Chemical Bonding And Molecular Structure Multiple Choice Questions
Question 1. The Sp3d2-hybridization of the central atom of a molecule would be
- Wouldsquareleadplanarto— geometry
- Tetrahedral geometry
- Trigonal bipyramidal geometry
- Octahedral geometry
Answer: 4. Octahedral geometry
One s, three p, and two d -orbitals mix up together to form six equivalent Sp3d2-hybrid orbitals. The molecules, in which these orbitals are involved, have octahedral geometry.
Question 2. Which of the following is paramagnetic
- N2
- NO
- CO
- O3
Answer: 2. No
From a consideration of electron distribution in molecular orbitals of NO, it is known that it has one unpaired electron. So, NO molecule is paramagnetic.
Question 3. In the electron-dot structure, calculate the formal charge from left to right nitrogen atom, \(\ddot{\mathrm{N}}=\mathrm{N}=\ddot{\mathrm{N}}-\)
- -1,-1+1
- -1,+1,-1
- +1,-1,-1
- +1,-1,+1
Answer: 2. -1,+1,-1
Formal charge = No. of valence electrons in the atom No. of unshared electrons \(-\frac{1}{2}\) No. of shared electrons.
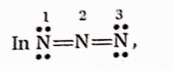
- Formal charge on N-atom (1) =5-4- (4 ÷ 2) = -1
- Formal charge on N-atom (2) = 5- 0- (8 ÷ 2) = 1
- Formal charge onN-atom (3) = 5- 4- (4÷ 2) = -1
Question 4. Which of the following Compounds has the maximum volatility
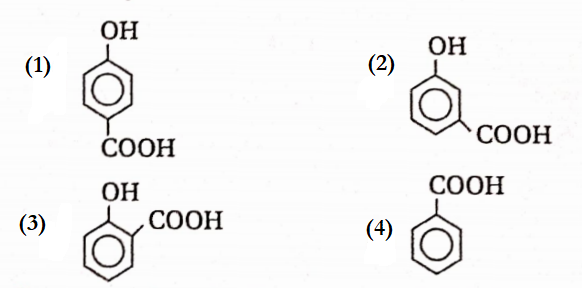
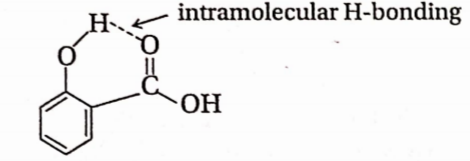
Answer: 3. ![]() In O-hydroxy carboxylic acid, the —OH and —COOH groups are situated at two adjacent carbon atoms of the ring and are involved in intramolecular H-bond formation.
In O-hydroxy carboxylic acid, the —OH and —COOH groups are situated at two adjacent carbon atoms of the ring and are involved in intramolecular H-bond formation.
So, these molecules exist as discrete molecules and consequently, the compound has maximum volatility.
Question 5. The number of acid protons in H3PO3 is
- 0
- 1
- 2
- 3
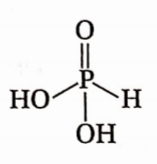
Answer: 3. 2 Number of the —OH group in H3PO3 is 2 and henceitis dibasic in nature.
Question 6. In 2-butene, which of the following statements is true—
- C1 —C2 bondis a sp3-sp3 tr – bond
- C2— C3 bond is a sp3-sp2 or- bond
- C1—C2 bond is a sp3-sp3 r-bond
- C1—C2 bond is a sp2-sp2 cr-bond
Answer: 3. C1—C2 bond is a sp3-sp2 r-bond
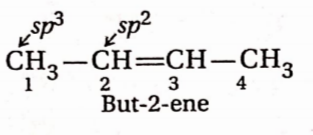
Question 7. The paramagnetic behavior of B2 Is due to the presence of—
- 2 unpaired electrons πb MO
- 2 unpaired electrons in π* MO
- 2 unpaired electrons in σ* MO
- 2 unpaired electrons in σb MO
Answer: 1. The electronic configuration of B2 (10 electrons) is \(K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\pi_{2 p x}\right)^1\left(\pi_{2 p y}\right)^1\)
Since the molecule contains two unpaired electrons in πbMO, it is paramagnetic.
Question 8. The state of hybridization of the central atom and the number of lone pairs over the central atom in POCl3 are-
- sp,O
- sp2O
- sp3,O
- dsp2,1
Answer: 3. The Geometrical shape of the POC13 molecule is tetrahedral. In POC13 central P-atom is sp3 hybridised and has no lone pair.
Question 9. CO is practically non-polar since—
- The cr -electron drift from c to o is almost nullified by the n -electron drift from 0 to c
- The tr -electron drift from o to c is almost nullified by the 7r -electron drift from c to o
- The bond moment is low
- There is a triple bond between c and O
Answer: 1. The cr -electron drift from c to o is almost nullified by the n -electron drift from 0 to c
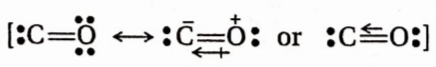
Oxygen donates an unshared pair of electrons to carbon and helps it to complete its octet by forming a dative n bond with it. As a result, a much stronger n -n-moment acts from oxygen to a carbon atom, and this moment is almost canceled by the cr -moment and the weak n -moment acting in the opposite direction. Hence, the polarity of the molecule is verylow.
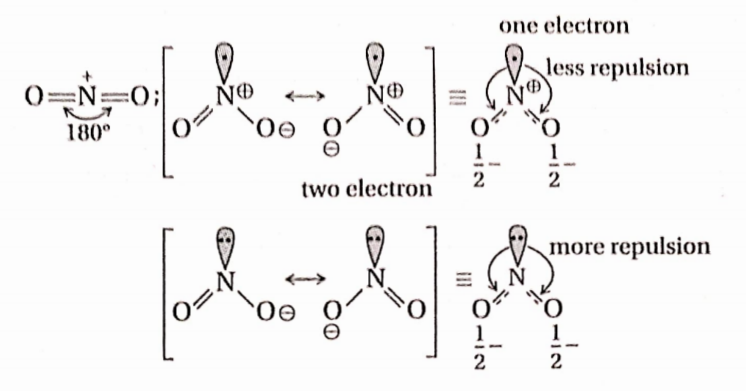
Question 10. The increasing order of the O —N —0 bond angle in the species NO2, NO+2, and NO–2is—
- \(\mathrm{NO}_2^{+}<\mathrm{NO}_2<\mathrm{NO}_2^{-}\)
- \(\mathrm{NO}_2<\mathrm{NO}_2^{-}<\mathrm{NO}_2^{+}\)
- \(\mathrm{NO}_2^{+}<\mathrm{NO}_2^{-}<\mathrm{NO}_2\)
- \(\mathrm{NO}_2<\mathrm{NO}_2^{+}<\mathrm{NO}_2^{-}\)
Answer: None; the correct order is \(\mathrm{NO}_2<\mathrm{NO}_2^{-}<\mathrm{NO}_2^{+}\)
Question 11. The ground state electronic configuration of the CO molecule is-
- \(1 \sigma^2 2 \sigma^2 1 \pi^2 3 \sigma^2\)
- \(1 \sigma^2 2 \sigma^2 3 \sigma^2 1 \pi^2 2 \pi^2\)
- \(1 \sigma^2 2 \sigma^2 1 \pi^2 3 \sigma^2 2 \pi^2\)
- \(1 \sigma^2 1 \pi^4 2 \sigma^2 3 \sigma^2\)
Answer: 4. \(1 \sigma^2 1 \pi^4 2 \sigma^2 3 \sigma^2\)
The ground state outer electronic configuration of the CO molecule is \(1 \sigma^2 1 \pi^4 2 \sigma^2 3 \sigma^2\)
Question 12. In diborane, the number of electrons that account for bonding die bridges is
- Six
- Two
- Eight
- Four
Answer: 4. Four
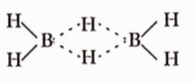
In diborane, each bridging B——H——B bond is formed by two electrons. Hence, four electrons account for bonding the bridges.
Question 13. In O2 and H2O2, the O — O bond lengths are 1.2lA and 1.48A respectively. In ozone, the average O —O bond length is
- 1.28A
- 1.18A
- 1.44A
- 1.526A
Answer: 1. Bond length is nearly average of O—O length in
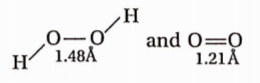
Question 14. In SOC12, the Cl—S—Cl and Cl—S—O bond angles are—
- 130°, 115°
- 106°, 96°
- 107°, 108°
- 96°, 106°
Answer: 4. 96°, 106°
IN SOC12, the Cl —S —Cl bond angle is 96° and the Cl — S —O bond angle is 106°, since multiple bonds create more repulsions than single bonds.
Question 15. The structure of XeF6 is experimentally determined to be a distorted octahedron. Its structure according to VSEPR theory is—
- Octahedron
- Trigonal bipyramid
- Pentagonal bipyramid
- Tetragonal bipyramid
Answer: 3. Pentagonal bipyramid
In XeF6, Xe is surrounded by 6 bond pairs and one lone pair. So, according to VSPER theory, the geometry (geometry of electron pairs) is pentagonal bipyramid.
Question 16. In the case of heteronuclear diatomics of the type AB, where A is more electronegative than B, bonding MO resembles the character of A more than that of B. The statement—
- Is False
- Is True
- Cannot Be Evaluated Since the Data Is Not Sufficient
- Is True Only FOr Certaqin Systems
Answer: 2. Cannot Be Evaluated Since Data Is Not Sufficient
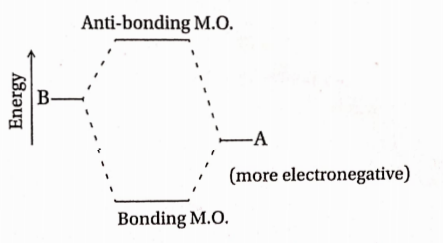
As A is more electronegative, there is less energy difference between the atomic orbital of A and bonding M.O. Hence, bonding M.O. resembles A more closely.
Question 17. The bond angle in NF3 (102.3°) is smaller than NH3 (107.2°). This is because of—
- Large size off compared to
- The large size compared to
- Opposite polarity of n in the two molecules
- Small size compared to a ton
Answer: 3. In NF3 molecules, the N—F bond pair is drawn
more towards the more electronegative F-atom. But in the NH3 molecule, the N—H bond pair is drawn more towards the more electronegative N-atom. Therefore, the extent of bp-bp repulsion in NH3 is more than that in NF3. As a consequence, the bond angle in NH3(107.2°) is greater than that of NF3(102.3°).
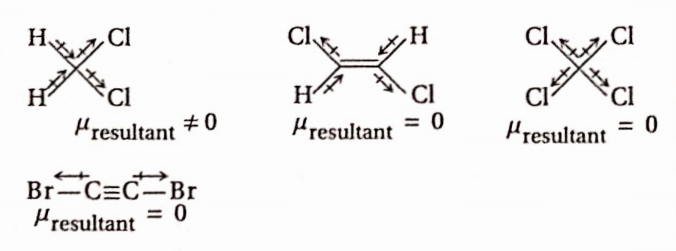
Question 18. The compound that will have a permanent dipole moment among the following is
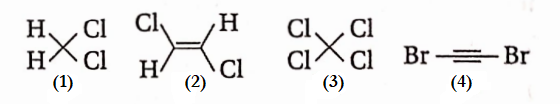
- 1
- 2
- 3
- 4
Answer: 1. Permanent dipole moment means a non-zero value of dipole moment. So, only compound (1) has a permanent dipole moment.
Question 19. Among the following structures, the one which is not a resonating structure of others is—
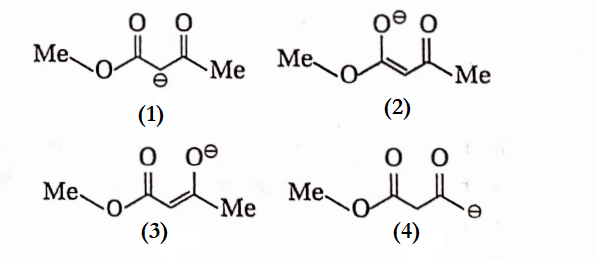
- 1
- 2
- 3
- 4
Answer:
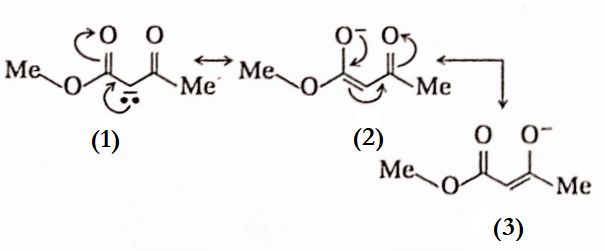
Structure (4) is not a resonance structure because it involves shifting a pair of electrons as well as an H-atom
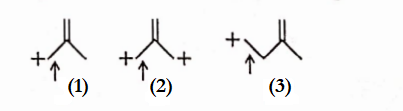
Question 20. The correct order of decreasing length ofthe bond as indicated by the arrow in the following structures is—
- 1>2>3
- 2>13
- 3>2>1
- 1>3>2
Answer: 3. In general, C=C and C—C bond lengths are respectively 1.33A and 1.54A
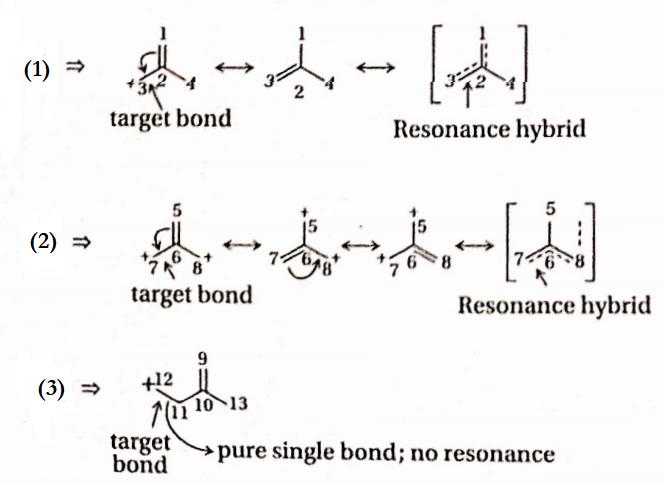
In structure I, n -electrons are delocalized over Ci — C2 and C2— C3 bonds. In structure II a pair of n electrons are delocalised Over C5-C6, C6-C7C6-C8
Question 21. The correct order of decreasing H—C—H angle in the following molecules is
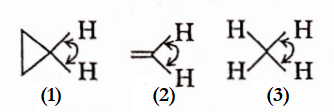
- 1>2>3
- 2>1>3
- 3>2>1
- 1>3>2
Answer: Overlapping is maximum when orbitals overlap “endon” i.e., via a -bonding, n -bonds overlap laterally. The overlap in cyclopropane is neither end-on nor lateral but in between. So, it is intermediate between cr -and n bonding.
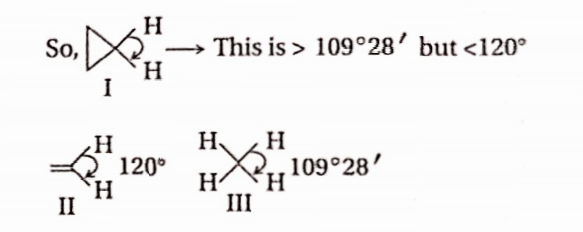
So, in order of decreasing H —C —H angle: 2 >1 > 3
Question 22. The correct order of decreasing length ofthe bond as indicated by the arrow in the following structures is—
- 1>2>3
- 2>1>3
- 3>2>1
- 1>3>2
Answer: 3. 3>2>1
Question 23. Thenumberoflone pairs of electrons on the central atoms of H2O, SnCl2 PCl3, and XeF2 respectively, are
- 2,1,1,3
- 2,2,1,3
- 3,1,1,2
- 2,1,2,3
Answer: 4. Number of lone pairs of electrons present in the hybrid orbital, L = H- X- D [Where H: no. of orbitals involved in the hybridization, X: no. of monovalent atoms surrounding the central atom, D: no. of bivalent atoms attached to the central atom.
Question 24. The number of car and n -bonds present between the two carbon atoms of calcium carbide are respectively
- 1σ, 1π- bond
- 1σ, 2π- bond
- 2σ, 1π- bond
- \(1 \sigma, 1 \frac{1}{2} \pi \text {-bond }\)
Answer: 2. 1σ, 2π- bond
In calcium carbide, two carbon atoms are bonded by a triple bond. Thus between two carbon atoms, 1 cr, and 2/r bonds are present.
Question 25. Which of the following molecules have a shape like CO2
- HgCl2
- SnCl2
- C2H2
- NO2
Answer: 1. HgCl2
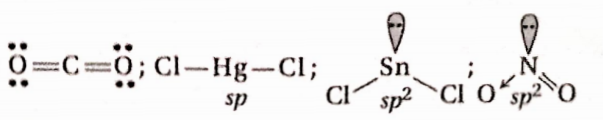
Question 26. The ground state magnetic property of B2 and C2 molecules will be—
- B, paramagnetic and C2 diamagnetic
- B, diamagnetic C2 paramagnetic
- Botii are diamagnetic
- Both are paramagnetic
Answer: 1. B, paramagnetic and C2 diamagnetic
MO electronic configuration of B2
⇒ \(K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\pi_{2 p_x}\right)^1\left(\pi_{2 p_y}\right)^1\)
Mo electronic configuration of C2
⇒ \(\operatorname{KK}\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\)
In BO, there are unpaired electrons present which is paramagnetic. But in C9 there is no impaired electron and henceitis diamagnetic.
Question 27. The shape of XeFg- is—
- Square pyramidal
- Triangularbipyramidal
- Planar
- Pentagonal bipramidal
Answer: 3. Planar
⇒ \(\text { Here, } \mathrm{H}=\frac{1}{2}(8+5-0+1)=7\)
∴ Central atom Xe: sp3d2 -hybridized
No. of lone pair of electronic Xe, L = (7-5-0) = 2
therefore XeF5 ionic planar.
Question 28. Which statements are correct for the peroxide ion—
- It has five filled anti-bonding molecular orbitals
- It is diamagnetic
- It has bond order one
- It is isoelectronic with neon
Answer: 1. MO electronic configuration of 0|~ (peroxide ion):
\(\left(\sigma_{1 s}\right)^2\left(\sigma_{1 s}^*\right)^2\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\sigma_{2 p_z}\right)^2\) \(\left(\pi_{2 p x}\right)^2\left(\pi_{2 p y}\right)^2\left(\pi_{2 p_x}^*\right)^2\left(\pi_{2 p_y}^*\right)^2\)Bond Order \(=\frac{N_b-N_a}{2}=\frac{10-8}{2}=1\)
Question 29. Which ofthe following has the strongest H-bond
- H-O…shape
- S-H….O
- F-H….F
- F-H….O
Answer: 3. F-H….F
Since fluorine is the most electronegative element F —H bond is more polar which forms the strongest H-bonding [F—H-—F] among the given compounds.
Question 30. B cannot form which ofthe following anions
- \(\mathrm{BF}_6^{3-}\)
- BH-4
- B(OH)-4
- BO-2
Answer: 1. \(\mathrm{BF}_6^{3-}\)
The stability of hydrides of group-15 decreases from NH3 to BiH3 due to an increase in the size of the central atom.
Question 31. Which of the following statements is wrong—
- Nitrogen cannot form an-inbound
- Single n-nbond is weaker than the single p-p bond
- N2O4 has two resonance structures
- The stability of hydrides increases from nh3 to bih3 due to the increase in size ofthe central atom.
Answer: 4. The stability of hydrides increases from NH3 to BiH3 due to the increase in size ofthe central atom.
Question 32. The Square Of IF7 is
- Square pyramid
- Trigonal bipyramid
- Octahedral
- Pentagonal bipyramid
Answer: 4. Pentagonal bipyramid
In IF7, the hybridization of the central atom is sp3d3 and its structure is a pentagonal bipyramid.
Question 33. The hybridization orbitals of N-atoinin NO-3, NO+2, and NH+4 are respectively—
- sp, sp2, sp3
- Sp2,sp,sp3
- Sp,sp3,s2
- sp2, sp3 sp
Answer: 2. Sp2,sp,sp3
Question 34. Among die following, die maximum covalent character is shown by
- FeCl2
- SnCl2
- AlCl3
- MgCl2
Answer: 3. AlCl3
The ionic potential (0) of the cations increases with the increase in cationic charge and decrease in cationic radii. Thus, the resulting compound possesses a more covalent
character. So, A1C13 exhibits maximum covalency.
Question 35. Iron exhibits +2 and +3 oxidation states. Which of the following statements about incorrect—
- Ferrous compounds are relatively more ionic than ferric compounds
- Ferrous compounds are less volatile than the corresponding ferric compounds
- Ferrous compounds are more easily hydrolyzed than the corresponding ferric compounds
- Ferrous oxide is more basic than ferric oxide
Answer: 3. Ferrous compounds are more easily hydrolyzed than the corresponding ferric compounds
The tendency of hydration increases with a decrease in the size of a cation. Ferrous ions are larger than ferric ions. Consequently, the ferric ion will be more easily hydrolyzed than the ferrous ion.
Question 36. Ortho-nitrophenol is less soluble in water than p – and m nitrophenols because—
- O-nitrophenol shows intramolecular-bonding
- O-nitrophenol shows intermolecular-bonding
- The melting point of o-nitrophenol is less than that of mand p-isomers
- O-nitrophenol is more volatile than those of m-and prisoners
Answer: 1. o-nitrophenol shows intramolecular H-bonding
In o-nitrophenol, —OH & —N02 groups are situated at two adjacent carbon atoms of the ring and involved in intramolecular bonding. So, o-nitrophenol is less soluble in water than p- and m- m-nitrophenol.
Question 37. The molecule having the smallest bond angle is
- AsCl3
- SbCl3
- PCl3
- NCL3
Answer: 2. SbCl3
The bond angle of a molecule increases with the increases in electronegativity or with the decrease in size of the central atom. Hence, the correct order of bond angle is: SbCl3 < ASC13 < PC13 < NCl3
Question 38. In which of the following pairs the two species are not isostructural
- PCI+6 and SiCl4
- PF5 and BrF5
- AlFg- and SF6
- CO6– and NO-3
Answer: 2. PF5 and BrF5
In molecule PF5, \(H=\frac{1}{2}\) [5 + 5- 0 + 0] = 5. Hybridisation of central atom(P): sp3d. Number of p lone pairs in the central atom (P): 0. Shape of the molecule: Trigonal bipyramidal.
For BrF5, Hybridisation of central atom (Br): sp3d2. Number of loner pairs in the central atom (Br): 1. Shape ofthe molecule: square pyramidal.
Question 39. The stability of the species Li2, Li2, and Li2 increases in the order of
- Li2<Li+2<Li-2
- Li22< Li+2 < Li2
- Li2<Li–2<Li+2
- Li2<Li2<Li2
Answer: 2. Li2< Li-2 < Li2
⇒ \(\mathrm{Li}_2:\left(\sigma_{1 s}\right)^2\left(\sigma_{1 s}^*\right)^2\left(\sigma_{2 s}\right)^2\)
⇒ \(\begin{aligned}
& \mathrm{Li}_2^{+}:\left(\sigma_{1 s}\right)^2\left(\sigma_{1 s}^*\right)^2\left(\sigma_{2 s}\right)^1 \\
& \mathrm{Li}_2^{-}:\left(\sigma_{1 s}\right)^2\left(\sigma_{1 s}^*\right)^2\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^1
\end{aligned}\)
\hline \mathbf{B . 0} & \mathbf{L i}_{\mathbf{2}} & \mathbf{L i}_{\mathbf{2}}^{+} & \mathbf{L i}_{\mathbf{2}}^{-} \\
\hline \frac{N_b-N_a}{2} & \frac{2-0}{2}=1 & \frac{1-0}{2}=0.5 & \frac{2-1}{2}=0.5 \\
\hline
\end{array}\)
Question 40. In which ofthe following pairs of molecules/ions, both the species are not likely to exist—
- \(\mathrm{H}_2^{+}, \mathrm{He}_2^{2-}\)
- \(\mathrm{H}_2^{-}, \mathrm{He}_2^{2-}\)
- \(\mathrm{H}_2^{2+}, \mathrm{He}_2\)
- \(\mathrm{H}_2^{-}, \mathrm{He}_2^{2+}\)
Answer: 3. \(\mathrm{H}_2^{2+}, \mathrm{He}_2\)
⇒ \(\mathrm{H}_2^{2+}:\left(\sigma_{1 s}\right)^0\)
⇒ \(\mathrm{He}_2:\left(\sigma_{1 s}\right)^2\left(\sigma_{1 s}^*\right)^2\)
\(\begin{array}{|c|c|c|}\hline \text { B.o. } & \mathbf{H}_2^{2+} & \mathbf{H e}_{\mathbf{2}} \\
\hline \frac{N_b-N_a}{2} & 0 & \frac{2-2}{2}=0 \\
\hline
\end{array}\)
Question 41. Which one of the following molecules is expected to exhibit diamagnetic behavior—
- C2
- N2
- O2
- S2
Answer: 1. C2
Question 42. The correct statement for the molecule, Csl3, is—
- It contains Cs+, I– and lattice I2 molecule
- It is a covalent molecule
- It contains Cs+ and I–2 ions
- It contains Cs3+ and I– ions
Answer: 3. It contains Cs+ and I-3 ions
Cs cannot show a +3 oxidation state. So, Csl3 is formulated as Cs+ and I3 ions. It is a typical ionic compound.
Question 43. For which of the following molecules is significant μ≠ 0-
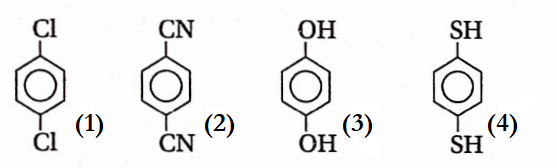
- 3 and 4
- only 1
- 1 and 2
- only 3
Answer: 1. 3 and 4
Question 44. Which of the following alkaline earth metal sulfates has its hydration enthalpy greater than its lattice enthalpy—
- BaSO4
- SRSO4
- CaSO4
- BeSO4
Answer: 1. BaSO4
In the NO4 ion, the N atom is sp -hybridized. O=N=0
Question 45. In which of the following molecule Orion the hybridization state of the-atom is sp —
- NO+2
- NO-2
- NO-3
- NO2
Answer: 4. NO2
There is no unpaired electron in the MO electronic configuration of the CO molecule. Thus, it is not paramagnetic.
Question 46. Which one of the following is not paramagnetic-
- O2
- B2
- NO
- CO
Answer: 4. CO
KC1 is an electrovalent compound, it exists as K+ and Cl- ions
Question 47. The total number of one pair of electrons I-3 ion is
- 9
- 3
- 13
- 6
Answer: 1. 9
Question 48. Which ofthe following compounds contain(s) no covalent bond(s)—KC1, PH3, O2, B2H6, H2SO4
- KCL
- KCl, B2H6
- KClB2H6,PH3
- KCl, H2SO4
Answer: 1. KCL
KC1 is an electrovalent compound, it exists as K+ and Cl- ions
Question 49. According to molecular orbital theory, which of the following will not be a viable molecule—
- H-2
- H2-2
- He2+2
- He+2
Answer: 2. MO electronic configuration of H2-2 \(\left(\sigma_{1 s}\right)^2\left(\sigma_{1 s}\right)^2\)
Therefore Bond Order \(=\frac{2-2}{2}=0\)
Question 50. In which of the following pairs of molecules/ions, do the central atoms have sp2 hybridization—
- NO-2 And NH3
- BF3 And NO-2
- NH-2 And H2O
- BF3 And NH-2
Answer: 2. BF3 And NO-2
\(\begin{array}{|c|c|c|}\hline \begin{array}{c}
\text { Molecule / } \\
\text { ion }
\end{array} & \text { Value of } \mathbf{H} & \begin{array}{c}
\text { Type of } \\
\text { hybridisation }
\end{array} \\
\hline \mathrm{BF}_3 & \mathrm{H}=\frac{1}{2}(3+3-0+0) & s p^2 \\
& =3 & \\
\hline \mathrm{NO}_2^{-} & \mathrm{H}=\frac{1}{2}(5+0-0+1) & s p^2 \\
\hline
\end{array}\)
Question 51. Considering the state of hybridization ofC-atoms, find out the molecule among the following which is linear-
- CH3—CH2—CH2—CH3
- CH3—CH=CH—CH3
- CH3—C=C—CH3
- CH2=CH—CH2—C=CH
Answer: 3. CH3—C=C—CH3
In the case of sp3, sp2, and sp hybridized carbons, the bond angle is 109°28′; 120° and 180° respectively. So, only image- is linear (excluding H-atoms)
Question 52. Which ofthe following structures is the most preferred and
hence of the lowest energy for SO3-
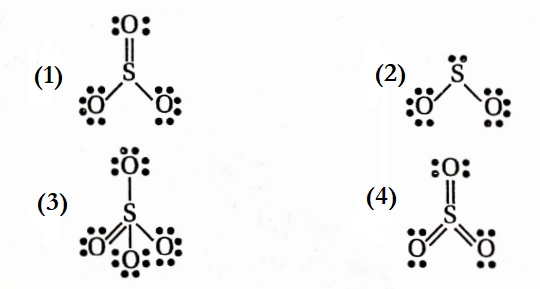
Answer: 4. Has a maximum number of covalent bonds and hence is of the lowest energy.
Question 53. The correct order of increasing bond length of C—H C—O, C—C, and C=C is
- C—H < C=C < C—O < C—C
- C—C < C=C < C—0 < C—H
- C—0<C—H<C—C<C=C
- C—H<C—0<C—C<C=C
Answer: 4. C—H<C—0<C—C<C=C
⇒ \(\begin{aligned}
& \mathrm{C}-\mathrm{H}<\mathrm{C}=\mathrm{C}<\mathrm{C}-\mathrm{O}<\mathrm{C}-\mathrm{C} \\
& 107 \mathrm{pm} \quad 134 \mathrm{pm} \quad 141 \mathrm{pm} \quad 154 \mathrm{pm}
\end{aligned}\)
Question 54. Which of the two ions from the list given below have the geometry that is explained by the same hybridization of orbitals, NO–2, NO–3, NH–2, NH–4, SCN–
- NO–2 and NO–3
- NH+4 and NO–3
- SCN– and NH–2
- NO–2 and NH–2
Answer: 1. Increasing the order of bond length is
⇒ \(\begin{array}{|c|c|c|c|c|c|}
\hline \text { Ions } & \mathrm{NO}_3^{-} & \mathbf{N O}_2^{-} & \mathbf{N H}_2^{-} & \mathbf{N H}_4^{+} & \mathbf{S C N}^{-} \\
\hline \text { Hybridisation } & s p^2 & s p^2 & s p^3 & s p^3 & s p \\
\hline
\end{array}\)
Question 55. Which has the minimum bond length—
- O+2
- O–2
- O22-
- O2
Answer: 1. O+2
⇒ \(\mathrm{O}_2^*: \mathrm{KK}\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\sigma_{2 p_z}\right)^2\left(\pi_{2 p_1}\right)^2\left(\pi_{2 p}\right)^2\left(\pi_{2 p}^*\right)^1\)
⇒ \(\begin{aligned}
& \mathrm{O}_2^{-}: K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\sigma_{2 p_z}\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\left(\pi_{2 p_x}^*\right)^2\left(\pi_{2 p_y}^*\right)^1 \\
& \mathrm{O}_2^{2-}: K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\sigma_{2 p_z}\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\left(\pi_{2 p_x^*}^*\right)^2\left(\pi_{2 p_y}^*\right)^2
\end{aligned}\)
⇒ \(\mathrm{O}_2: K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\sigma_{2 p_z}\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\left(\pi_{2 p_x}^*\right)^1\left(\pi_{2 p_y}^*\right)^1\)
Question 56. Four diatomic species are listed below. Identify the correct order in which the bond order is increasing
- \(\mathrm{NO}<\mathrm{O}_2^{-}<\mathrm{C}_2^{2-}<\mathrm{He}_2^{+}\)
- \(\mathrm{O}_2^{-}<\mathrm{NO}<\mathrm{C}_2^{2-}<\mathrm{He}_2^{+}\)
- \(\mathrm{C}_2^{2-}<\mathrm{He}_2^{+}<\mathrm{O}_2^{-}<\mathrm{NO}\)
- \(\mathrm{He}_2^{+}<\mathrm{O}_2^{-}<\mathrm{NO}<\mathrm{C}_2^{2-}\)
Answer: 4. \(\mathrm{He}_2^{+}<\mathrm{O}_2^{-}<\mathrm{NO}<\mathrm{C}_2^{2-}\)
NO(7=8=15)
⇒ \(K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\sigma_{2 p_z}\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\left(\pi_{2 p_x}^*\right)^1\)
Question 57. During the change of O2 to O2 ion, the electron adds on which one ofthe following orbitals—
- π* -orbitals
- π – orbitals
- σ* orbital
- σ- orbital
Answer: 1. π* -orbitals
⇒ \(\mathrm{O}_2: K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\sigma_{2 p_z}\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\left(\pi_{2 p_x}^*\right)^1\left(\pi_{2 p_y}^*\right)^1\)
⇒ \(\mathrm{O}_2^{-}: K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\sigma_{2 p_z}\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\left(\pi_{2 p_x}^*\right)^2\left(\pi_{2 p y}^*\right)^1\)
Thus, the electron goes into the π*-orbital.
Question 58. The pair of species with the same bond order is
- \(\mathrm{O}_2^{2-}\)
- \(\mathrm{O}_2^{+}, \mathrm{NO}^{+}\)
- NO, CO
- N2, O2
Answer: 1. \(\mathrm{O}_2^{2-}\)
⇒ \(\mathrm{O}_2^{2-}: K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\sigma_{2 p_z}\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\left(\pi_{2 p_x}^*\right)^2\left(\pi_{2 p_y}^*\right)^2\)
⇒ \(\mathrm{B}_2: K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\pi_{2 p_x}\right)^1\left(\pi_{2 p_y}\right)^1\)
\(\begin{array}{|c|c|c|}\hline \text { Bond order } & \mathbf{O}_2^{2-} & \mathbf{B}_2 \\
\hline \frac{N_b-N_a}{2} & \frac{8-6}{2}=1 & \frac{4-2}{2}=1 \\
\hline
\end{array}\)
Note: B.O. of O2 = 2.5, N0+ = 3, NO = 2.5 CO = 3, N2 = 3 and O2 = 2]
Question 59. Which of the following species contains three bond pairs and one lone pair around the central atom—
- H2O
- BF2
- NH-2
- PCl2
Answer: 4. PCl3
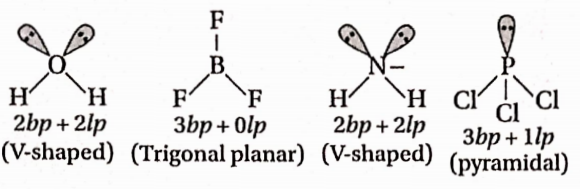
Question 60. Bond order of 1.5 is shown by—
- O+2
- O-2
- O2-2
- O2
Answer: 2. O-2
⇒ \(\mathrm{O}_2^{+}: K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\sigma_{2 p_z}\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\left(\pi_{2 p_x}^*\right)^1\)
⇒ \(\begin{aligned}
& \mathrm{O}_2^{+}: K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\sigma_{2 p_z}\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\left(\pi_{2 p_x}^*\right)^2\left(\pi_{2 p_y}^*\right)^1 \\
& \mathrm{O}_2^{2-}: K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\sigma_{2 p_z}\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\left(\pi_{2 p_x^*}^*\right)^2\left(\pi_{2 p_y}^*\right)^2 \\
& \mathrm{O}_2: K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\sigma_{2 p_z}\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\left(\pi_{2 p_x}^*\right)^1\left(\pi_{2 p_y^*}\right)^1
\end{aligned}\)
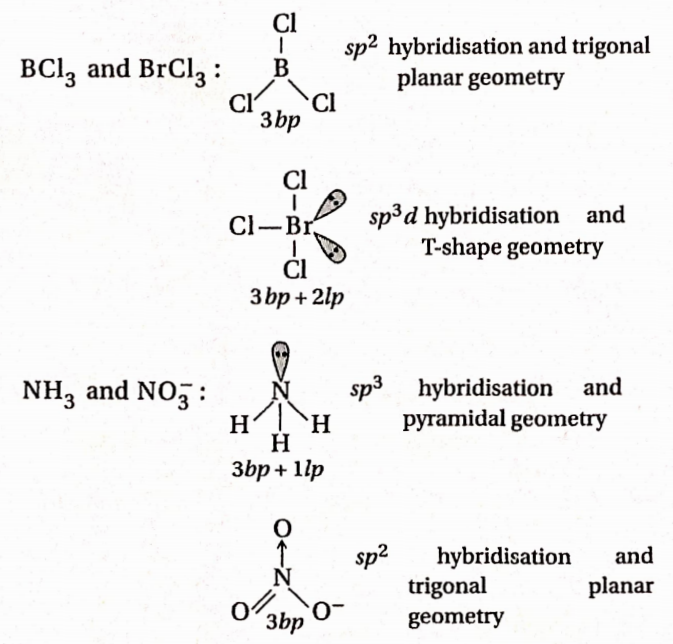
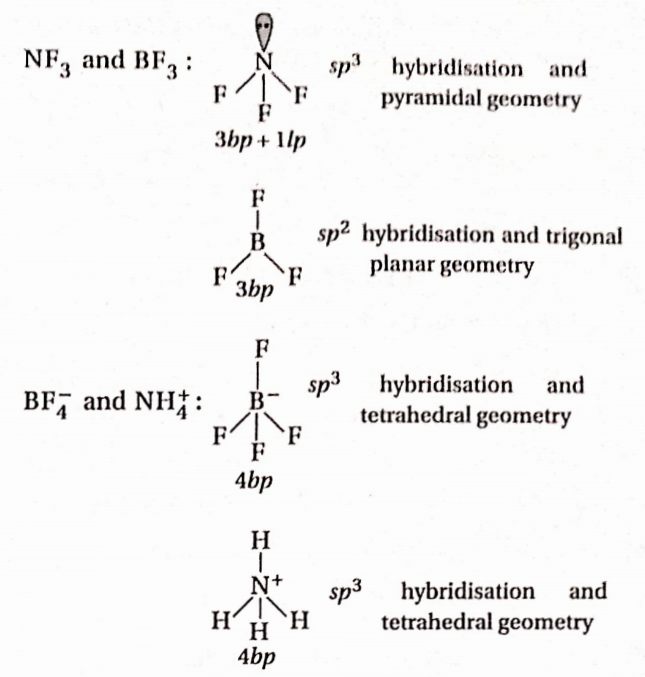
\hline \begin{array}{c}
\text { Bond } \\
\text { order }
\end{array} & \mathbf{O}_2^{+} & \mathbf{O}_2^{-} & \mathbf{0}_2^{2-} & \mathbf{O}_2 \\
\hline \frac{N_b-N_a}{2} & \frac{8-3}{2}=2.5 & \frac{8-5}{2}=1.5 & \frac{8-6}{2}=1 & \frac{8-4}{2}=2 \\
\hline
\end{array}\)
Question 61. The following pairs are isostructural-
- BC13 and BrCl3
- NH3 and NO3-
- NF3 and BF3
- BF4 and NH+4
Answer: 4. BF2 and NH+4
If some bond pairs and lone pairs are the same for the given pairs, they are isostructural.
Question 62. Which contains no n -bond—
- SO2
- NO2
- CO2
- H2O
Answer: 4. H2O
Question 63. Which ofthe following lanthanoid ions is diamagnetic (Atnos. Ce=58, Sm=62,Eu=63, Yb70)-
- EU2+
- Yb2+
- Ce2+
- Sm2+
Answer: 4. Sm2+
⇒ \(\begin{aligned}
& ; \mathrm{Sm}^{2+}(\mathrm{Z}=62):[\mathrm{Xe}] 4 f^6 \\
& \mathrm{Yb}^{2+}(\mathrm{Z}=70):[\mathrm{Xe}] 4 f^{14} \\
& \mathrm{Ce}^{2+}(\mathrm{Z}=58):[\mathrm{Xe}] 4 f^1 5 d^1 \\
& \mathrm{Eu}^{2+}(\mathrm{Z}=63):[\mathrm{Xe}] 4 f^7
\end{aligned}\)
Question 64. Which of the following is paramagnetic—
CN–
NO+
CO
O–2
Answer: 4. O–2
MO electronic configuration of O-2(17):
⇒ \(K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\sigma_{2 p_z}\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi 2_{p_y}\right)^2\left(\pi_{2 p_x}^*\right)^2\left(\pi_{2 p_y}^*\right)^1\)
Owing to the presence of one unpaired electron, it is paramagnetic in nature.
Question 65. Which of the following is a polar molecule—
- SiF4
- XeF4
- BF3
- SF4
Answer: 4. SF4
SF4 has sp3d -hybridization and see-saw shape with 4 bond pairs and 1 lone pair and resultant u=0.
Question 66. XeF2 is isostructural with-
- SbCl3
- BaCL2
- TeF2
- ICI-2
Answer: 4. ICI-2
Question 67. Which species has a plane triangular shape-
- N3
- NO-3
- NO2
- CO2
Answer: 2. NO-3
Question 68. Which has the maximum dipole moment—
- CO2
- CH4
- NH3
- NF3
Answer: 3. NH3
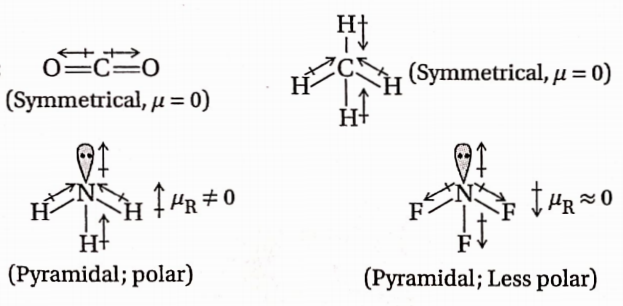
In NH3, H is less electronegative than N and hence dipole moment of each N —H bond is towards N and creates a high net dipole moment.
Question 69. The formation ofthe oxide ion O2-(g), form oxygen atom requires first an exothermic and then an endothermic step as shown below
⇒ \(\begin{aligned}
& \mathrm{O}(\mathrm{g})+e^{-} \rightarrow \mathrm{O}^{-}(\mathrm{g}) ; \Delta H_f^0=-141 \mathrm{~kJ} \cdot \mathrm{mol}^{-1} \\
& \mathrm{O}^{-}(\mathrm{g})+e^{-} \rightarrow \mathrm{O}^{2-}(\mathrm{g}) ; \Delta H_f^0=+780 \mathrm{~kJ} \cdot \mathrm{mol}^{-1}
\end{aligned}\)
Thus, the process of formation of O2- in the gas phase is unfavorable even though O2- is isoelectronic with neon. It is because—
- Electron repulsion outweighs the stability gained by achieving noble gas configuration
- O-ion has a comparatively smaller size than o-atom
- Oxygen is more electronegative
- The addition of electronic o results in large-size ion
Answer: 1. Electron repulsion outweighs the stability gained by achieving noble gas configuration
Electron repulsion predominates over the stability gained by achieving noble gas configuration. Hence, the formation of O2- in the gas phase is unfavorable.
Question 70. In which of the following pairs, both the species are not isostructural—
- SiCl4, Pcl+4
- Diamond, siC
- NH3,PH3
- XeF4, XeO4
Answer: 4. XeF4, XeO4
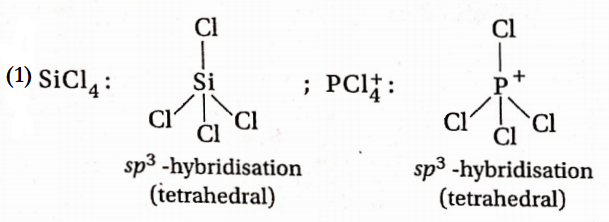
Diamond and silicon carbide (SiC), are both isostructural because their central atom is sp3 hybridized and both have tetrahedral arrangements.
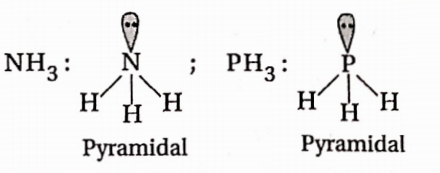
Both NH3 and PH3 have pyramidal geometry.
XeF4 has sp3d2 hybridisation while XeO4 has sp3 hybridisation.

Hence, XeF4 and XeO4 are notisostructural.
Question 71. Decreasing order of stability is-
- \(\mathrm{O}_2^{+}>\mathrm{O}_2>\mathrm{O}_2^{-}>\mathrm{O}_2^{2-}\)
- \(\mathrm{O}_2^{2-}>\mathrm{O}_2^{-}>\mathrm{O}_2>\mathrm{O}_2^{+}\)
- \(\mathrm{O}_2>\mathrm{O}_2^{+}>\mathrm{O}_2^{2-}>\mathrm{O}_2^{-}\)
- \(\mathrm{O}_2^{-}>\mathrm{O}_2^{2-}>\mathrm{O}_2^{+}>\mathrm{O}_2\)
Answer: 1. \(\mathrm{O}_2^{+}>\mathrm{O}_2>\mathrm{O}_2^{-}>\mathrm{O}_2^{2-}\)
Order of stability ∞ bond order.
therefore The order of stability ofthe given species,
⇒ \(\mathrm{O}_2^{+}>\mathrm{O}_2>\mathrm{O}_2^{-}>\mathrm{O}_2^{2-}\)
Bond order: 2.5 2 1.5 1
Question 72. Which one of the following compounds §hows the presence of intramolecular hydrogen bond-
- HCN
- Cellulose
- Conc.Acetic Acid
- H2O2
Answer: 2. Cellulose
Only cellulose can perform intramolecular bonding whereas the other compounds can perform intermolecular-bonding only.
Question 73. Which of the following pairs of ions are isoelectronic and isostructural-
- \(\mathrm{ClO}_3^{-}, \mathrm{SO}_3^{2-}\)
- \(\mathrm{CO}_3^{2-}, \mathrm{NO}_3^{-}\)
- \(\mathrm{ClO}_3^{-}, \mathrm{CO}_3^{2-}\)
- \(\mathrm{SO}_3^{2-}, \mathrm{CO}_3^{2-}\)
Answer: 2. \(\mathrm{CO}_3^{2-}, \mathrm{NO}_3^{-}\)
Both CO2–3 and NO3 have a total of 32 electrons and both are triangular planar shape
Question 74. Among the following, which one is a wrong statement—
- Pn-dn bonds are present in SO2
- SeF4 and CH4 have the same shape
- 1+3 has bent geometry
- PH3 and BiCl5 do not exist
Answer: 2. SeF4 and CH4 have same shape
The shape of CH4(sp3) is regular tetrahedron. However, in SeF4, the Se atom is sp3d-hybridized -hybridised and the presence of two lone pairs makes the molecule see-saw shaped.
Question 75. The hybridizations of atomic orbitals of nitrogen in NO+2 NO-3 and NH+4 respectively are-
- Sp2, sp3 and sp
- sp, sp2 and sp3
- sp2, sp and sp3
- sp, sp3 and sp2
Answer: 2. sp, sp2 and sp3
⇒ \(\mathrm{NO}_2^{\oplus}(s p) \text {-linear; } \mathrm{NO}_3^{-}\left(s p^2\right) \rightarrow \text { trigonal planar }\mathrm{NH}_4^{\oplus}\left(s p^3\right) \text {-tetrahedral }\)
Question 76. Consider the molecules CH, NH3, and H20. Which of the given statements is false—
- The h—c—h bond angle in ch4, the h—n—h bond angle in nh3 & the h—o —h bond angle in h,0 are all greater than 90°
- The h—o —h bond angle in h20 is larger than the h—c—h bond angle in ch4
- The h—o —h bond anglein h20 is smaller than the h —n—h bond anglein nh3
- The h—c—h bond angle in ch4 is larger than the h—n—h bond anglein nh
Answer: 2. The h—o —h bond angle in h20 is larger than the h—c—h bond angle in ch4
Question 77. Predict the correct order among the following—
- Lone pair-lone pair > lone pair-bond pair > bond pair-bond pair
- Lone pair-lone pair > bond pair-bond pair > lone pairlonepair
- Bond pair-bond pair > lone pair-bond pair > lone pairlonepair
- Lone pair-bond pair > bond pair-bond pair > lone pairlonepair
Answer: 1. Lone pair-lone pair > lone pair-bond pair > bond pair-bond pair
According to VSEPR theory, Ip — Ip repulsion > Ip- bp repulsion > bp-bp repulsion.
Question 78. The charge-forming electron pair in the carbonation CH3C=C exists in
- sp -orbital
- 2p-orbital
- sp3 -orbital
- sp2 -orbital
Answer: 1. sp -orbital
CH3CHC-, triply bonded carbon atoms are sp hybridized. Thus forming an electron pair is in sp orbital
Question 79. Match the compound given in column 1 with the hybridization and shape given in column 2 and mark the correct option:
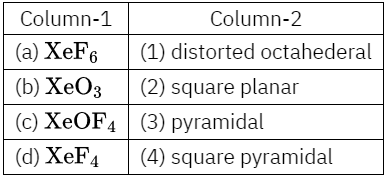
Code: 1 2 3 4
- 4-1- 2- 3
- 1- 3- 4- 2
- 1- 2- 4-3
- 4- 3- 1 – 2
Answer: 2. 1- 3- 4- 2
Question 80. Which of the following pairs of species have the bond order
- O2, NO+
- CN–, CO
- N2,O2–
- CO, NO
Answer: 2. Total no. of electrons present in CN = 14
- Total no. electrons present in CO = 14
- MO electronic configuration of CO
⇒ \(\left(\sigma_{1 s}\right)^2\left(\sigma_{1 s}^*\right)^2\left(\sigma_{2 s}\right)^2\left(\sigma_{2 p_z}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\)
MO electronic Configuration of CN-
⇒ \(\left(\sigma_{1 s}\right)^2\left(\sigma_{1 s}^*\right)^2\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\left(\sigma_{2 p_z}\right)^2\)
⇒ \(\text { Bond order of } \mathrm{CO}=\frac{1}{2}(8-2)=3\)
⇒ \(\text { Bond order of } \mathrm{CN}^{-}=\frac{1}{2}(8-2)=3\)
Question 81. The species, having angles of 120° is—
- ClF3
- NCL3
- BCl3
- PH3
Answer: 3. BCl3
Question 82. Match the interhalogen compounds of column I with the geometry in column 2 and assign the correct code:
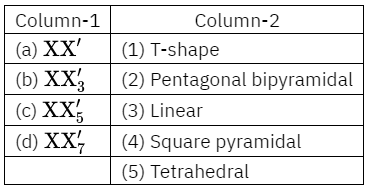
Code: (1) (2) (3) (4)
- 3-1- 4- 2
- 5- 4- 3- 2
- 4- 3 – 2- 1
- 3- 4 -1 -2
Answer: 1. 3-1- 4- 2
- XX ⇒ linear (sp3d)
- XX3 ⇒ T-shape (sp3d)
- XXg ⇒ square pyramidal (sp3d2)
- XXy ⇒ pentagonal bipyramidal (sp3d3)
Question 83. Consider the following species: CN+, CN-, NO, and CN Which one of these will have the highest bond order—
- CN
- NO
- CN+
- CN–
Answer: 4. CN–
- \(\text { B.O. of } \mathrm{NO}=\frac{10-5}{2}=2.5\)
- \(\text { B.O. of } \mathrm{CN}^{-}=\frac{10-4}{2}=3 \text {; }\)
- \(\text { B.O. of } \mathrm{CN}=\frac{9-4}{2}=2.5 \text {; }\)
- \(\text { B.O. of } \mathrm{CN}^{+}=\frac{8-4}{2}=2\)
Question 84. In the structure of C1F3, the number of lone pairs of electrons on central atom ‘Cl’ is-
- Three
- One
- Four
- Two
Answer: 4. Two
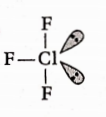
Question 85. The decreasing order of bond angle is—
- BeCl2 > NO2 > SO2
- BeCl2 > SO2 > NO2
- SO2 > BeCl2 > NO2
- SO2>NO2>BeCl2
Answer: 1. BeCl2 > NO2 > SO2
Compound \(\begin{aligned}
& \mathrm{BeCl}_2>\mathrm{NO}_2>\mathrm{SO}_2 \\
& 180^{\circ} \quad 132^{\circ} \quad 119.5^{\circ} \\
&
\end{aligned}\)
Question 86. The dipole moment is minium in—
- NH3
- NF3
- SO2
- BF3
Answer: 4. BF3
BF3 has zero dipole moment.
Question 87. In BF3, the B —F bond length is 1.30 A when BF3 is allowed to be treated with mMe3 N, it forms an adduct, Me3 N→BF3, and the bond length of —F in the adduct is-
- Greater than 1.30A
- Smaller than 1.30A
- Equal to 1.30A
- None of these
Answer: 1. Greater than 1.30A
In BF3, there is backbonding in between fluorine and boron due to the presence of -orbital in boron.
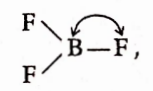 back bonding imparts double-bond characteristics
back bonding imparts double-bond characteristics
As BF3 forms adduct, the back bonding Is no longer present and thus double bond characteristic disappears. Hence, the bond becomes a bit longer than earlier (1.30A).
Question 88. The total number of antibonding electrons present in O2 will be
- 6
- 8
- 4
- 2
Answer: 1. MO electronic configuration of O2
\(\begin{aligned}& \left(\sigma_{1 s}\right)^2\left(\sigma_{1 s}^*\right)^2\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\sigma_{2 p_z}\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2 \\
& \left(\pi_{2 p_x}^*\right)^1\left(\pi_{2 p_y}^*\right)^1
\end{aligned}\)
Hence, the correct B.O. is O+2 → O-2 → 2-2
Question 89. Which ofthe following represents the correct bond order
- \(\mathrm{O}_2^{+}<\mathrm{O}_2^{-}>\mathrm{O}_2^{2-}\)
- \(\mathrm{O}_2^{+}<\mathrm{O}_2^{-}>\mathrm{O}_2^{2-}\)
- \(\mathrm{O}_2^{2-}>\mathrm{O}_2^{+}>\mathrm{O}_2^{-}\)
- \(\mathrm{O}_2^{+}>\mathrm{O}_2^{-}>\mathrm{O}_2^{2-}\)
Answer: 4. \(\mathrm{O}_2^{+}>\mathrm{O}_2^{-}>\mathrm{O}_2^{2-}\)
Question 90. In the O3 molecule, the formal charge on the central O-atom is—
- 0
- -1
- -2
- +1
Answer: 4. +1
Lewis gave the structure of the O3 molecule as
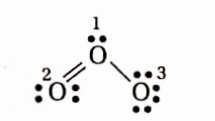
Using the relation, Formal charge = [Total no, of valence electrons in the free atom] – [Total no. of non-bonding (lone pair) electrons]-\(\frac{1}{2}\)[Total no. of bonding (shared) electrons]
The formal charge on central O -atom i.e., no. 1 = +1
Question 91. Four diatomic species are listed below in different sequences. Which of these represents the correct order of their increasing order
- \(\mathrm{C}_2^{2-}<\mathrm{He}_2^{+}<\mathrm{NO}<\mathrm{O}_2^{-}\)
- \(\mathrm{He}_2^{+}<\mathrm{O}_2^{-}<\mathrm{NO}<\mathrm{C}_2^{2-}\)
- \(\mathrm{O}_2^{-}<\mathrm{NO}<\mathrm{C}_2^{2-}<\mathrm{He}_2^{+}\)
- \(\mathrm{NO}<\mathrm{C}_2^{2-}<\mathrm{O}_2^{-}<\mathrm{He}_2^{+}\)
Answer: 2. \(\mathrm{He}_2^{+}<\mathrm{O}_2^{-}<\mathrm{NO}<\mathrm{C}_2^{2-}\)
According to molecular orbital theory, the energy level ofthe given molecules are
⇒ \(\mathrm{C}_2^{2-}-K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\left(\sigma_{2 p_z}\right)^2\)
⇒ \(\begin{aligned}
& \text { B.O. }=\frac{1}{2}[10-4]=3 \\
& \mathrm{He}_2^{+}-\left(\sigma_{1 s}\right)^2\left(\sigma_{1 s}^*\right)^1
\end{aligned}\)
⇒ \(\begin{aligned}
& \text { B.O. }=\frac{1}{2}[2-1]=\frac{1}{2}=0.5 \\
& \mathrm{O}_2^{-}-K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\sigma_{2 p_z}\right)^2 \\
& \quad\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\left(\pi_{2 p_x}^*\right)^2\left(\pi_{2 p_y}^*\right)^1
\end{aligned}\)
⇒ \(\begin{aligned}
& \text { B.O. }=\frac{1}{2}[10-7]=1.5 \\
& \text { NO : }-K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\sigma_{2 p_z}\right)^2 \\
& \qquad\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\left(\pi_{2 p_x}^*\right)^1\left(\pi_{2 p_y}^*\right)^0
\end{aligned}\)
⇒ \(\text { B.O. }=\frac{1}{2}[8-3]=2.5\)
So, the correct order of their increasing bond order is He+2 < O-2 < NO < C²-2
Question 92. Which of the following molecules has more than one lone pair
- SO2
- XeF2
- SiF4
- CH4
Answer: 2. XeF2
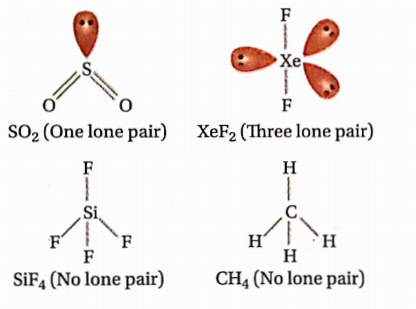
Question 93. The ASF5 molecule is trigonal bipyramidal. The hybrid orbitals used by the As atoms for bonding are—
- \(d_{x^2-y^2}, d_{z^2}, s, p_x, p_y\)
- \(d_{x y}, s, p_x, p_y, p_z\)
- \(d_{x^2-y^2}, s, p_x, p_y\)
- \(s, p_x, p_y, p_z, d_z^2\)
Answer: 4. \(s, p_x, p_y, p_z, d_z^2\)
AsF5 has sp3d hybridisation. In sp2d hybridization, the dz2 orbital is used along with the ‘s’ and three ‘p’ orbitals to form three equatorial bonds and two equally strong axial bonds for a trigonal bipyramid
Question 94. H2O is polar, whereas BeF2 is not because—
- Electronegativity ofF is greater than that of O
- H2O involves H-bonding, whereas, BeF2 is a discrete molecule
- H2O is angular and BeF2 is linear
- H2O is linear and BeF2 is angular
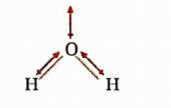
Answer: 3. H2O is angular and BeF2 is linear
Because of the linear shape, dipole moments cancel each other in BeF2 (F—Be—F) and thus, it is non-polar, whereas H2O is V-shaped and hence, it is polar
Question 95. Which of the following have the same hybridization but are not isostructural
- C1F3 and l-3
- BrF3 and NH3
- CH4 and NH+4
- XeO3 and NH3
Answer: 1. C1F3 and l-3
- C1F3 (sp3d, T-shape); I3 (sp3d, linear)
- BrF3 (sp3d, T-shape); NH3 (sp3, pyramidal)
- CH4 (sp3, Tetrahedral); NH3 (sp3, Tetrahedral)
- XeO3 (sp3, Pyramidal); NH3 (sp3, Pyramidal)
Question 96. Which of the following pairs have different hybridization and the same shape—
- \(\mathrm{NO}_3^{-} \text {and } \mathrm{CO}_3^{2-}\)
- SO2 and NH2-
- XeF2 and CO2
- H2O and NH3
Choose The Correct Option
- 1 and 4
- 2 and 4
- 2 and 3
- None of these
Answer: 3. 2 and 3
- NO-3 (sp2, trigonal planar);
- CO2-3 (sp2, trigonal planar);
- Same hybridization and the same shape.
- SO2 (sp2, bent); NH2 (sp2, bent)
Different hybridization but the same shapes.
- XeF2 (sp2d, linear); CO2 (sp, linear)
- Different hybridization but the same shapes.
- H2O (sp3angular); NH3 (sp3, pyramidal)
Question 97. Which of the following is correct regarding bond angles—
- SO2
- H2S<SO2
- SO2<H2S
- SBH3<NO+2
Choose the correct one
- 1 and 4
- 2, 1 and 4
- 1 and 3
- None of these
Answer: 1. 1 and 4
⇒ \(\begin{array}{ccccr}
\mathrm{SbH}_3 & \mathrm{H}_2 \mathrm{O} & \mathrm{H}_2 \mathrm{~S} & \mathrm{SO}_2 & \mathrm{NO}_2^{+} \\
91.3^{\circ} & 104.5^{\circ} & 109.5^{\circ} & 120^{\circ} & 180^{\circ}
\end{array}\)
Question 98. Which pair of molecules does not have an identical structure-
- I-3,BeF2
- O3,SO2
- BF2,ICI3
- BrF-4,XeF4
Answer: 3. BF3, ICI3
- BF3 —Trigonal planar
- IC13 —T-shape
Question 99. Which ofthe following order is correct—
- A1C13 < MgCl2 < NaCl: polarising power
- CO > CO2 > HCO-2 > CO2-3: bond length
- BeCl2 < NF3 < NH3 :dipole moment
- H2S > NH3 > SiH4 > BF3: bond angle
Answer: 3. A1C13 < MgCl2 < NaCl: polarising power
The polarising power of cations increases with the increasing charge.
⇒ \(\stackrel{(+1)}{\mathrm{NaCl}}<\stackrel{(+2)}{\mathrm{MgCl}_2}<\stackrel{(+3)}{\mathrm{AlCl}_2}\)
⇒ \(\text { Bond order } \propto \frac{1}{\text { Bond length }}\)
\(\text { Bond order }=\frac{\text { Bond order of each } \mathrm{C}-\mathrm{O} \text { bond }}{\text { Total no. of resonating structures }}\)⇒ \(\begin{aligned}
& \mathrm{CO} \rightarrow \mathrm{C} \equiv \mathrm{O} \Rightarrow \frac{2+1}{1}=3.0 \\
& \mathrm{CO}_2 \rightarrow \mathrm{O}=\mathrm{C}=\mathrm{O} \Rightarrow \frac{2+2}{2}=2.0
\end{aligned}\)
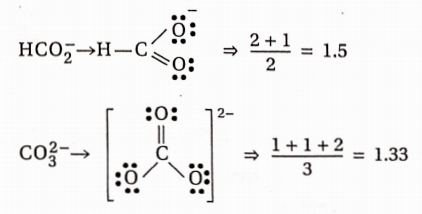
Hence, the decreasing order of bond length is, CO2-3 > HCO2> CO2 > CO
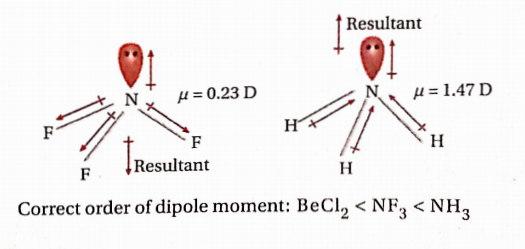
The correct order of bond angle:
BF3 > SiH4 > NH3 > H2S
120° 109°28/ 107° 94°
Question 100. Which of the following has the maximum % of s- s-character
- N2H2
- N2H4
- NH3
- NH-2
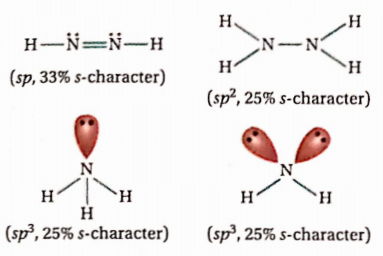
Answer: 1. N2H2
Question 101. Which of the following pairs does not have the same bond order-
- N2 and Cn-
- o+2 and no
- F-2 and O+2
- B2–2 and CN+
Answer: 1. N2 and Cn-
M.O. electronic configuration of N2(14):
⇒ \(\left(\sigma_{1 s}\right)^2\left(\sigma_{1 s}^*\right)^2\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\left(\pi_{2 p_z}\right)^2\)
⇒ \(\text { B.O. }=\frac{1}{2}\left(\mathrm{~N}_b-\mathrm{N}_a\right)=\frac{1}{2}(10-4)=3.0\)
M.O. electronic configuration of CN-(14):
⇒ \(\begin{aligned}
& \quad\left(\sigma_{1 s}\right)^2\left(\sigma_{1 s}^*\right)^2\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_z}\right)^2 \\
& \text { B.O. }=\frac{1}{2}(10-4)=3.0
\end{aligned}\)
M.O. electronic configuration of O2(15):
⇒ \(K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\sigma_{2 p_z}\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\left(\pi_{2 p_x}^*\right)^1\)
⇒ \(\text { B.O. }=\frac{1}{2}(10-5)=2.5\) \text { B.O. }=\frac{1}{2}(10-5)=2.5
M.O. electronic configuration of NO(15):
⇒ \(K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\sigma_{2 p_z}\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\left(\pi_{2 p_x}^*\right)^1\)
⇒ \(\text { B.O. }=\frac{1}{2}(10-5)=2.5\)
M.O. electronic configuration of F-2( 19):
⇒ \(\begin{aligned}
& K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\sigma_{2 p_z}\right)^2\left(\pi_{2 p_x}\right)^2 \\
& \left(\pi_{2 p_y}\right)^2\left(\pi_{2 p_x}^*\right)^2\left(\pi_{2 p_y}^*\right)^2\left(\sigma_{2 p_z}\right)^1 \\
&
\end{aligned}\)
⇒ \(\text { B.O. }=\frac{1}{2}(10-8)=1.0\)
M.O. electronic configuration of O²2-(18):
⇒ \(\begin{aligned}
& K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\sigma_{2 p_z}\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\left(\pi_{2 p_x}^*\right)^2\left(\pi_{2 p_y}^*\right)^2 \\
& \text { B.O. }=\frac{1}{2}(10-8)=1.0
\end{aligned}\)
M.O. electronic configuration of B²-2(12):
⇒ \(K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\)
⇒ \(\text { B.O. }=\frac{1}{2}(8-4)=2.0\)
M.O. electronic configuration of CN+(12):
⇒ \(K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2 ; \text { B.O. }=\frac{1}{2}(8-4)=2.0\).

