opaque polythene bottle or a wax-lined colored glass bottle away from light and at low temperature.
Acidic nature:
Pure H2O2 turns blue litmus red but its dilute solution is neutral to litmus. Thus, it behaves as a very weak acid. In fact, it is a slightly stronger acid (Ka = 1.55 X 10–12 at 25°C) (Ka = 1.0 x 10-14 at 25°C). Since it contains at than water two ionizable or replaceable H-atoms, it reacts with bases like NaOH to form two types of salts, for example, hydroperoxides (acidic salt) and peroxides (normal salts). This property of H2O2 is known as peroxidizing property.
⇒ \(\mathrm{H}_2 \mathrm{O}_2 \rightleftharpoons \mathrm{H}^{+}+\mathrm{HO}_2^{-} \text {(Hydroperoxide ion) }\)
⇒ \(\mathrm{H}_2 \mathrm{O}_2 \rightleftharpoons 2 \mathrm{H}^{+}+\mathrm{O}_2^{2-} \text { (Peroxide ion) }\)
⇒ \( \mathrm{NaOH}+\mathrm{H}_2 \mathrm{O}_2 \rightarrow \mathrm{NaHO}_2 \text { (acidic salt) }+\mathrm{H}_2 \mathrm{O}\)
Sodium hydroperoxide
⇒ \( 2 \mathrm{NaOH}+\mathrm{H}_2 \mathrm{O}_2 \longrightarrow \mathrm{Na}_2 \mathrm{O}_2+\mathrm{H}_2 \mathrm{O}\)
Sodium peroxide
H2O2 cannot decompose carbonates to CO2. H2O2 is weaker add than carbonic add (H2CO3) so it cannot decompose carbonate or bicarbonate salts to liberate CO2
Oxidizing and reducing properties: H2O2 is an oxidizing agent. But in the presence of other oxidizing agents, It behaves as a reducing agent. These oxidising and reducing properties are exhibited both In acidic and alkaline solutions. The oxidation state of oxygen in H2O2 is. It lies in between the tire oxidation state of oxygen in H2O or OH– (-2) and that of O2 (zero). So the oxidation state of oxygen in H2O2 may decrease to\(-2\left(\mathrm{H}_2^{-1} \mathrm{O}_2 \rightarrow \mathrm{H}_2^{-2} \mathrm{O}^{\mathrm{O}} \text { or } \stackrel{-2}{\left.\mathrm{OH}^{-}\right)}\right.\) or increase to zero \(\left(\mathrm{H}_2 \mathrm{O}_2^{-1} \rightarrow \stackrel{0}{\mathrm{O}_2}\right)\) and bacause of this,h2o2 is formed to exhibit both oxidising and reducing properties.
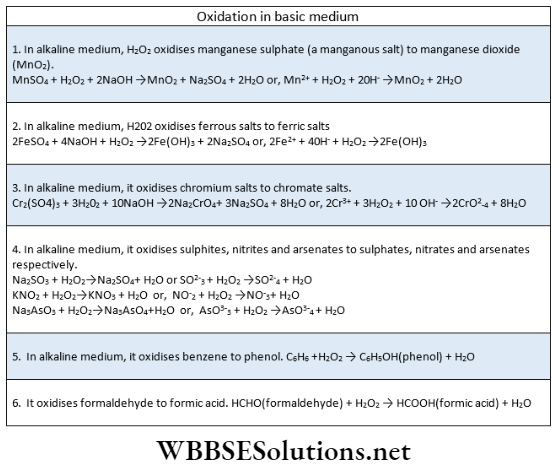
Oxidizing properties: H2O2 can act as an oxidizing agent in both acidic and basic mediums.
In acidic medium: \(\mathrm{H}_2 \mathrm{O}_2+2 \mathrm{H}^{+}+2 e \rightarrow 2 \mathrm{H}_2 \mathrm{O}\)
In basic medium: \(\mathrm{H}_2 \mathrm{O}_2+2 e \rightarrow 2 \mathrm{OH}^{-}\)
Restoration of the color of oil paintings: H2O2 is used to restore the original color of old oil paintings.
- In oil paintings, lead white, a mixture of basic lead acetate, lead carbonate, lead sulfate, etc. is used.
- Lead white, if left exposed to open air for a long time, the compounds present in it react with H2S of air to form insoluble black lead sulfide and as a result, the oil painting gets blackened.
- If the oil painting is washed with an H2O2 solution, lead sulfide init is oxidized to white lead sulfate, and the brightness of the oil painting is regained.
⇒ \(\mathrm{PbCO}_3+\mathrm{H}_2 \mathrm{~S} \rightarrow \mathrm{PbS} \downarrow \text { (black) }+\mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}\)
⇒ \(\mathrm{PbSO}_4+\mathrm{H}_2 \mathrm{~S} \rightarrow \mathrm{PbS}(\text { black }) \downarrow+\mathrm{H}_2 \mathrm{SO}_4 ;\)
⇒ \(\mathrm{Pb}\left(\mathrm{CH}_3 \mathrm{COO}\right)_2+\mathrm{H}_2 \mathrm{~S} \rightarrow \mathrm{PbS}(\text { black }) \downarrow+2 \mathrm{CH}_3 \mathrm{COOH}\)
⇒ \(\mathrm{PbS}(\text { black })+4 \mathrm{H}_2 \mathrm{O}_2 \rightarrow \mathrm{PbSO}_4(\text { white })+4 \mathrm{H}_2 \mathrm{O}\)
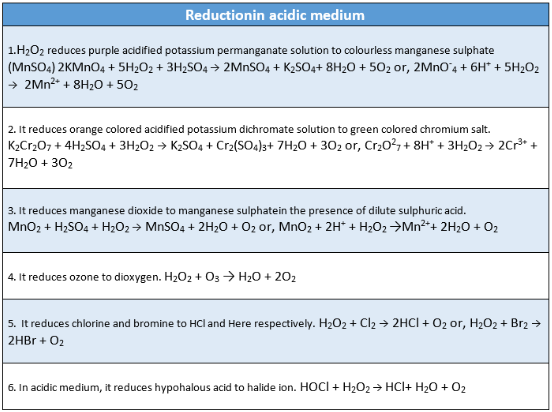
Reducing properties: H2O2 can act as a reducing agent in both acidic and alkaline mediums.
In acidic medium: \(\mathrm{H}_2 \mathrm{O}_2 \rightarrow 2\mathrm{H}^{+}+\mathrm{O}_2+2 e\)
In alkaline medium: \(\mathrm{H}_2 \mathrm{O}_2+2 \mathrm{OH}^{-} \rightarrow 2 \mathrm{H}_2 \mathrm{O}+\mathrm{O}_2+2 e\)
Antichlor property of H2O2: Since H2O2 destroys Cl2 by reducing it to HCl, it is used to remove excess chlorine after bleaching operations in the textile industry. It is known as the chlorine-destroying or antichlor property of H2O2.
Bleaching action: Hydrogen peroxide is used for bleaching delicate articles like ivory, feathers, silk, wool, etc. The bleaching action of H2O2 is due to oxidation of the coloring matter by nascent oxygen liberating on decomposition: H2O2 H2O+[O]
Colouring matter + [O]→ colorless matter
Formation of additional compounds: H2O2 combines with some salts to form additional compounds.
For example, Na2SO4 -H2O2 9H2O; NaBO2-H2O2-3H2O; (NH4)2SO4-H2O2 etc.
These additional compounds are known as perhydrates (hydrates where the water molecules have been replaced by H2O2). Also, it reacts with alkenes to form glycols.
CH2=CH2 + H2O2 → HOCH2CH2OH [ethylene glycol]
Identification And Uses Of H2O2
Identification of H2O2:
1. Perchromic acid test: H2O2 is added to K2Cr2O? solution acidified with dilute H2SO4 and the mixture is shaken with ether. Perchromic acid or chromium pentoxide (CrO5), produced by the reaction of H2O2 with acidified K2Cr2O7 solution, dissolves in ether through the formation of an addition compound that turns the ether layer blue.
K2Cr2O7 + H2SO4 + 4H2O2K2SO4 + 2CrO5 + 5H2O
2. When H2O2 is treated with an acidified solution of titanium salt, the orange color is produced due to the formation of pertitanic acid (H2TiO4).
Ti(SO4)2 + H2O2 + 2H2O → H2TiO4 + 2H2SO4
3. H2O2 liberates iodine from an acidified solution of KI. The liberated iodine turns the starch solution blue
2KI + 2HCl + H2O2 → I2 + 2KCl + 2H2O
Starch + I2 → Deep blue colored complex
Uses of hydrogen peroxide:
- The most important industrial application of hydrogen peroxide acts as a bleaching agent for fine and delicate materials like silk, wool, ivory, paper pulp, leather, oils fats, etc.
- Its dilute solution is used to impart a golden color to hair.
- It is used as an antichlor for removing chlorine from articles bleached by chlorine.
- The dilute solution of H2O2 is used as an antiseptic for washing wounds. This solution is known as perhydrol.
- It is used for restoring the color of old oil paintings.
- It is largely used as an oxidizing agent in the laboratory. A mixture of H2O2 and FeSO4 is called Fenton’s reagent. It is used to oxidize many organic compounds.
- A mixture of H2O2 and hydrazine hydrate with copper (II) catalyst is used as a rocket propellant.
- It is used for preparing chemicals like sodium peroxoborate and peroxocarbonate which are largely used as brighteners in high-quality detergents.
- It is used in the synthesis of hydroquinone, tartaric acid, and certain food products and pharmaceuticals [e.g, cephalosporin) etc.
- H2O2 is being increasingly used to control pollution by—treatment of domestic and industrial effluents, oxidation of cyanides, restoration of aerobic conditions to sewage wastes, etc.
Volume Strength: The volume strength of an H2O2 solution indicates the volume in mL of oxygen that will be evolved at STP on the complete decomposition of lmL of the H2O2 solution. Thus, ’20 volume’ H2O2 solution means that lmL of that solution yields 20mL of oxygen at STP as a result of its complete decomposition.
Percentage Strength: The percentage strength of an H2O2 solution indicates the amount of H2O2 in gram present in 100 mL of this solution. Thus, 30% H2O2 solution means that 100 mL of that solution contains 30 g of H2O2.
Relation between volume strength & percentage strength:
H2O2( 68 g) → 2H2O + O2 [22400 mL at STP]
The equation states that 68 g H2O2 yields 22400 mL O2 at STP.
∴ lg of H2O2 yields 22400/68 mL or 329.4 mL of O2 at STP. Now, if 100 mL of H2O2 solution contains lg of H2O2, then the strength of the solution is 1%.
Hence 100 mL 1% H2O2 solution on being completely decomposed liberates 329.4 mL of O2 at STP.
∴ 1 mL of 1% H2O2 solution on being completely decomerate 329.4/100 mL or 3.249 mL of O2 at STP.
∴ The strength of 1% of H2O2 solution is ‘3.294 volume
Thus, ‘x volume’ H2O2 solution = \(\frac{x}{3.294} \%\) or 0.3036x
H2O2 solution, i.e., if the volume strength of any H2O2 solution is known, then its percentage strength can readily be calculated by multiplying its volume strength by 0.3036. It thus follows that the x% H2O2 solution is stronger than the ‘ x volume’ H2O2 solution.
Relation between volume strength and normal strength:
The equivalent mass of H2O2 \(=\frac{68}{32} \times 8=17.0\) [From the dissociation equation of H2O2 (2H2O2 -> 2HaO + 02) it becomes clear that 8g of O2 is obtained from 17g of H2O2]
Now, 68g of H2O2 produces 22400 mL of O2 at STP.
or, 17g H2O2 produces\(\frac{22400}{68} \times 17=5600 \mathrm{~mL}\) of O2 at STP.
The strength of an H2O2 solution will be (N) if 1000 mL of that solution contains 17g of H2O2.
Thus 1000 mL of (N) H2O2 solution will produce 5600 mL O2 at STP.
or, lmL(N) H2O2 solution will produce 5.6mL O2 at STP.
∴ The volume strength of (N) H2O2 solution =5.6 or, the volume strength of x (N) H2O2 solution = 5.6 x. The volume strength of the H2O2 solution of any normality can be obtained by multiplying its normal strength by 5.6.
1. Volume strength
= 5.6 x normality
⇒ \(=5.6 \times \frac{\text { percentage strength }}{17} \times 10\)
⇒ \(=5.6 \times \frac{\text { strength in gram per litre }}{17}\)
2. Volume strength
= 11.2 x molarity
⇒ \(=11.2 \times \frac{\text { percentage strength }}{34} \times 10\)
Numerical Examples
Question 1. Determine the strength of’10 volume H2O2 solution in
- Gram per liter,
- Normality and
- Percentage strength.
Answer: 2H2O2(68 g) 2H2O + O2 [22.4L at STP]
1. Now, 10 volume H2O2 solution means that at STP 10 mL O2 is obtained from 1 mL of this solution.
∴ 22400 mL O2 at STP is obtained from
\(\frac{22400}{10}=2240 \mathrm{~mL} \mathrm{H}_2 \mathrm{O}_2\)
∴ 2240 mL of H2O2 solution contains 68g of H2O2
∴ 1000 mL H2O2 solution\(=\frac{68 \times 1000}{2240}=30.36 \mathrm{~g} \mathrm{H}_2 \mathrm{O}_2\)
So, strength of10 volume H2O2 solution = 30.36 g-L-1.
2. Amount of H2O2 present in 1000 mL solution = 30.36 g.
∴ In normality the strength of10 volume H2O2solution
= 30.36/17 = 1.7858 (N)
3. The amount of H2O2in 1000 mL solution = 30.36 g
∴ Amount of H2O2 in 100 mL of the solution
= 30.36 x 100/1000 =3.036 g.
∴ Percentage strength of H2O2 solution = 3.036
2. Determine the volume strength of 1.5 (N) H2O2.
Answer: 1L of 1.5 (N)H2O2 solution contains 1.5 x 17 =25.5 g
1 mL of l.5 (N) H2O2 solution contains 25.5/1000=0.0255g.
Now 68g H2O2 liberates 22400 ml, O2 STP.
∴ 0.0255g I-I202 liberates\(22400 \times \frac{0.0255}{68}=8.4 \mathrm{~mL} \mathrm{O}_2 \text { at STP. }\)
Therefore, the volume strength of 1.5 (N) H2O2 solution =8.4.
Question 3. Determine the volume strength of a 6.07% H2O2 solution.
Answer: 6.07% H2O2 solution means 100 mL of the solution contains 6.07g of H2O2.
∴ \(1 \mathrm{~mL} \text { solution contains } \frac{6.07}{100}=0.0607 \mathrm{~g} \mathrm{H}_2 \mathrm{O}_2 \text {. }\)
2H2O2(68 g) -> 2H2O + O2 [22400 mL at STP]
Now, 68 g H2O2 liberates 22400 mL O2 at STP.
∴ 0.0607 g H2O2 liberates\(\frac{22400 \times 0.0607}{68} \approx 20 \mathrm{~mL} .\)
Thus, 1 mL H2O2 solution produces 20 mL O2 at STP.
Hence, the volume strength of 6.07% H2O2 solution is ’20 volume.
Question 4. The strengths of the three H2O2 solutions are 10, 15, and 20 volumes respectively. 0.5L of each of these solutions is mixed and an equal amount of water is added to it. Determine the volume strength of the mixed solution.
Answer:
Given
The strengths of the three H2O2 solutions are 10, 15, and 20 volumes respectively. 0.5L of each of these solutions is mixed and an equal amount of water is added to it.
Volume strength = 5.6 x normality
∴ The normality of the first solution, N1 = 10/5.6, the normality of the second solution, N2 – 15/5.6, and the normality of the third solution, N3 = 20/5.6.
Now, if the volumes of the first, second, third, and mixed solutions are V1, V2, V3, and VR respectively, and if the normality of the mixed solution is NR, then
V1V1 + N2V2 + N3V3 = NRVP
\(\text { or, } \quad \frac{10}{5.6} \times \frac{1}{2}+\frac{15}{5.6} \times \frac{1}{2}+\frac{20}{5.6} \times \frac{1}{2}=N_R \times 3\)
⇒ \(\text { or, } \quad N_R=\frac{(5+7.5+10)}{5.6 \times 3}=1.339\)
Therefore, the volume strength of the mixed solution
=NR X 5.6 = 1.339 X 5.6 = 7.5 volume.
Uses of Hydrogen in Industry: Class 11 Overview
Question 5. 20mL of a H2O2 solution after acidification required 20 mL of N/10 KMn04 solution for complete oxidation. Calculate the percentage and volume strength of the H2O2 solution.
Answer:
Given
20mL of a H2O2 solution after acidification required 20 mL of N/10 KMn04 solution for complete oxidation.
From die given data, for H2O2 solution, V1 = 20mL and for KMnO4 solutions V2 = 20mL,\(N_2=\frac{N}{10}\)
Applying the normality equation, N1V1 = N2V2
⇒ \(\text { or, } \quad 20 \times N_1=20 \times \frac{1}{10} \quad \therefore N_1=0.1(\mathrm{~N})\)
Thus, the normality of the H2O2 solution = 0.1(N).
Now, amount of H2O2 in 1 L solution = 0.1 x 17 = 1.7g
∴ The amount of H2O2 in 100 mL of the solution \(=\frac{1.7 \times 100}{1000}=0.17 \mathrm{~g}\)
∴ The percentage strength of the solution = 0.17 %.
Now, 68g of H2O2 produces 22400 mL of O2 at STP.
∴ \(\text { 1.7g of } \mathrm{H}_2 \mathrm{O}_2 \text { produces } \frac{22400}{68} \times 1.7=560 \mathrm{~mL} \mathrm{O}_2 \text { at STP. }\)
This 1.7g of H2O2 is present in 1000 mL of H2O2 solution.
Hence, 1000 mL of H2O2 solution gives 560 mL of O2 at STP.
∴ \(1 \mathrm{~mL} \mathrm{H}_2 \mathrm{O}_2 \text { solution gives } \frac{560}{1000}=0.56 \mathrm{~mL} \text { of } \mathrm{O}_2 \text { at STP. }\)
Question 14. Arrange the following: CaH2, BeH2, and TiH2 in order of increasing electrical conductance. LiH, NaH, and CH in order of increasing ionic character.If —D, D—D, and F—F in order of increasing bond dissociation enthalpy. NaH, MgH2, and H2O in order of Increasing reducing properties.
Answer: Being a covalent hydride BeH2 does not conduct electricity at all. Being an Ionic hydride CaH2 conducts electricity in the fused state while TiH2, being a metallic hydride, conducts electricity at room temperature, Thus, the order of increasing electrical conductance is: BeH2 < CaH2 < TiH2.
The electronegativity of the alkali metals decreases down the group from Li to Cs. Therefore, the ionic character of their hydrides also increases in the same order, l.e., LIH < NaH < CsH.
The bond dissociation enthalpy of the: F—F: bond is the lowest (242.6 kj. mol-1) and this is due to the high concentration of electron density around each F atom in the form of three unshared pairs which have significant repulsive interactions. Again, because of the marginally smaller size of D as compared to H, the bond dissociation enthalpy of the D—D bond (443.35 kj-mol-1) is slightly higher than that of the H —H bond (435.88 kj-mol-1). Hence, the bond dissociation enthalpy increases in the order: of F —F < H —H < D —D.
NaH, being an ionic hydride, is a more powerful reducing agent than the covalent hydrides MgH2 and H2O. MgH2 is a stronger reducing agent than H2O because the bond dissociation enthalpy of the Mg—H bond is much lower than that of the O —H bond. Therefore, the reducing property increases in the order: H2O < MgH2 < NaH
Question 15. Compare the structures of H2O and H2O2.
Answer:
Comparing the structures of H2O with the structures of H2O2
The oxygen atom in water is sp3 -sp3-hybridized. The two O —H bonds are sp3-s sigma bonds. The H —O —H bond angle is 104.5°. This value is a little less than the tetrahedral angle (109°28/) because of stronger lone pair-lone pair and lone pair-bond pair repulsions than bond pair-bond pair repulsion.
Thus, water is a bent molecule. Each oxygen atom in H2O2 is also sp3 hybridized. The O —0 bond is a sp3-sp3 sigma bond and the two O —H bonds are sp3-s sigma bonds. The two O —H bonds are, however, present in different planes. In the gas phase, the dihedral angle between the two planes (i.e., the planes containing H —O —O system) is 111.5°. So, the molecule has an open-booklike structure.
Question 16. What do you understand by the term ‘auto-protolysis’ of water? What is its significance?
Answer:
Self-ionization of water is called auto-protolysis. Self-ionization of water can be expressed by the given equation—
⇒ \(\begin{aligned}
& \mathrm{H}_2 \mathrm{O}(l)+\mathrm{H}_2 \mathrm{O}(l) \rightleftharpoons \mathrm{H}_3 \mathrm{O}^{+}(a q)+\mathrm{OH}^{-}(a q) \\
& \text { Acid-1 Base-2 Acid-2 Base-1 } \\
&
\end{aligned}\)
Water exhibits amphoteric properties because of protolysis. Thus water reacts with both acids and bases. It usually acts as a base in the presence of an acid stronger than it and acts as an acid in the presence of a base stronger than it. For example,
\(\underset{\text { Acid-1 }}{\mathrm{H}_2 \mathrm{O}(l)}+\underset{\text { Base-2 }}{\mathrm{NH}_3(a q)} \longrightarrow \underset{\text { Acid-2 }}{\mathrm{NH}_4^{+}(a q)}+\underset{\text { Base-1 }}{\mathrm{OH}^{-}(a q)}\)
\(\underset{\text { Base-1 }}{\mathrm{H}_2 \mathrm{O}(l)}+\underset{\text { Acid-2 }}{\mathrm{HCl}(a q)} \longrightarrow \underset{\text { Acid-1 }}{\mathrm{H}_3 \mathrm{O}^{+}(a q)}+\underset{\text { Base-2 }}{\mathrm{Cl}^{-}(a q)}\)
Question 17. Consider the reaction of water with F2 and suggest, In terms of oxidation and reduction, which species are oxidized/reduced.
Answer: \(
2 \mathrm{~F}_2(g)+2 \mathrm{H}_2 \mathrm{O}(l) \rightarrow \mathrm{O}_2(g)+4 \mathrm{H}^{+}(a q)+4 \mathrm{~F}^{-}(a q)
Oxidant Reductant\)
⇒ \(
3 \mathrm{~F}_2(g)+3 \mathrm{H}_2 \mathrm{O}(\mathrm{g}) \rightarrow \mathrm{O}_3(\mathrm{~g})+6 \mathrm{H}^{+}(a q)+6 \mathrm{~F}^{-}(a q)
Oxidant Reductant\)
In these reactions, water acts as a reductant and itself gets oxidized to oxygen or ozone. In this case, highly electronegative fluorine acts as an oxidant and gets reduced to F-
Question 18. Complete the following chemical reactions. Classify the above into [a] hydrolysis, [b] redox and [c] hydration reactions
Answer: \(\mathrm{PbS}(s)+4 \mathrm{H}_2 \mathrm{O}_2(a q) \longrightarrow \mathrm{PbSO}_4(s)+4 \mathrm{H}_2 \mathrm{O}(l)\)
\(\begin{aligned}
2 \mathrm{MnO}_4^{-}(a q)+5 \mathrm{H}_2 \mathrm{O}_2(l)+6 \mathrm{H}^{+}(a q) \\
2 \mathrm{Mn}^{2+}(a q)+8 \mathrm{H}_2 \mathrm{O}(l)+5 \mathrm{O}_2(g)
\end{aligned}\)
\(\mathrm{CaO}(s)+\mathrm{H}_2 \mathrm{O}(\mathrm{g}) \longrightarrow \mathrm{Ca}(\mathrm{OH})_2(a q)\)
\(\begin{aligned}
& \mathrm{AlCl}_3(\mathrm{~g})+6 \mathrm{H}_2 \mathrm{O}(l) \\
& {\left[\mathrm{Al}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}(a q)+3 \mathrm{Cl}^{-}(a q)} \\
&
\end{aligned}\)
⇒ \(\begin{array}{r}
{\left[\mathrm{Al}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}(a q)+\mathrm{H}_2 \mathrm{O}(l) \longrightarrow} \\
{\left[\mathrm{Al}\left(\mathrm{H}_2 \mathrm{O}\right)_5(\mathrm{OH})\right]^{2+}(a q)+\mathrm{H}_3 \mathrm{O}^{+}(a q)}
\end{array}\)
Reactions 1 and 2 are redox reactions. Reactions 3 and 5 are hydrolysis reactions. The reaction is a hydration reaction.
Hydrogen Warm-Up Type Questions
Question 1. Name the isotopes of hydrogen and state their mass ratio.
Answer: The three isotopes of hydrogen are— Protium \({ }_1^1 \mathrm{H}\) or H, Deuterium \({ }_2^1 \mathrm{H}\) or D, Tritium \({ }_3^1 \mathrm{H}\) or T Their mass ratio is protium: deuterium: tritium =1:2:3.
Question 2. What is the source of solar energy?
Answer:
Source of solar energy
The main source of solar energy is the given nuclear fusion reaction
\(4{ }_1^1 \mathrm{H} \rightarrow{ }_2^4 \mathrm{He}+2{ }_{+1} e^0 \text { (positron) + Energy }\)
Question 3. Although Fe is placed above hydrogen in the electrochemical series, dihydrogen is not obtained by its reaction with nitric acid. Explain with reasons.
Answer: HNO3 being a strong oxidizing agent oxidizes
dihydrogen into the water and itself gets reduced to nitrogen dioxide \(\mathrm{Fe}+6 \mathrm{HNO}_3 \rightarrow \mathrm{Fe}\left(\mathrm{NO}_3\right)_3+3 \mathrm{NO}_2+3 \mathrm{H}_2 \mathrm{O}\)
Question 4. How can one prepare H2 gas from water by using a reducing agent?
Answer: Reaction between metals such as Na or. JC (strong reducing agents) and water produce hydrogen gas \(2 \mathrm{Na}+2 \mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{NaOH}+\mathrm{H}_2 \uparrow\)
Question 5. Name two compounds, in one ofwhich hydrogen is in +1 and the other in -1 oxidation state.
Answer: In HC1, hydrogen is in a +1 oxidation state and in NaH it is in a -1 oxidation state
Question 6. Holli dihydrogen and carbon monoxide burn in the air with a blue flame. How will you distinguish between them?
Answer: Dihydrogen burns with a blue flame in the air to form water vapor, turning white anhydrous CuSO4 into hydrated copper sulfate (CuSO4-5H2O). However, carbon monoxide on combustion forms CO2 which does not bring about any change in CuSO4
Question 7. What characteristics do you expect from an electron-deficient and an electron-rich hydride to their structures?
Answer: Electron-deficient hydrides are electron acceptors, i.e., they act as Lewis acids whereas electron-rich hydrides are electron donors, i.e., Lewis bases. For example, B2H6 is a Lewis acid while NH3 is a Lewis base.
Question 8. Why the boiling point of HF is higher than that of other hydrogen halides?
Answer: Due to the formation of strong intermolecular hydrogen bonding, the boiling point of HF is higher than that of other hydrogen halides.
Question 9. How can you separate H2 or D2 from He?
Answer: Red hot palladium is cooled in an atmosphere of He mixed with H2 or D2. Consequently, a large amount of H2 or D2 gets adsorbed by palladium but not He. When palladium is heated, occluded H2 or D2 gels are liberated as free hydrogen or deuterium.
Question 10. Why ionic or salt-like hydrides are used to dry organic solvents?
Answer: Ionic or salt-like hydrides are used to dry organic solvents because they readily react with water to form the corresponding metal hydroxide along with the evolution of H2 8as- The solvent is then separated from the metallic hydroxide by distillation.
Question 11. Why concentration of D2O increase when electrolysis of water is carried out for a long time?
Answer: Electrolysis of H2O occurs at a faster rate than D2O because the bond dissociation energy of the O—H bond is greater than that of the O—D bond. So, electrolysis of ordinary water for a prolonged time results in an increase in concentration of D2O
Question 12. How would you prepare deuterium peroxide (D2O2)?
Answer: Deuterium peroxide (D2O2) can be prepared by the reaction between barium peroxide (BaO2) and deuterosulphuric acid (D2SO4) BaO2 + D2SO4 → BaSO4 + D2O2
Question 13. How will you prepare deuteroammonia (ND3) from N2?
Answer: Magnesium burns in nitrogen to produce magnesium nitride which further reacts with D2O to produce ND3 (deuteroammonia)
⇒ \(\begin{gathered}
3 \mathrm{Mg}+\mathrm{N}_2 \rightarrow \mathrm{Mg}_3 \mathrm{~N}_2 \\
\mathrm{Mg}_3 \mathrm{~N}_2+6 \mathrm{D}_2 \mathrm{O} \rightarrow 3 \mathrm{Mg}(\mathrm{OD})_2+2 \mathrm{ND}_3
\end{gathered}\)
Question 14. How will you prove thathypophosphorusacid (H3PO2) is a monobasic acid?
Answer: When H3PO2 is treated with D2O, only one of its hydrogen atoms is replaced by D. So, it can be said that only one H-atom remains attached to O-atom in hypophosphorous acid (H3PO2). Therefore, it is a monobasic acid
Question 15. Sodium chloride is less soluble in heavy water than ordinary water—why?
Answer: As the dielectric constant of D2O is less than that of H2O, NaCl (sodium chloride) is less soluble in heavy water than ordinary water.
Question 16. Explain why the water obtained after passing hard water through cation exchange resins is acidic.
Answer: When hard water is passed through an organic ion exchange resin, the water obtained is acidic because all the metal ions present in water are exchanged with H+ ions of the resin. As a result, the resulting water is free of cations and has a high concentration of H+ ions. So, the water turns blue litmus paper red.
Question 17. A sugar solution prepared in distilled water is passed successively through cation and anion exchange resins. What will be the taste ofthe collected water and why?
Answer: ion- exchange cannot remove sugar(non-electrolyte) from water. therefore, when a sugar solution is passed successively through cation and anion exchange resins after being collected tastes sweet.
Question 18. The hardness of water in a tube well is 300 ppm. What do you mean by this statement?
Answer: The statement means that in million parts by mass of the sample of water from the tube, the well contains salts causing its hardness which are equivalent to 300 parts by mass of calcium carbonate.
Question 19. Will the water obtained by passing hard water through anion exchange resin, form lather with soap? Why?
Answer: As the sample of water is not free from Ca2+ and Mg2+ ions, it will not form a lather with soap easily.
Hydrogen Production Methods: Class 11 Notes
Question 20. A sample of water contains MgS04 and urea. How can they be eliminated easily?
Answer: They can be eliminated by a simple distillation method.
Question 21. It is better to preserve H2O2 in a polythene bottle than in a
glass bottle—why?
Answer: The decomposition of H2O2 is accelerated by the presence of glass, sunlight, and basic substances. So, H2O2 is preserved in a polythene bottle rather than a glass bottle.
Question 22. What do you understand by the expression ’30 volume H2O2 solution?
Answer: ’30 volume H2O2 solution’ means that 1 mL of that solution yields 30 mL of oxygen at STP as a result of its complete decomposition.
Question 23. what do you mean by 20% H2O2 solution?
Answer: 20% H2O2 solution means that momT. of that solution contains 20g of H2O2
Question 24. Calculate the percentage strength of6.588 volume H2O2
Answer: Percentage strength of solution= \(\frac{\text { volume strength } \times 34}{11.2 \times 10}\)
⇒ \(=\frac{6.588 \times 34}{11.2 \times 10}=1.99=\frac{6.588 \times 34}{11.2 \times 10}=1.99\)
Hydrogen Very Short Answer Type Questions
Question 1. Explain why concentrated HCI is not used in the laboratory preparation of H2 gas.
Answer: Concentrated HCI is not used for the laboratory preparation of dihydrogen because HCI, being highly volatile, gets mixed with dihydrogen.
Question 2. Write down the name and formula of a compound that on electrolysis produces dihydrogen at the anode.
Answer: Sodium hydride (NaH)
Question 3. What is syngas?
Answer:
Syngas
All mixtures of CO and H2 irrespective of their composition are called synthesis gas or syngas
Question 4. Which isotope of hydrogen is used as a tracer in organic reactions?
Answer: Deuterium 21D is usually used as a tracer in determining the mechanism of organic reactions.
Question 5. Explain why dihydrogen is not suitable for balloons.
Answer: As dihydrogen, the lightest substance known, is a highly inflammable gas, it is not suitable for balloons.
Question 6. Which bond between two atoms has the highest bond dissociation enthalpy?
Answer: Bond dissociation enthalpy ofthe H —H bond is highest.
Question 7. Explain why H2 is more reactive than D2.
Answer: This is because the H —H bond dissociation enthalpy is less than the D —D bond dissociation enthalpy.
Question 8. What change is expected to take place when vegetable oils are hydrogenated?
Answer: Carbon-carbon double bonds are converted to carbon- V carbon single bonds.
Question 9. Which isotope of hydrogen is used in nuclear rectors?
Answer: Deuterium (21H or D )
Question 10. Why are ionic hydrides used as solid fuels?
Answer: When heated, ionic hydrides decompose to evolve dihydrogen gas which ignites readily.
Question 11. The densities of ionic hydrides are greater than that of the metal from which they are formed—why?
Answer: This is because hydride ions (H) occupy the holes in the lattice ofthe metal without distorting the metal lattice.
Question 12. Give examples of two interstitial hydrides.
Answer: CuH and FeH.
Question 13. Which gaseous compound on treatment with dihydrogen produces methanol?
Answer: Carbon monoxide (CO).
Question 14. Give the chemical reaction that occurs when hydrogen is used as a rocket fuel.
Answer: \(\mathrm{H}_2(g)+\frac{1}{2} \mathrm{O}_2(g) \rightarrow \mathrm{H}_2 \mathrm{O}(l)+286 \mathrm{~kJ}\)
Question 15. A sample of water containing KC1 does not behave as hard water, but a sample of water containing CaCI2 or MgCl2 behaves as hard water—why?
Answer: The potassium salt of soap is soluble in water and forms a lather while calcium or magnesium salt of soap is insoluble in water and does not form a lather.
Question 16. What is EDTA, a compound used to determine the hardness of water?
Answer: EDTA is the disodium salt of ethylenediamine tetraacetic acid[NaO2C(COOH)NCH2CH2N(C00H)COONa].
Question 17. Can distilled water be called deionized water?
Answer: Distilled water can be called deionized water because it does not contain any cations and anions.
Question 18. What is the difference between the water softened by the permit process and the water softened by the organic ion exchangers?
Answer: Although the water softened by the permit process contains no cation, it contains various anions (e.g., Cl-, SO- etc.). However, the water softened by the organic ion exchangers contains no cations and anions.
Question 19. What will be the hardness of a sample of water, 106 g of which contains i mol A12(SO4)3?
Answer: 50ppm
Question 20. What is Calgon?
Answer:
Calgon
Sodiumhexametaphosphate, Na2[Na4(PO3)g]
Question 21. Give the chemical formula of the permit.
Answer: Na2Al2Si2O8 x H2O.
Question 22. What is the main source of heavy water?
Answer: Ordinary water is the main source of heavy water.
Question 23. Can sea animals survive in distilled water?
Answer: Sea animals cannot survive in distilled water because distilled water contains no salt and dissolved oxygen.
Question 24. Although D2O resembles H2O chemically, it is a toxic substance—why?
Answer: Dilute solution of H2O2,
Question 25. What is the trade name of hydrogen peroxide used as an antiseptic?
Answer: Perhydrol.
Question 26. What is the strength in the normality of an ‘11.2 volume’ H2O2 solution?
Answer: 2(N) H2O2 solution.
Hydrogen Reactions with Other Elements: Key Points
Question 28. Name a compound that suppresses the decomposition of H2O2.
Answer: Acetanilide (PhNHCOCH3).
Question 29. H2O2 molecule has an open-book-like structure. What is the angle between the two pages of the book in the gas phase?
Answer: 111.50.
Question 30. Name an organic compound without peroxo bond that is used to manufacture H2O2.
Answer: 2-ethylanthraquinol
Question 25. why H2O2 is a better oxidant than water?
Answer: H2O2 is a better oxidant than water because H2O2 being unstable readily dissociates to form stable water molecules along with the evolution of O2 gas. 2H2O2 2H2O + O2 + Heat
Question 1. What is heavy water? Why is it so-called?
Answer: Deuterium oxide is commonly known as heavy water because its density is higher than that of ordinary water, i.e., it is heavier than ordinary water.
Question 2. A water sample contains 1 millimole of Mg2+ ion per liter. Calculate the hardness of the water sample in ppm units.
Answer: 1 millimole Mg2+=1 millimole of MgCl2 s 0.095 g of MgCL,.
1 1. or 1000 g or 103 g of water contains 0.1 g of MgCl2 106g of water contains 0.095 x 103 = 95g of MgCl2 The degree of the hardness of water is 95 ppm
Question 3. BaO2 is a peroxide but MnO2 is not a peroxide— explain?
Answer: There is peroxide linkage in the BaO2 molecule ( —O —O—). But there is no such bonding present in MnO, molecule (O —Mn=0). Thus MnO2 is not a peroxide.
Hydrogen Short Answer Type Questions
Question 1. Why do most of the reactions of H2 occur at much higher temperatures?
Answer: Due to the much higher bond dissociation enthalpy of the H—H bond, dihydrogen is quite stable and relatively inert at temperature. It dissociates into atoms at about 5000K. For this reason, most of the reactions of dihydrogen occur at much higher temperatures.
Question 2. What characteristics do you expect from electron-deficient hydrides to their structure and chemical reactivity?
Answer: Electron-deficient hydrides have less number of electrons in the valence shell of the central atom and so, their mononuclear units do not satisfy the usual Lewis octet rule. Due to a deficiency of electrons, these hydrides act as Lewis acids and form complex entities with Lewis bases such as NH3, H- ion, etc.
⇒ \(\mathrm{B}_2 \mathrm{H}_6+2 \mathrm{NH}_3 \rightarrow\left[\mathrm{BH}_2\left(\mathrm{NH}_3\right)_2\right]^{+}\left[\mathrm{BH}_4\right]^{-} \text {; }\mathrm{B}_2 \mathrm{H}_6+2 \mathrm{LiH} \rightarrow 2 \mathrm{Li}^{+}\left[\mathrm{BH}_4\right]^{-} \text {(Lithium borohydride) }\)
Question 3. Explain why it is harmful to bathe in heavy water and use it for drinking purposes.
Answer: Being a very hygroscopic substance, heavy water (D2O) absorbs water from the body and thereby damages body cells. Also, it retards some cellular processes such as mitosis, cell division, and various enzyme-catalyzed reactions. For these reasons, it is harmful to bathe in heavy water and use it for drinking purposes
Question 4. Explain why the thermal stability of H2O2 is verylow.
Answer: The bond dissociation enthalpy of the O —O bond presenting H2O2 molecule is very low (35kcal. mol-1) i.e., the bond is very weak. For this reason, the thermal stability of H2O2 is extremely low.
Question 5. How the presence of H- ions be confirmed in ionic hydrides?
Answer: In the molten state, ionic hydrides conduct electricity with the liberation of dihydrogen at the anode. This confirms the presence of hydride (H ) ions in them.
Question 6. How do you separate 2 allotropic forms of hydrogen?
Answer: Ordinary hydrogen is a mixture of 75% ofortho and 25% of para-isomer at room temperature. On passing through activated charcoal kept at 20K, the para-isomer is adsorbed leaving behind the ortho-isomer. From the charcoal surface, para-hydrogen can be released by reducing pressure.
Question 7. Mention the difference in chemical characteristics of the two hydrides obtained when hydrogen combines with two elements having atomic numbers 17 and 20.
Answer: The highly electronegative Cl atomhaving atomic number 17 combines with hydrogen to form the covalent hydride H —Cl. On the other hand, the highly electropositive Ca atom having atomic number 20 combines with hydrogen to form the ionic hydride CaH2
Question 8. Two samples of hard water contain the same cations, Ca2+ & Mg2+. One is marked as temporary and the other as permanent. In which respect do they differ?
Answer: The two samples of magnesium of water salts are different present. with The sample of watermarked as temporary hard water containing bicarbonates of Ca2+ and Mg2+ ions while the sample marked as permanent hard water containing chlorides and sulfates of Ca2+ and Mg2+ ions.
Question 9. Tube-well water, if left for some time, assumes a brownish turbidity—explain.
Answer: Tube-well water sometimes contains soluble ferrous bicarbonate [Fe(HCO3)2]. This compound, on aerial oxidation, is converted into brown ferric hydroxide, Fe(OH)3, which remains in water as colloidal suspension, and because of this, water assumes a brownish turbidity.
⇒\(4 \mathrm{Fe}\left(\mathrm{HCO}_3\right)_2+2 \mathrm{H}_2 \mathrm{O}+\mathrm{O}_2 \rightarrow \underset{\text { (brown) }}{4 \mathrm{Fe}(\mathrm{OH})_3}+8 \mathrm{CO}_2\)
Question 10. Write the reactions for the Preparation of H2O2 from two sodium salts and preparation of D2O2 from potassium persulphate
Answer: \(\begin{aligned}
& \mathrm{Na}_2 \mathrm{O}_2+2 \mathrm{NaH}_2 \mathrm{PO}_4 \rightarrow 2 \mathrm{Na}_2 \mathrm{HPO}_4+\mathrm{H}_2 \mathrm{O}_2 \\
& \text { Sodium dihydrogen Disodium hydrogen } \\
& \text { phosphate phosphate } \\
&
\end{aligned}\)
Question 11. Between deionized water and distilled water which one is more pure and why?
Answer: Both deionized and distilled water are free from ions. Yet distilled water is superior to deionized water in terms of purity. This is because deionized water contains a small amount of dissolved silica and CO2 along with some germs and organic impurities. However, distilled water prepared in a glass apparatus does not contain any impurities other than trace amounts of silica and CO2.
Question 12. Why is Na2O2 used for purifying air in submarines and crowded places?
Answer: Na2O2 reacts with CO2 of air to evolve O2 (2Na2O2 + 2CO2 →2Na2CO3+ O2). For this reason, Na2O2 is used for the purification of air in submarines and in crowded places.
Question 13. Comment on the reactions of dihydrogen with Chlorine Sodium and Copper (2) oxide.
Answer: Dihydrogen reduces chlorine (Cl) to chloride ion (Cl-) and itself gets oxidized to form H+ ions. These two ions (H+ and Cl-) share an electron pair between themselves to form a covalent molecule of hydrogen chloride (HC1)
⇒ \(\mathrm{H}_2(g)+\mathrm{Cl}_2(g) \rightarrow 2 \mathrm{HCl}(g)\)
Sodium reduces dihydrogen to hydride ion (H-) and itself gets oxidized to sodium ion (Na+). In this reaction, an electron gets completely transferred from Na toH thereby forming ionic sodium hydride (NaH).
⇒\(2 \mathrm{Na}(s)+\mathrm{H}_2(s) \xrightarrow{\Delta} 2 \mathrm{Na}^{+} \mathrm{H}^{-}(s)\)
Dihydrogen reduces copper(2) oxide to metallic copper while itself gets oxidized to form a covalent molecule of H2O
⇒ \(\stackrel{+2}{\mathrm{CuO}}(\mathrm{s})+\stackrel{0}{\mathrm{H}}{ }_2(s) \xrightarrow{\Delta} \stackrel{0}{\mathrm{Cu}}(\mathrm{s})+\stackrel{+1}{\mathrm{H}}_2 \stackrel{-2}{\mathrm{O}}(l)\)
Question 14. An ionic alkali metal hydride has a covalent character to some extent and it does not react with oxygen and chlorine. This hydride is used in the synthesis of another hydride. Write the formula of the hydride and what happens when it reacts with A12CI6.
Answer:
Given
An ionic alkali metal hydride has a covalent character to some extent and it does not react with oxygen and chlorine. This hydride is used in the synthesis of another hydride.
Since the alkali metal hydride possesses sufficient covalent character, the hydride is of the smallest alkali metal, Li, i.e. the hydride is LiH. As LiH is quite stable, it does not react with oxygen and chlorine. Lithium hydride reacts with A12C16 to form lithium aluminum hydride (LiAH4) which is extensively used as a reagent in the synthesis of different organic compounds.
⇒ \(8 \mathrm{LiH}+\mathrm{Al}_2 \mathrm{Cl}_6 \longrightarrow 2 \mathrm{LiAlH}_4+6 \mathrm{LiCl}\)
Question 15. Sodium reacts with dlliydrogcn to form a crystalline ionic solid. It is non-volatile and a non-conductor of electricity. It also reacts vigorously with water to liberate H2 gas. Write the formula of the Ionic solid and give a reaction between the lids solid & water. What happens when the ionic solid in its molten stater Is electrolysed?
Answer:
Given
Sodium reacts with dlliydrogcn to form a crystalline ionic solid. It is non-volatile and a non-conductor of electricity. It also reacts vigorously with water to liberate H2 gas.
Sodium reacts with dihydrogen to form sodium hydride which is a crystalline ionic solid \(2 \mathrm{Na}+\mathrm{H}_2 \xrightarrow{\Delta} 2 \mathrm{Na}^{+} \mathrm{H}^{-}\)
Sodium hydride reacts with water as follows—
⇒ \(2 \mathrm{NaH}+2 \mathrm{H}_2 \mathrm{O} \longrightarrow 2 \mathrm{NaOH}+2 \mathrm{H}_2\)
Sodium hydride, in its solid state, does not undergo electrolysis. However, in its molten state, undergoes electrolysis. However, in its molten state, NaH undergoes electrolysis to liberate dihydrogen (H2) at the anode and metallic sodium at the cathode
⇒ \(\mathrm{Na}^{+} \mathrm{H}^{-}(\text {molten }) \xrightarrow{\text { Electrolysis }} \underset{\text { Cathode }}{2 \mathrm{Na}(\text { molten })}+\underset{\text { anode }}{\mathrm{H}_2(g)}\)
Question 16. Why is seawater not used in boilers?
Answer: Sea water is hard water. It contains Mg(HCO3)2 and Ca(HCO3)2, which on boiling, form a hard heat-insulating thick layer or scale of MgCO3 and CaCO3 on the inner surface ofthe boiler. Consequently, much heat is required to raise the temperature of the boiler, and thus, fuel economy is adversely affected.
Again at much higher temperatures, the boiler scales and the metal of the boiler expand unequally. Due to such uneven expansion, cracks are formed on the scales. When hot water comes in contact with the hot metal surface of the boiler through these cracks, it is suddenly converted into steam. Due to the high pressure, thus developed, the boiler may burst leading to accidents.
Again, MgCl2 and MgSO4 present in seawater undergo hydrolysis to form HCI and H2SO4 which degrade the metallic component of the boiler. So, seawater is not used in boilers.
Hydrogen Isotopes: Understanding for Class 11
Question 17. The values of melting point, enthalpy of vaporization, and viscosity of H2O and D2O are given below: From the given data, determine which liquid has a greater magnitude of intermolecular forces of attraction
Answer: The magnitude of intermolecular forces of attraction depends on the magnitudes of melting point, enthalpy of vaporization, and viscosity of the liquid. As, these parameters have a higher value in the case of D2O, the magnitude of intermolecular forces of attraction is greater for D2O than H2O.
Question 18. How will you prepare heavy water from ordinary water? Explain its principle
Answer: Heavy water (D2O) is prepared by prolonged electrolysis of ordinary water. As water is not a good conductor of electricity, an alkaline solution of water [~0.5(N) NaOH] is used for electrolysis. The bond dissociation energy of the O —H bond is less than that of the O —D bond. So, electrolysis of H2O occurs at a faster rate and more easily than D2O. Consequently, the amount of D2O in ordinary water increases. Pure D20 is obtained when the amount of residual liquid decreases
Question 19. Can phosphorus form PHg with its outer electronic configuration of 3s23p3?
Answer: Phosphorus cannot form PH5 although it shows +3 and +5 oxidation states. Dihydrogen acts as a weak oxidizing agent due to the high bond dissociation enthalpy of H—Hbond (435.88 kJ-moH) and slightly negative electron-gain enthalpy (-73 kj mol-1). So, dihydrogen can oxidize phosphorus to a +3 oxidation state but not to its highest oxidation state of +5. Therefore, phosphorous can form only PH3 and not PH5.
Question 20. How will you prepare dinitrogen from HNO3?
Answer: Magnesium and manganese react with a very dilute solution of HN03(2%) to form dihydrogen.
⇒ \(\begin{aligned}
& \mathrm{Mg}+2 \mathrm{HNO}_3 \rightarrow \mathrm{Mg}\left(\mathrm{NO}_3\right)_2+\mathrm{H}_2 \uparrow \\
& \mathrm{Mn}+2 \mathrm{HNO}_3 \rightarrow \mathrm{Mn}\left(\mathrm{NO}_3\right)_2+\mathrm{H}_2 \uparrow
\end{aligned}\)
Question 21. Do you think the hydrides of N, O, and F will have lower boiling points than the hydrides of their corresponding group members? State reasons?
Answer: The hydrides of the elements N, O, and F are NH3, H2O, and HF respectively. These hydrides are expected to have lower boiling points than that of their corresponding group members (PH3, H2S & HC1) when their masses are considered. However, because of the high electronegativity of N, O, and F, their hydrides undergo extensive hydrogen bonding (intermolecular). As a result, boiling points of NH3, H2O, and HF are much higher than the hydrides of their corresponding group members, i.e., PH3, H2S, and HC1 respectively.
Question 22. KF reacts with HF to form the compound, KF-2HF. Discuss the probable structure of the compound.
Answer: The h-bond in the HF molecule is very strong. When KF gets added to HF, one F” ion forms H — bond with two HF molecules to form H2F3 ion [F—-H —F—H —F —]“
Question 23. Calculate the amount of energy liberated due to combustion of 4g dihydrogen.
Answer: Amount of energy liberated due to combustion of 1 mol, i.e., 2g dihydrogen = 242 kj .mol-1.
⇒ \(\left[\mathrm{H}_2(\mathrm{~g})+\frac{1}{2} \mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{H}_2 \mathrm{O}(\mathrm{g}) ; \Delta \mathrm{H}=-242 \mathrm{~kJ} \cdot \mathrm{mol}^{-1}\right]\) Amount of energy liberated due to 4g dihydrogen = 242×2 = 484 kj-mol-1
Question 24. Under what conditions, water reacts with calcium cyanamide and what are the products formed due to this reaction?
Answer: Superheated steam reacts with calcium cyanamide under high pressure to form ammonia gas and calcium carbonate as a result of hydrolysis
⇒ \(\mathrm{CaNCN}\left(\text { Calcium cyanamide) }+3 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{CaCO}_3+2 \mathrm{NH}_3\right.\)
Question 2. Write the names of isotopes of hydrogen. What is the mass ratio of these isotopes?
Answer: In nature, there are three isotopes of hydrogen. These are
protium \(\left({ }_1^1 \mathrm{H}\right)\) deuterium \(\left[{ }_1^2 \mathrm{H} \text { or } \mathrm{D}\right]\) and tritium \(\left[{ }_1^3 \mathrm{H} \text { or } \mathrm{T}\right]\).
The mass ratio of \({ }_1^1 \mathrm{H},{ }_1^2 \mathrm{H} \text { and }{ }_1^3 \mathrm{H}\) is 1: 2: 3.
Question 3. Why does hydrogen occur in a diatomic form rather than in a monoatomic form under normal conditions?
Answer: A hydrogen atom has only one electron and thus, it has one electron less than the stable configuration of the nearest noble gas helium. Thus, to achieve a stable configuration it shares its single electron with the electron of other H -atoms to form a stable diatomic molecule (H2 ).
Question 6. Complete the following reactions
⇒ \(\mathrm{H}_2(\mathrm{~g})+\mathrm{M}_m \mathrm{O}_0(\mathrm{~s}) \xrightarrow{\Delta}\)
⇒ \(\mathrm{CO}(g)+\mathrm{H}_2(g) \xrightarrow[\text { catalyst }]{\Delta}\)\(\mathrm{C}_3 \mathrm{H}_8(g)+3 \mathrm{H}_2 \mathrm{O}(g) \xrightarrow[\text { catalyst }]{\Delta}\) \(\mathrm{Zn}(s)+\mathrm{NaOH}(a q) \xrightarrow{\text { heat }}\)
Answer: \(o \mathrm{H}_2(\mathrm{~g})+\mathrm{M}_m \mathrm{O}_o(s) \xrightarrow{\Delta} m \mathrm{M}(s)+o \mathrm{H}_2 \mathrm{O}(l)\)
\(\mathrm{CO}(\mathrm{g})+2 \mathrm{H}_2(\mathrm{~g}) \xrightarrow[\text { catalyst }]{\Delta} \mathrm{CH}_3 \mathrm{OH}(l)\)
\(\mathrm{C}_3 \mathrm{H}_8(\mathrm{~g})+3 \mathrm{H}_2 \mathrm{O}(\mathrm{g}) \xrightarrow{\mathrm{Nl}, 1270 \mathrm{~K}} 3 \mathrm{CO}(\mathrm{g})+7 \mathrm{H}_2(\mathrm{~g})\)
⇒ \(\mathrm{Zn}(s)+2 \mathrm{NaOH}(a q) \xrightarrow{\Delta} \underset{\text { Sodium zincate }}{\mathrm{Na}_2 \mathrm{ZnO}_2(a q)}+\mathrm{H}_2(g)\)
Question 7. Discuss the consequences of high enthalpy of the H —H bond in terms of the chemical reactivity of dihydrogen.
Answer: The 2 —2 bond length is very small (74 pm) because the H-atom has a very small atomic size. Consequently, the H—H bond dissociation endialpyis very high (435.9 kj-mol-1) which makes hydrogen completely inert at ordinary temperature. However, at higher temperatures, the H—H bond undergoes dissociation in the presence of a catalyst to form hydrides with metals & non-metals.
Hydrogen Solved WBCHSE Scanner
Question 8. What characteristics do you expect from an electron-deficient hydride concerning its structure and chemical reactions?
Answer: There is an insufficient number of electrons in the valence shell of the central atom. So, in this type of hydride, the valence shell of the central atom does not have a complete octet configuration. For example, in BH3, the valence shell of B has six electrons and the hydrides are trigonal planarian shape.
Due to electron deficiency, this type of hydrides act as Lewis acids, i.e., they accept electron pairs. For example, H3B + NH3→ H3B→NH3
To compensate for the electron deficiency, the hydrides form dimers, trimers, and polymers and attain stability.
For example, B2H6, B4H10, (A1H3)b
Electron-deficient hydrides are extremely reactive. They easily react with both metals and non-metals.
Question 9. Do you expect the carbon hydrides of the (CMH2n + 2) to act as ‘Lewis’ acid or base? Justify your answer.
Answer: The hydrides of carbon of the type CnH2n + 2 are electron-precise hydrides, i.e., they have an exact number of electrons in the valence shell of the central atom to write their conventional Lewis structures. Therefore, they do not tend to either gain or lose electrons and consequently, they do not act as Lewis acids or bases.
Question 10. What do you understand by the term “nonstoichiometric hydrides”? Do you expect this type of hydride to be formed by alkali metals? Justify your answer.
Answer:
Nonstoichiometric hydrides
Hydrides of d and f-block elements that are deficient in hydrogen and in which the ratio of the metal to hydrogen is fractional are called non-stoichiometric hydrides.
The alkali metals, being highly reducing in nature, transfer their lone pair of electrons to the H-atom forming Hions. Since a hydride ion H- is formed by the complete transfer of an electron, the ratio of metal to hydrogen is always fixed in these hydrides and their compositions correspond to a simple whole-number ratio. Hence, the alkali metals form only stoichiometric hydrides.
Question 11. How do you expect the metallic hydrides to be useful for hydrogen storage? Explain.
Answer: Some transition metals like palladium (Pd), Platinum (Pt), etc., adsorb a large volume of hydrogen on their surface (as Hatoms) forming hydrides. Due to the inclusion of H-atoms, the metal lattice expands and thus becomes less stable.
Therefore, when such metal hydrides are heated, they decompose to release dihydrogen and change back to a metallic state in finely divided form. The dihydrogen evolved in this manner and can be used as a fuel. Thus, the transition metals or their alloys can be used to store as well as for transportation of hydrogen used as a fuel.
Question 12. Among NH3 H2O and HF, which would you expect to have the highest magnitude hydrogen bonding & why?
Answer: The strength of a hydrogen bond depends upon the atomic size mid the electronegativity of the atom to which the 2-atom Is covalently linked. Smaller size and higher electronegativity of the other atom favor the formation of stronger H -bonding and that Is due to an increase In the magnitude of bond polarity. Among N, I’, and O atoms, V has the lowest atomic size and the highest electronegativity. Hence the 2 — F bond is maximum polar and as a result, it will have the highest magnitude of H-bonding.
Question 13. Saline hydrides are known to react with water violently producing fire. Can CO2, a well-known fire extinguisher, be used in this case? Explain.
Answer: The saline hydrides (Example; NaH, CaH2, etc.), react with water violently to yield the corresponding metal hydroxides with the evolution of H2 gas. The liberated H2 gas undergoes spontaneous combustion causing fire and this is because of the highly exothermic nature of the combustion reaction.
⇒ \(\begin{gathered}
\mathrm{NaH}(s)+\mathrm{H}_2 \mathrm{O}(l) \rightarrow \mathrm{NaOH}(a q)+\mathrm{H}_2(\mathrm{~g}) ; \\
2 \mathrm{H}_2+\mathrm{O}_2 \rightarrow 2 \mathrm{H}_2 \mathrm{O}(l) ; \Delta H^0=-286 \mathrm{~kJ} \cdot \mathrm{mol}^{-1}
\end{gathered}\)
In this case, CO2 cannot used as a fire extinguisher because its gels are reduced by the hot metal hydride to form formate ions.
⇒ \(\mathrm{NaH}+\stackrel{+1}{\mathrm{CO}_2} \rightarrow \stackrel{+1+2}{\mathrm{HCOONa}}\)
Question 19. Is demineralized or distilled water useful for drinking purposes? If not, how can it be made useful?
Answer: Although very pure, demineralized or distilled water is not useful for drinking purposes and this is because it does not contain even useful minerals. To make it useful for drinking purposes, useful minerals in proper amounts should be added to demineralized or distilled water.
Question 20. Describe the usefulness of water in biosphere and biological systems
Answer:
The usefulness of water in biosphere and biological systems
Water is extremely necessary for the existence of different forms of life on Earth. It constitutes about 65-70% of the body weight of plants and animals. Since water has a high specific heat, thermal conductivity, surface tension, dipole moment, and dielectric constant compared to other liquids, it plays a vital role in the biosphere.
Due to the high heat of vaporization of water, it can moderate the body temperature. Water also indirectly helps in climate control.
In the living body, water helps to transport minerals, nutrients, and enzymes to the different parts of the body and also affects the process of metabolism. Water is a vital component in the process of photosynthesis. Therefore, waterways a significant role in the biosphere.
Question 21. Knowing the properties of H2O and D2O, do you think that D2O can be used for drinking purposes?
Answer: Drinking heavy water (D2O) is harmful to the human beings. Heavy water being highly hygroscopic, absorbs water from different parts of the body. As a result, the body cells may get destroyed. Apart from this, heavy water slows down different biochemical reactions such as mitosis, cell division, etc.
Question 22. What is the difference between the terms ‘hydrolysis’ and ‘hydration’?
Answer:
Hydrolysis refers to the reaction of salt with water to form acidic or basic solutions. For example:
⇒ \(
\mathrm{Na}_2 \mathrm{CO}_3+2 \mathrm{H}_2 \mathrm{O} \rightleftharpoons 2\left[\mathrm{Na}^{+}+\mathrm{OH}^{-}\right]+\mathrm{H}_2 \mathrm{CO}_3 \text {; }
(basic solution)\)
⇒ \(
\mathrm{NH}_4 \mathrm{Cl}+\mathrm{H}_2 \mathrm{O} \rightleftharpoons\left[\mathrm{H}^{+}+\mathrm{Cl}^{-}\right]+\mathrm{NH}_4 \mathrm{OH}
(acidic solution)\)
Hydration, on the other hand, refers to the addition of H2O to ions or molecules to form hydrated ions or hydrated salts. For example
⇒ \(\underset{\text { Salt }}{\mathrm{NaCl}(s)+\mathrm{H}_2 \mathrm{O}} \rightarrow \underbrace{\mathrm{Na}^{+}(a q)+\mathrm{Cl}^{-}(a q)}_{\text {Hydrated ions }}\)
⇒ \(\underset{\text { Anhydrous salt (colourless) }}{\mathrm{CuSO}_4(s)+5 \mathrm{H}_2 \mathrm{O}(l)} \rightarrow \underset{\text { (Hydrated salt blue) }}{\mathrm{CuSO}_4 \cdot 5 \mathrm{H}_2 \mathrm{O}(s)}\)
Question 23. What do you expect the nature of hydrides to be if formed by elements of atomic numbers 15, 19, 23, and 44 with dihydrogen? Compare their behavior towards water.
Answer:
The element with Z = 15 is non-metal phosphorus and hence it forms the covalent hydride PH3.
The element with Z = 19 is the alkali metal potassium and hence it forms the saline or ionic hydride K+HT.
The element with Z = 23 is the transition metal vanadium of group-3 and hence it forms the interstitial hydride (VH1.6).
The element with Z = 44 is the transition metal ruthenium (Ru) belonging to group 8. It does not form anhydride (hydride gap).
Only the ionic hydride, KH reacts with water evolving dihydrogen.
Question 24. Do you expect different products in solution when aluminum (3) chloride and potassium chloride are treated separately with Normal water Acidified water and Alkaline water? Write equations wherever necessary.
Answer:
In normal water: KC1, being a salt of a strong acid and strong base, does not undergo hydrolysis in normal water. It simply dissociates to form K+(aq) and Cl-(aq) ions.
⇒ \(\mathrm{KCl}(s) \xrightarrow{\text { water }} \mathrm{K}^{+}(a q)+\mathrm{Cl}^{-}(a q)\)
On the other hand, A1C13, being a salt of a weak base Al(OH)3 and a strong acid HC1, undergoes hydrolysis giving an acidic solution
⇒ \(\mathrm{AlCl}_3(s)+3 \mathrm{H}_2 \mathrm{O}(l) \rightarrow \mathrm{Al}(\mathrm{OH})_3(s)+3 \mathrm{H}^{+}(a q)+3 \mathrm{Cl}^{-}(a q)\)
In acidified water: The H+ ions react with Al(OH)3 to form Al3+(aq) ions and H20. Therefore, in acidic water, A1C13 exists as A13+(aq) and Cl-(aq) ions.
⇒ \(\mathrm{AlCl}_3(s) \xrightarrow{\text { acidified } \mathrm{H}_2 \mathrm{O}} \mathrm{Al}^{3+}(a q)+3 \mathrm{Cl}^{-}(a q)\)
Potassium chloride, KC1 simply dissociates to give K+(aq) and Cl-(aq) ions
⇒ \(\mathrm{KCl}(s) \xrightarrow{\text { acidified water }} \mathrm{K}^{+}(a q)+\mathrm{Cl}^{-}(a q)\).
In alkaline water: A1(OH)3 reacts with OH ions to form soluble tetra hydroxo aluminate complex or meta-aluminateion (A1O-2).
Question 25. What do you understand by the terms: hydrogen economy hydrogenation ‘syngas’?
Answer: Hydrogenation is the process of addition of hydrogen to unsaturated organic compounds to form saturated organic compounds. For hydrogenation of oils.
Hydrogen Higher Order Thinking Skill Questions
Question 1. What is ‘hydrogenite’? Mention its use.
Answer:
‘Hydrogenite’
A mixture of silicon, caustic soda, and slaked lime is called hydrogenite. Dihydrogen can be prepared by heating hydrogenate with water.
⇒ \(\begin{aligned}
& \mathrm{Si}+2 \mathrm{NaOH}+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Na}_2 \mathrm{SiO}_3+\mathrm{H}_2 \uparrow \\
& \mathrm{Si}+\mathrm{Ca}(\mathrm{OH})_2+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{CaSiO}_3+2 \mathrm{H}_2 \uparrow
\end{aligned}\)
Question 2. What is denoted by [HgO4]+ ion? Explain
Answer:
[HgO4]+ ion
High charge density and high hydration energy of the H+ ion (proton) cause the H+ ion to remain solvated to form a hydroxonium ion or hydronium ion (H3O+). Hydronium ion again forms hydrogen bonds with three water molecules and remains solvated. Therefore, in water, a proton forms [H3O(H2O)3]+ or [H9O4]+ ion.
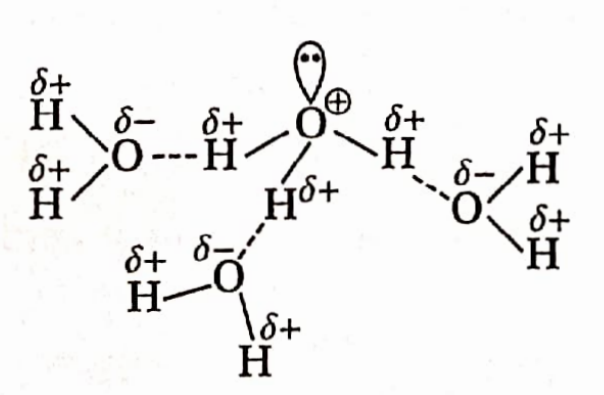
Question 3. Pure para-hydrogen is available but not pure ortho¬ hydrogen. Explain.
Answer:
Pure para-hydrogen is available but not pure ortho¬ hydrogen.
At ordinary temperature, ordinary hydrogen is a mixture of 75% of ortho and 25% of para-isomer. With the decrease in temperature, the amount of ortho-hydrogen decreases while that of para-hydrogen increases. At 20K, pure para-hydrogen is obtained. As para-hydrogen is more stable, it is found in the pure form. However, if the temperature is increased, the amount of ortho-isomer of dihydrogen increases but at 400K or above, the ratio of ortho-and para-isomer is fixed (3: 1). Therefore, pure para-hydrogen is available but not pure ortho-hydrogen.
Question 4. How many hydrogen-bonded water molecule(s) are associated with CuSO4-5H2O?
Answer: Only one molecule of water, which remains outside the brackets (coordination sphere) is linked by a hydrogen bond to SO2-4 as shown below. The remaining four water molecules are associated with Cu2+ ions by coordination bonds.
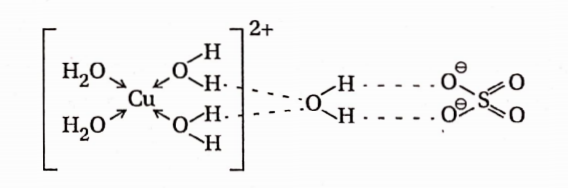
Question 5. Write down the reaction between H2O2 and hydrazine (NH2NH2) in the presence of a Cu(2) catalyst. Mention the use of this reaction.
Answer: A highly concentrated solution (about 40%) of H2O2 (called high test peroxide) oxidizes hydrazine (N2H4) in the presence of Cu(II) into nitrogen gas, itself being oxidized to water (steam)
⇒ \(\stackrel{-2}{\mathrm{~N}_2} \mathrm{H}_4(l)+2 \mathrm{H}_2{ }^{-1} \mathrm{O}_2(l) \xrightarrow[\text { catalyst }]{\mathrm{Cu}(\mathrm{II})} \stackrel{0}{\mathrm{~N}}(\mathrm{~g})+4 \mathrm{H}_2 \stackrel{-2}{\mathrm{O}}(\mathrm{g})+\text { heat }\)
The reaction is highly exothermic and is accompanied by a large increase in volume which in turn generates high pressure. Due to this, the reaction is employed for propelling rockets.
Hydrogen Multiple Choice Questions
Question 1. At absolute zero—
- Only para-hydrogen exists
- Only ortho-hydrogen exists
- Both ortho- and para-hydrogen exist
- Neither para- nor ortho-hydrogen exists
Answer: 1. Only para-hydrogen exists
Question 2. In which of the following reaction dihydrogen acts as an oxidizing agent—
- F2 +H2→2H
- Cl2 + H2→2hc1
- N2 + 3H2→2nH3
- 2Na + H2→2naH
Answer: 4. 2Na + h2→2naH
Question 3. Which of the following halogens has the least affinity towards hydrogen—
- H
- Cl2
- Br2
- F2
Answer: 1. H
Question 4. Which of the following compounds on electrolysis produces hydrogen—
- Dil. H2sO4
- Dil. Solution of NaOH
- Ba(oh)2 solution
- Koh solution
Answer: 3. Ba(oh)2 solution
Question 5. Thermal stability of gr.-15 Hydrides follows the order—
- Ash3 > ph3 > nh3 > sbh3 > bih3
- Nh3 > ph3 > ash3 > sbh3 > bih3
- Nh3 > ash3 > ph3 > sbh3 > bih3
- Bih3 > sbh3 > ash3 > ph3 > nh3
Answer: 1. Ash3 > ph3 > nh3 > sbh3 > bih3
Question 6. The correct order of vaporization enthalpy of the following hydride is—
- Nh3<ph3<ash3
- Ash3<ph3<nh3
- Ph3<ash3<nh3
- Nh3<ash3<ph3
Answer: 3. Ph3<ash3<nh3
Question 7. Interstitial hydrides are formed by—
- 5-Block elements
- P-block elements
- R f-block elements
- Intert gas elements
Answer: 3. Rf-block elements
Question 8. The correct descending order of thermal stability of alkali metals hydrides is—
- Lih > nah > kh > rbh > csh
- Csh > rbh > kh > nah > lih
- Nah > kh > lih > csh > rbh
- Csh > lih > kh > nah > rbh
Answer: 1. Lih > nah > kh > rbh > csh
Question 9. Solubility of nacl in the solvents h3O and d2O is—
- Equal in both
- More in d2O
- More in H2O
- Only in H2O
Answer: 3. More in D20
Question 10. Degree of hardness of 1 sample water containing 0.002 mol mgsO4 is—
- 20 Ppm
- 200 Ppm
- 2000 Ppm
- 120 Ppm
Answer: 2. 200 Ppm
Question 11. Which of the following reacts with water to produce electron-precise hydrides—
- Ca3p2
- A14c3
- Mg3n2
- None of these
Answer: 2. A14c3
Question 12. Which of the following couples reacts with water to produce the same gaseous product—
- K and kO2
- Ca and cah2
- Na and na2O2
- Ba and baO2
Answer: 2. Ca and cah2
Question 13. Which of the following compounds contain free hydrogen—
- Water
- Marsh gas
- Water gas
- Acid
Answer: 3. Water gas
Question 14. Which of the following reacts with metallic sodium to produce hydrogen—
- CH4
- C2H6
- C2H4
- C2H2
Answer: 4. C2h2
Question 18. Semi-water gas is—
- Co + H2 + N2
- H2 + ch4
- Co + H2 + O2
- Co + H2
Answer: 1. Co + H2 + N2
Question 10. Which of the following metals does not react with cold water but liberates H2 with boiling water—.
- Na
- K
- Pt
- Fe
Answer: 2. K
Question 17-. Volume of ’10 volume’ h2O2 required to convert 0.01 mol PBS into pbsO4 is—
- 11.2 ml
- 22.4 ml
- 33.6 ml
- 44.8 ml
Answer: 4. 44.8 ml
Question 18. On dilution of h2O2, the value of dielectric constant—
- Increases
- Remains same
- Decreases
- None of these
Answer: 1. Increases
Question 19. By which of the following water gets oxidized to oxygen—
- C1O2
- KmnO4
- H2O2
- F2
Answer: 4. F2
Question 20. Which of the following does not get oxidized by h2O2 —
- Na2sO3
- Pbs
- Ki
- O3
Answer: 4. O3
Question 21. The temperature at which the density of d2O is maximum is—
- 9°C
- 11.5°c
- 15.9°c
- 20°C
Answer: 2. 11.5°c
Question 22. Which of the following undergoes disproportionation reaction with water—
- SO3
- F2
- Cl2
- N2
Answer: 3. Cl2
Question 23. The non-inflammable hydrides—
- Nh3
- Ph3
- Ash3
- Sbh3
Answer: 1. Nh3
Question 24. The Triplepoint of water is—
- 203K
- 193K
- 273K
- 373K
Answer: 3. 273K
Question 25. The process by which hydrogen is prepared by the reaction of silicon, iron alloy, and NaOH is—
- Wood process
- Haber’s process
- Silicol process
- Bosch process
Answer: 3. Silicol process
Question 26. An element reacts with hydrogen to form a compound a, which on reaction with water liberates hydrogen again. The element is—
- Cl
- Cs
- Se
- N2
Answer: 2. Cs
Question 27. Only one element of which of the following groups forms metal hydride—
- Gr-6
- Gr-7
- Gr-8
- Gr-9
Answer: 1. Gr-6
Question 28. An acidic solution of which of the following turns orange in the presence of h2O2 —
- BaO2
- Na2O2
- TiO2
- PbO2
Answer: 3. TiO2
Question 29. In the following reaction the isotopic oxygens—
2MnO4 + 3h2O8 2mnO2 + 3O2 + 2H2O + 2Oh-
- Both get converted into O2
- Both get converted into Oh-
- Both get converted into mnO2
- One of them gets converted to O2, another to mnO2
Answer: 1. Both get converted into O2
Question 30. X on electrolysis produces y which on vacuum distillation produces h202. The numbers of peroxo linkage present in x and y are—
- 1,1
- 1, 2
- 0 > 1
- 0, 0
Answer: 3. 0 > 1
Question 31. The compound which on electrolysis in its molten or liquid state liberates hydrogen at anode is—
- Noah
- Cah2
- Hc1
- H2O
Answer: 2. Cah2
Question 32. Which of the following couples exhibit the maximum isotope effect—
- \({ }_1^1 \mathrm{H},{ }_1^2 \mathrm{D}\)
- \({ }_8^{16} \mathrm{O},{ }_8^{18} \mathrm{O}\)
- \({ }_{17}^{35} \mathrm{Cl},{ }_{17}^{37} \mathrm{Cl}\)
- \({ }_6^{12} \mathrm{C},{ }_6^{14} \mathrm{C}\)
Answer: 1. \({ }_1^1 \mathrm{H},{ }_1^2 \mathrm{D}\)
Question 33. Which of the following emits by tritium—
- Neutron
- Y-ray
- 3-Particle
- O-particle
Answer: 3. 3-Particle
Question 34. Oxidation of benzene by H2O9 in the presence of ferrous sulfate produces—
- Phenol
- Cyclohexane
- Anisole
- Benzaldehyde
Answer: 1. Phenol
Question 35. The oxidation state of cr in the product obtained by the reduction of k2cr2O7 by atomic hydrogen is—
- +6
- +2
- 0
- +3
Answer: 4. +3
Question 38. Which of the following does not get reduced by h2 in its aqueous solution—
- Cu2+
- Fe3+
- Zn2+
- Ag+
Answer: 1. Cu2+
Question 37. Which of the following compounds has a similar odor as that of h2O2 —
- Caustic soda
- Chloroform
- Alcohol
- Nitric acid
Answer: 4. Nitric acid
Question 38. Which of the following compounds reacts with atomic hydrogen to form formaldehyde—
- Co
- CO2
- Ch4
- C2H2
Answer: 1. Co
Question 39. Which of the following isotopes of hydrogen is the most reactive—
- \({ }_1^1 \mathrm{H}\)
- \({ }_2^1 \mathrm{H}\)
- \({ }_3^1 \mathrm{H}\)
- All the isotopes are equally reactive
Answer: 1. \({ }_1^1 \mathrm{H}\)
Question 40. When equal amounts of zn are allowed to react separately with excess h2SO4 and excess NaOH, then the ratio of the volumes of hydrogen produced for the first and the second case respectively is—
- 1:2
- 2:1
- 4:9
- 1:1
Answer: 4. 1:1
Question 41. Which of the following hydrides of s-block elements have a polymeric structure—
- Lih
- Beh2
- Nah
- Mgh2
Answer: 2. Beh2
Question 42. Which of the following statements is true—
- If z= 15, the element forms a covalent hydride
- If z = 23, the element forms an ionic hydride
- If z= 19, the element forms an ionic hydride
- If z = 44, the element forms a metalic hydride
Answer: 1. If z= 15, the element forms a covalent hydride
Question 43. Which of the following hydrides are polynuclear hydrides—
- Nah
- C3h8
- N2h4
- H2
Answer: 2. C2H8
Question 44. Which of the following statements is correct—
- Metallic hydrides are hydrogen-deficient
- Metallic hydrides are conductors of heat and electricity
- Ionic hydrides in their solid state do not conduct electricity
- Ionic hydrides on electrolysis in their molten state produce h2 at the cathode.
Answer: 1. Metalic hydrides are hydrogen deficient
Question 45. Which of the following ions get exchanged with the na+ ion of zeolite when the zeolite is added to the hard water—
- H+ ion
- Ca2+ ion
- SO4 ion
- Mg2+ ion
Answer: 2. Ca2+ ion
Question 46. Which of the following reactions are neurolysis—
- 2Na + 2D2O → 2NaOD + D2
- AIcI3 + 3D2O →AI(OD)3 + 3DCI
- Ca + 2D2O→Ca(OD)2 + D2
- Fe2(SO4)3 + 6D2O→2Fe(OD)3 + 3D2SO4
Answer: 2. AIcI3 + 3D2O →AI(OD)3 + 3DCI
Question 47. Which of the following reactions are redox reactions —
- H2O+SO2→H2SO
- CaO + H2O → Ca(OH)2
- 2Na + 2H2O → 2NaOH + H2
- 2F2 + 2H20 →O2 + 4HF
Answer: 3. 2Na + 2H2O → 2NaOH + H2
Question 40. In which of the following reactions H2O2 acts as a reductant—
- CgHg + H2O2 C6H5OH + H2O
- PbS + 4H2O2→PbSO4 + 4H2O
- NaOBr +H2O2→NaBr + H2O +O2
- 2MnO–4+6H+→+ 5H2O2→2Mn2+ + 8H2O + 5O2
Answer: 3. NaOBr +H2O2→NaBr + H2O +O2
Question 49. Which of the following properties are the same for metal and its hydride—
- Hardness
- Metallic lustre
- Electrical conductance
- Magnetic property
Answer: 1. Hardness
Question 50. The correct orders are—
- H2 < D2 < T2 : boiling point
- H2 < D2 < T2 : freezing point
- H2 < D2 < T2 : latent heat of vaporisation
- T20 > H2O > D20 : dissociation constant
Answer: 1. H2 < D2<T2 : boiling point
Question 51. Which of the following reacts with zinc to produce hydrogen gas—
- Dil. Hc1
- Hot naoh solution
- Cold water
- Cone. H2SO4
Answer: 1. Dil. Hc1
Question 52. Which of the following properties have a greater magnitude in D2O than that in h2O —
- Viscosity
- Surface tension
- Dielectric constant
- Latent heat of vaporization
Answer: 1. Viscosity
Question 53. Which of the following metal hydrides get reduced by hydrogen—
- Cuo
- Pb3O4
- Na2O2
- MgO
Answer: 1. Cuo
Question 54. Multimolecular covalent hydrides of 5-block are—
- Lih
- Beh2
- Nah
- Mgh2
Answer: 2. Beh2
Question 55. The oxidation numbers of the most electronegative element in the product were obtained due to the reaction between baO2 and dil. H2sO4 are—
- -1
- -2
- 0
- +1
Answer: 1. -1
Question 55. Which of the following compounds decreases the rate of decomposition of h2O2 —
- Co(nh2)2
- PbNHCOCH3
- MnO2
- (Cooh)2
Answer: 1. Co(nh2)2
Question 57. Which of the following produces h2O2 on hydrolysis—
- Pernitric acid
- Perchloric acid
- Perdisulphuric acid
- Caro’s acid
Answer: 1. Pernitric acid
Question 58. Choose the correct statements—
- The concentration of 20 volume h2O2 solution is 60.7g
- Volume strength of 2(n)h2O2 solution is 15
- Volume strength of 2(n)h2O2 solutions 11.2
- The concentration of 20 volume h2O2 solution is 50.7g
Answer: 1. Concentration of 20-volume h2O2 solution is 60.7g
Question 59. Choose the correct alternative—
- A mixture of HCI and hair is formed when chlorine reacts with cold water
- Arrange color of the k2cr2O7 solution turns blue when it reacts with H2O2
- Under low pressure, isopropyl alcohol reacts with a small amount of H2O2 to produce formaldehyde
- Hydrolith produces black coloured product when it reacts with pbs04
Answer: 1. Mixture of hc1 and hair is formed when chlorine reacts with cold water
Question 60. Which of the following alternatives is not true—
- Correct order of reactivity of H2 towards the halogens is: cl2 > br2 > i2 > f2
- The concentration of H2O2 used in rockets is 90%
- H2 gets more readily absorbed on the surface of pt-metal than D2
- Conversion of atomic hydrogen into molecular hydrogen is an exothermic process
Answer: 1. Correct order of reactivity of H2 towards the halogens is: cl2 > br2 > i2> f2
Question 61. The normality of ’30 volume’ of H2O2 is—
- 2.678 (N)
- 5.336 (N)
- 8.034 (N)
- 6.685 (N)
Answer: 2. 5.336 (N)
Volume strength = 5.6 x normality or, 30= 5.6 x normality or, noemality \(=\frac{30}{5.6} \mathrm{~N}=5.357 \mathrm{~N}\) The normality of 30 volumes of H2O2 is 5.357N.
Question 62. When H2O2 is shaken with an acidified solution of K2Cr2O7 in the presence of ether, the ether layer turns blue due to the formation of— 2-
- Cr2O3
- Cr2(SO4)3
- CrO4
- CrO5
Answer: 4. CrO5
Question 63. A commercial sample of H2O2 is labeled as 10V. Its % strength is nearly—
- 3
- 6
- 9
- 12
Answer: 1. 6
Question 64. In an aqueous alkaline solution, two-electron reduction of HO¯² given—
- HOθ
- H2O
- O2
- O¯2
Answer: 1. HOθ
Question 65. Which statement is not correct for ortho- and para-hydrogen—
- They have different boiling points
- Ortho-form is more stable than para-form
- They differ in their nuclear spin
- The ratio of ortho to para-hydrogen changes with a change in temperature
Answer: 2. Ortho-form is more stable than para-form
At normal or high temperatures, ortho-hydrogen is more stable than para-hydrogen but at very low temperatures para-hydrogen is more stable than orthohydrogen.
Question 66. At room temperature, the reaction between water and fluorine produces
- HF and H2O2
- HF,O2 and F2O2
- F+Θ, O2 and H+⊕
- HOF and HF
Answer: 3. F+Θ, O2 and H+⊕
⇒ \(\mathrm{F}_2+\mathrm{H}_2 \mathrm{O} \xrightarrow{\text { room temperature }} \underset{\substack{\downarrow \\ \mathrm{H}^{+}+\mathrm{F}^{-}}}{\mathrm{HF}}+\mathrm{O}_2\)
Question 67. Very pure hydrogen (99.9%) can be made by which of the following processes—
- Mixing natural hydrocarbons of high molecular weight
- Electrolysis of water
- Reaction of salt-like hydrides with water
- Reaction of methane with steam
Answer: 3. Reaction of salt-like hydrides with water
Question 68. In which of the following reactions, H2O2 acts as a reducing agent—
- \(\mathrm{H}_2 \mathrm{O}_2+2 \mathrm{H}^{+}+2 e \rightarrow 2 \mathrm{H}_2 \mathrm{O}\)
- \(\mathrm{H}_2 \mathrm{O}_2-2 e \rightarrow \mathrm{O}_2+2 \mathrm{H}^{+}\)
- \(\mathrm{H}_2 \mathrm{O}_2+2 e \rightarrow 2 \mathrm{OH}^{-}\)
- \(\mathrm{H}_2 \mathrm{O}_2+2 \mathrm{OH}^{-}-2 e \rightarrow \mathrm{O}_2+2 \mathrm{H}_2 \mathrm{O}\)
Choose The Correct Option
- 2,4
- 1,2
- 3,4
- 1,3
Answer: 1. 2,4
Question 69. From the following statements regarding H202, choose the incorrect statement—
- It has to be stored in plastic or wax-lined glass bottles in dark
- It has to be kept away from dust
- It can act only as an oxidizing agent
- It decomposes on exposure to light
Answer: 3. It can act only as an oxidizing agent
Question 70. Which one of the following statements about water is false—
- Water can act both as an acid and as a base
- There is extensive intramolecular hydrogen bonding in the condensed phase
- Ice formed by heavy water sinks in normal water
- Water is oxidized to oxygen during photosynthesis
Answer: 2. There is extensive intramolecular hydrogen bonding in the condensed phase
Water molecules are associated with the formation of intermolecular hydrogen bonding
Question 71. Hydrogen peroxide oxidises [Fe(CN)6]4- to [Fe(CN)6]3- in acidic medium but reduces [Fe(CN)6]3- to [Fe(CN)6]4- in alkaline medium. The other products formed are, respectively—
- H2O and (H2O + O2)
- H2O and (H2O + OH-)
- (H2O + O2) and H2O
- (H22O + O2) and (H2O + OH-)
Answer: 1. H2O and (H2O + O2)
⇒ \(\left.2\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{4-}+\mathrm{H}_2 \mathrm{O}_2+2 \mathrm{H}^{+} \longrightarrow \longrightarrow \mathrm{Fe}(\mathrm{CN})_6\right]^{3-}+2 \mathrm{H}_2 \mathrm{O}\)
Alkaline medium:
⇒ \(\begin{aligned}
2\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-}+2 \mathrm{OH}^{-}+ & \mathrm{H}_2 \mathrm{O}_2 \\
& {\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{4-}+2 \mathrm{H}_2 \mathrm{O}+\mathrm{O}_2 }
\end{aligned}\)
Question 72. Which of the following electron-deficient-
- (BH3)2
- PH3
- (Ch3)2
- (SiH3)2
Answer: 1. (BH3)2
Question 73. The reaction of aqueous KMnO4 with H2O2 conditions gives—
- Mn4+ and O2
- Mn2+ and O2
- Mn2+ and O3
- Mn2+ and MnO2
Answer: 2. Mn2+ and O2
Question 74.
- H2O2 + O3→H2O + 2O2
- H2O2 + Ag2O→2Ag + H2O + O2
The role of hydrogen Peroxide in the above reactions is respectively—
- Oxidsing in 1 and reducing in 2
- Reducing in 1 and oxidising in 2
- Reducing in 1 and 2
- Oxidizing in 1 and 2
Answer: 3. Reducing in 1 and 2
Question 75. Which of the following statements about hydrogen is incorrect—
- Hydrogen has three isotopes of which tritium is the most common
- Hydrogen never acts as cationic ionic salts
- Hydronium ion, H3O exists freely in solution
- Dihydrogen does not act as a reducing agent
Answer: 1. Hydrogen has three isotopes of which tritium is the most common
Question 76. In ice, the oxygen atom is surrounded—
- Tetrahedrally by 4 hydrogen atoms
- Octahedrally by 2 oxygen and 4 hydrogen atoms
- Tetrahedrally by 2 hydrogen 2 oxygen atoms
- Octahedrally by 6 hydrogen atoms
Answer: 1. Tetrahedrally by 4 hydrogen atoms
X-ray studies have shown that in ice, four hydrogen atoms tetrahedrally surround each oxygen atom.
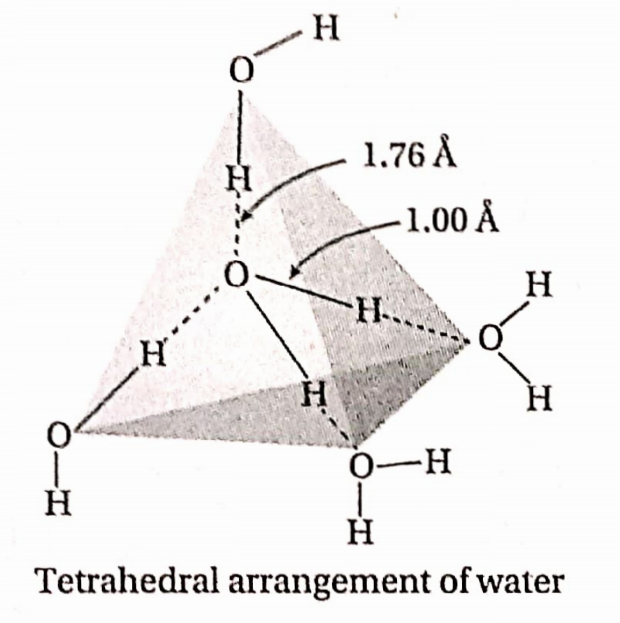
Question 77. Predict the product of the reaction of I2 with H2O2 in basic mL of H2O2
medium—
- I‾
- i2O3
- IO2
- I¯3
Answer: 1. I-
⇒ \(\begin{array}{r}
\mathrm{I}_2(s)+\mathrm{H}_2 \mathrm{O}_2(a q)+2 \mathrm{OH}^{-}(a q) \longrightarrow \downarrow \\
2 \mathrm{I}^{-}(a q)+2 \mathrm{H}_2 \mathrm{O}(l)+\mathrm{O}_2(g)
\end{array}\)
Question 78. The strength of H2O2 is 15.18 g. L-1, then it is equal to—
- 1 volume
- 10 volume
- 5 volume
- 7 volume
Answer: 3.05 volume
Question 79. Which of the following reactions increases the production of dihydrogen from synthesis gas-
- \(\mathrm{CH}_4(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}(\mathrm{g}) \xrightarrow[\mathrm{Ni}]{\mathrm{1270K}} \mathrm{CO}(\mathrm{g})+\mathrm{H}_2(\mathrm{~g})\)
- \(\mathrm{C}(\mathrm{g})+\mathrm{H}_2 \mathrm{O}(\mathrm{g}) \xrightarrow{1270 \mathrm{~K}} \mathrm{CO}(\mathrm{g})+\mathrm{H}_2(\mathrm{~g})\)
- \(\mathrm{CO}(\mathrm{g})+\mathrm{H}_2 \mathrm{O}(\mathrm{g}) \xrightarrow[\text { Catalyst }]{673 \mathrm{~K}} \mathrm{CO}_{2(\mathrm{~g})}+\mathrm{H}_{2(\mathrm{~g})}\)
- \(\mathrm{C}_2 \mathrm{H}_6(\mathrm{~g})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{g}) \xrightarrow[\mathrm{Ni}]{\frac{1270 \mathrm{~K}}{\longrightarrow}} 2 \mathrm{CO}+5 \mathrm{H}_2\)
Answer: 3. \(\mathrm{CO}(\mathrm{g})+\mathrm{H}_2 \mathrm{O}(\mathrm{g}) \xrightarrow[\text { Catalyst }]{673 \mathrm{~K}} \mathrm{CO}_{2(\mathrm{~g})}+\mathrm{H}_{2(\mathrm{~g})}\)
The production of dihydrogen can be increased by reacting the carbon monoxide of syngas with steam in the presence of iron chromate as a catalyst.
⇒ \(\mathrm{CO}_{(\mathrm{g})}+\mathrm{H}_2 \mathrm{O}_{(\mathrm{g})} \xrightarrow[\text { Catalyst }]{673 \mathrm{~K}} \mathrm{CO}_{2(\mathrm{~g})}+\mathrm{H}_{2(\mathrm{~g})}\)
This is called the water-gas shift reaction.
Question 80. Which ofthe following produces hydrogen
- Mg + H2O
- H2S2O8 + H2O
- BaO2 + HCl
- Na2O2 + 2HC1
Answer: 1. Alkali and alkaline earth metals react with water to produce hydrogen gas and metal hydroxides. This occurs due to high electropositive character ofthe metals
⇒ \(\mathrm{Mg}+2 \mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{Mg}(\mathrm{OH})_2+\mathrm{H}_2\)
Question 81. H2O2 can be obtained when the following reacts with H2SO4 except with-
- BaSO2
- PbO2
- Na2O2
- SrO2
Answer: 2. PbO2
H2O2 is prepared by the reaction of peroxide with H2SO4.PbO2 is a dioxide. Hence, it does not give H2O2 with dilute H2SO4
Hydrogen Very Short Answer Type Questions
Question 1. Which is the lightest gas known?
Answer: Dihydrogen;
Question 2. Which isotope of hydrogen is radioactive?
Answer: Tritium \(\left({ }_1^3 \mathrm{H}\right)\)
Question 3. Give examples of an ionic, a covalent, and a metallic hydride.
Answer: CaH2 (ionic); NH3 (covalent) & CrH (metallic)
Question 4. What is hydrolith?
Answer: Calcium hydride (CaH2)
Question 5. Name the two nuclear spin isomers of dihydrogen.
Answer: Ortho-hydrogen and para-hydrogen;
Question 6. Give an example of an electron-deficient hydride in which three centre-two
electron bonds are present.
Answer: B2H6
Question 7. Which gaseous compound on treatment with dihydrogen produces methanol?
Answer: Carbon monoxide
Question 8. How will you prove that a colorless liquid is water?
Answer: White anhydrous CuSO4 becomes blue in contact with water
Question 9. What is the unit for expressing the degree of hardness of water?
Answer: ppm (parts per million);
Question 10. write the names of two chemical substances which are used for removing
dissolved oxygen from water meant for the boiler.
Answer: Hydrazine (NH2NH2) and sodium sulphite (Na2SO3);
Question 11. Why is heavy water used in atomic reactors?
Answer: It is used as a moderator
Question 12. Name a solid and a liquid absorbent of water.
Answer: Cone. H2SO4 and P2O5
Question 13. Which chemical is commercially known as ‘perhydrol’?
Answer: H2O2 solution
Question 14. What is called ‘hyper lol or horizon’?
Answer: A compound of hydrogen peroxide and urea is called hyper lol (NH2CONH2-H2O2);
Question 15. What is the die volume strength of a molar solution of H2O2?
Answer: 11.2 volume
Question 16. Which organic reagent is used for the manufacture of H2O2?
Answer: 2-ethylanthraquinol
Question 17. 10 volume of H2O2 = x(N)H2O2 .What is the value of
Answer: X = 56
Question 18. What are how water molecules are bonded to the anhydrous salt
to form hydrates?
Answer: Coordinate bond and H bond.
Hydrogen Fill In The Blanks
Question 1. The radioactive isotope of hydrogen is____________.
Answer: Tritium
Question 2. When NaH is electrolysed,____________ is obtained at the anode.
Answer: Dihydrogen
Question 3. Syngas is a mixture of hydrogen and____________.
Answer: Carbon monoxide
Question 4. para-hydrogen is____________ stable than ortho-hydrogen.
Answer: More
Question 5. The oxygen atom in the water molecule is____________ hybridized.
Answer: SP3
Question 6. H2O undergoes electrolysis____________than D20.
Answer: Faster
Question 7. Heavy water is used as a____________in nuclear reactors.
Answer: Moderate
Question 8. Temporary hardness is also known as ____________hardness.
Answer: Carbonate
Question 9. Rainwater is____________water but sea water is water.
Answer: Soft, Hard
Question 10. The reaction between CaC2 and D2O forms____________.
Answer: C2D2
Question 11. D2O2 can be prepared by electrolysing____________by d2o.
Answer: K2S2O8
Question 12. H2O2____________ isslighdy acid than water.
Answer: Stronger
Question 13. Decomposition of H2O2 is suppressed by ____________
Answer: Urea
Question 14. Boiling point of H2O2 is____________ than water.
Answer: Higher
Question 15. The mixture of & H2O2 is known as Fenton’s____________ reagent.
Answer: FeSO4
Question 16. Volume strength of 1.5(N) H2O2 is ____________
Answer: 8.4
Hydrogen Numerical Examples
Question 1. 1L of a sample of hard water contains 1 mg CaCl2 and 1 mg MgCl2. Estimate the degree of hardness of this sample of water
Answer:
Given
1L of a sample of hard water contains 1 mg CaCl2 and 1 mg MgCl2.
Molecular mass of CaCl2 =111
Now lllg of CaCl2=100g of CaCO3
∴ 1 mg CaCl2 \(\equiv \frac{100 \times 0.001}{111} \mathrm{~g}\) of CACO3
= 9×10-4 g of CACO3 = 0.9 mg of CaCO3
The molecular mass of MgCl2 = 95
Now, 95g of MgCl2=100g of CaCO3
⇒ \(1 \mathrm{mg} \text { of } \mathrm{MgCl}_2 \equiv \frac{100}{95} \mathrm{mg}\)
CaCO3 = 1.05mg of CaCO3
An equivalent amount of CaCO3 corresponding to CaCl2 and MgCl2 present l Lor 103g of hard water
= (0.90 + 1.05)mg = 1.95mg [1L water = 103g = 106mg water]
The mass of the equivalent amount of CaC03 corresponding to CaCl2 and MgCl2 presenting 106mg of water is 1.95 mg.
Hence, the degree of hardness of the given sample is 1.95 ppm.
Hence, the hardness of that sample of water is 75 ppm.
Question 2. Determine the strength of ’30 volume’ H2O2 in normality
Answer: Volume strength = 5.6 x normality
or, 30 = 5.6 x normality or, normality \(=\frac{30}{5.6} \mathrm{~N}=5.35 \mathrm{~N}\)
∴ The normality of 30 volumes of H2O2 is 5.35N
Question 3. Determine the volume (in liter) of O2 obtained at STP when 0.1 liter of 2(M)H2O2 solution is decomposed.
Answer: \(\begin{array}{cc}
2 \mathrm{H}_2 \mathrm{O}_2 & 2 \mathrm{H}_2 \mathrm{O}+\mathrm{O}_2 \\
(2 \times 34) \mathrm{g}=68 \mathrm{~g} & 22.4 \mathrm{~L}(\text { at STP) }
\end{array}\)
Now, 1L1(M) H2O2 = 34g of H2O2
1L2(M) H2O2 →(2 X 34)g of H2O2
0.1L 2(M) H2O22 = (2 X 34 X 0.1 )g of H2O2 →Kg of H2O2
Again at STP, 68g of M2O2 produces 22.4L of O2
∴ 6.8 g of H2O2 produces \(\frac{22.4}{68} \times 6.8 \mathrm{~L} \text { of } \mathrm{O}_2\)
Question 4. When 100 ml of tube-well water is titrated using methyl orange as an indicator, it requires 15 ml of 0.01 (N) HCl. Estimate the hardness of that sample of water.
Answer: 15 mL 0.01 (N) HCI = 1 mL 0.15(N) HCI 1000 ml HCL=\(\mathrm{HCl} \equiv \frac{100}{2} \mathrm{~g} \mathrm{CaCO}_3\)
1 mL 0.15 (N) HCI \(\begin{aligned}
& =50 \times \frac{1}{1000} \times 0.15 \mathrm{~g}^{-} \text {of } \mathrm{CaCO}_3 \\
& =7.5 \times 10^{-3} \mathrm{~g} \text { of } \mathrm{CaCO}_3
\end{aligned}\)
Therefore, 100, or 100g of that sample of water contains some hardness-producing substance which is equivalent to 7.5 x 10-3g of CaCO3. 106g of water contains the hardness-producing substance equivalent to \(\frac{7.5 \times 10^{-3} \times 10^6}{100}=75 \mathrm{~g} \text { of } \mathrm{CaCO}_3\)
Question 5. Calculate the amount of H2O2 present in 600 mL of 10-volume H2O2 solution.
Answer: 10 volume H2O2 means that 1 mL of H2O2 solution will produce 10 mL of O2 at STP
\(\begin{gathered}
2 \mathrm{H}_2 \mathrm{O}_2 \longrightarrow 2 \mathrm{H}_2 \mathrm{O}+\mathrm{O}_2 \\
\left(2^{\prime} 34\right) \mathrm{g}=68 \mathrm{~g} \quad 22400 \mathrm{~mL} \text { (at STP) }
\end{gathered}\)
At STP, 10 mL O2 is obtained from 1 mL of 10vol H2O2 solution
22400 mL of O2 is obtained from \(\frac{22400}{10}\) of 10 ml of 10 vol; H2O2 solution =2240 mL of H2O2 2240 mL of H2O2 solution contains 68g of H2O2
100 mL of H2O2 solution contains \(\frac{68 \times 100}{2240}\) =3.03g of H2O2.
So, 600 mL of H2O2 solution contains \(=\frac{3.03 \times 600}{100} \mathrm{~g}\) of H2O2 = 18.18g of H2O2 =18.2g of H2O2
Question 6. An excess of acidic KI solution is added to 25 mL of a H2O2 solution when iodine is liberated. 20 mL of 0.1(N) sodium thiosulfate solution is required to titrate the liberated iodine. Calculate the percentage strength, volume strength, and strength in normality of the H2O2 solution.
Answer:
Given
An excess of acidic KI solution is added to 25 mL of a H2O2 solution when iodine is liberated. 20 mL of 0.1(N) sodium thiosulfate solution is required to titrate the liberated iodine.
25 x (N) = 20 x 0.1(N)
⇒ \(x=\frac{20 \times 0.1}{25}=\frac{2}{25}=0.08(\mathrm{~N})\)
Amount of H2O2 in 25mL0.08(N) H2O2 solution
1000 mL 1(N) H2O2 solution = 17g H2O2 25mL0.08(N) H2O2 solution \(\equiv \frac{17 \times 25 \times 0.08}{1000}=0.034 \mathrm{~g}\) H2O2
100ml 0.08(N) H2O2 solution contains \(=\frac{0.034 \times 100}{25}\)
= 0.136gH2O2
% strength of H2O2 solution = 0.136
⇒ \(\begin{array}{cr}
2 \mathrm{H}_2 \mathrm{O}_2 \\
2 \times 34=68 \mathrm{~g} & 2 \mathrm{H}_2 \mathrm{O}+\mathrm{O}_2 \\
& 22.4 \mathrm{~L} \text { at STP }
\end{array}\)
68g H2O2 gives→ 22.4 L O2 at STP
0.034g H2O2 solution \(\begin{aligned}
\rightarrow & \frac{22.4 \times 0.034}{68} \text { Litre } \mathrm{O}_2 \text { at STP } \\
& =0.0112 \mathrm{Litre}_2 \text { at STP } \\
& =11.2 \mathrm{~mL} \mathrm{O}_2 \text { at STP }
\end{aligned}\)
25mLH2O2 solution gives 11.2mLO2 atSTP
lmL H2O2 solution given \(\frac{11.2}{25}=0.448 \mathrm{~mL} \mathrm{O}_2\) at stp.
Volume strength ofthe given H2O2 solution = 0.448.