Chemical Bonding And Molecular Structure Short Answer Type Questions
Question 1. Name the energy that is released during the formation of an ionic crystal.
Answer: Lattice energy
Question 2. Out of NaCI and MgO, which one has higher lattice energy?
Answer: MgO (each ion carries two unit charge).
Question 3. Elements belonging to which groups of the periodic table combine to form electrovalent compounds?
Answer: Highly electropositive metals of groups- 1 and 2 combine with the electronegative elements of groups- 15, and 16 and 7 to form electrovalent compounds.
Question 4. A+ and B2+ ions are isoelectronic, then which of the following information regarding their size is correct:
- A+ > B2+
- A+ < B2+
- A+ = B2+
Answer: A+>B2+
Question 5. Arrange the following isoelectronic ions in increasing order of their ionic radii: X+, Y2+, A, and B2-.
Answer: Y2+ < X- < A- < B2-
Question 6. Designate the following changes as exothermic or endothermic:
- A(g) A+(g) + e–
- B(g) + e→ B—(g)
- X(s) → X(g)
- A+(g) + B–(g)→ AB
Answer: Endothermic; and exothermic.
Question 7. What is the coordination number of A+ and B– ions if the geometry of the ionic crystal (AB) is cubic?
Answer: The coordination number of A+ and B– ions will be 8
Question 8. Out of NaF, KCl, and MgO, which of the compounds exhibit isomorphism?
Answer: NaF and MgO are the two isomorphous compounds [Na– (2,8), F– (2,8); Mg2+ (2,8), O3 (2,8)]
Question 9. What is the geometry of the ionic crystal if the value of r+/r(radius ratio of the monovalent cation and anion) is in the range 0.225-0.414?
Answer: Tetrahedral
Question 10. Out of Sn2+ and Sn4+, which one is more stable?
Answer: Sn2+ ion is more stable due to the inert pair effect.
Question 11. The values dielectric constants of the solvents, A and B are x and y respectively. If \(\frac{1}{x}<\frac{1}{y}\), t lies in which solvent an ionic compound is more soluble?
Answer: if \(\frac{1}{x}<\frac{1}{y}\). Consequently, an ionic compound is more soluble in solvent A possessing a higher value of the dielectric constant.
Question 12. For the salt CaF2, ΔH°lattice > ΔH°hyd. Predict whether this salt is soluble in water or not.
Answer: CaF2 is insoluble in water.
Question 13. Which out of NaCl and CHC13 reacts with AgNO3 solution to give a precipitate of AgCl?
Answer: NaCl (being an electrovalent compound, it gives Cl–).
Question 14. Which of the following are hypervalent compounds? CO2,CIF3, SO2, IF5.
Answer: IF5 and ClF3 (the central atom has more than eight electrons in its valence shells)
Question 15. How many types of bonds are present in LiAlH4?
Answer: 3 types—electrovalent, covalent, and coordinate covalent bonds.
Question 16. Give examples of an anion and a cation which are isostructural with BF3 and CH4 respectively.
Answer: NO-3 (trigonal planar) and NH+4 (tetrahedral).
Question 17. Arrange in order of decreasing size: sp, sp2, sp3
Answer: sp3 > sp2 > sp.
Question 18. Give the hybridization of P in PCl5. Why are axial bonds longer as compared to equatorial bonds?
Answer: sp3d. This is due to greater repulsion on the axial bond pairs by the equatorial bond pairs.
Question 19. Mention the change in hybridization (if any) of the Al -atom in the reaction: AlCl3 + Cl– -> AlCl4.
Answer: In AICI3, Al-atom is sp3 – hybridised, but in AlCl3, Al- atom is sp3,-hybridized.
Question 20. Is there any change in his hybridization of II and N atoms as a result of the following reaction?
\(\mathrm{BF}_3+\mathrm{NH}_3 \rightarrow \mathrm{F}_3 \mathrm{~B} \cdot \mathrm{NH}_3\)
Answer: In the BF3 molecule, the B -atom is sp2 -hybridized, and In the Nil, molecule, the N -atom Is sp3 -hybridized. In the product molecule, both B and N atoms are sp3-hybridized.
Question 21. Although the O-atoms In water and diethyl ether are sp3 -hybridized, the H —O —H and Et —O —Et bond angles arc different —why?
Answer: Because of steric hindrance between two relatively bulkier C2H5 groups, the Et—O—Et bond angle is greater than the H—O—H bond angle.
Question 22. Draw the resonance structures of N2O obeying the octet rule
Answer: \(: \ddot{\mathrm{N}}=\stackrel{+}{\mathrm{N}}=\ddot{\mathrm{O}}: \longleftrightarrow: \mathrm{N} \equiv \stackrel{+}{\mathrm{N}}-\ddot{\mathrm{O}}:\)
Question 23. Which Of the following hybrid orbitals possess two types of angles?
- sp3,
- sp2,
- sp,
- sp3d,
- sp3d2,
- sp3d3
Answer: sp3d and sp3d3
Question 24. Which of the following arcs isostructural?
\(\text { (2) } \mathrm{SO}_4^{2-}, \text { (2) } \mathrm{NO}_3^{-}, \text {(3) } \mathrm{NH}_4^{+}, \text {(4) } \mathrm{CO}_3^{2-} \text {, (5) } \mathrm{PO}_4^{3-}\)
Answer: BF3, NO3¯, and CO3²¯ are isostructural (trigonal planar and all the central atoms are sp² -hybridized) and SO32-, NH+4, and PO4-3 are isostructural (tetrahedral and all the central atoms are sp³ -hybridized).
Question 25. Give an example of an anion that is Isostructural with BF3.
Answer: [BeF]3
Question 26. Out of SF6 and SCI2, S has higher electronegativity in which of the compounds and why?
Answer: The electronegativity of S in SF6 is higher because SF6 S has a higher oxidation state.
Question 27. Arrange in the order of increasing ionic character C—H, F—H, Br—II, Na—I, K—F, and Li—Cl
Answer: C—H < Br—H < F—H < Li —Cl < Na —I < K—F
Question 28. What is of a molecule, AB2 if it has a definite dipole moment?
Answer: \(\left(_B \backslash {A}\backslash_B\right)\).
Question 29. The dipole moment of HF is 2.0 D. Calculate its value in coulomb-metre (c.m)
Answer: 0.6674 x 10-29 c.m.
Question 30. N2O is polar even though it is linear- why?
Answer: The linear molecule N2O is polar asitisnot symmetrical.
Question 31. Arrange halobenzenes (C6Hg—X, X = F, Cl, Br, I) in the order of their increasing polarity.
Answer: C6H5F < C6H5Cl < C6H5Br < C6H5l.
Question 32. The dipole moment of a molecule, u = e x d. What is the value of d in the case of CCl4?
Answer: The resultant bond moment of the 2C—Cl bond is equal and opposite to that of the remaining 2C—Cl bonds. Hence there is no net dipole moment and so the value of u is zero.
Question 33. Arrange the following in the order of decreasing strengths: N —H–N, O —H—O, F—H—F.
Answer: F —H…F > O —H…O > N —H—N.
Question 34. Arrange the following interactions in the order of their increasing strengths: covalent bond, H- bonding, dipole-dipole interaction, and ran der Waals forces.
Answer: Wander Waals forces < dipole-dipole interaction < hydrogen bonding < covalent bond.
Question 35. Which of the following combinations of orbitals produce n -n-molecular orbitals?
- 2pz– 2pz
- 2ps + 2pz
- 2z + 2pz
- 2py + 2py
Answer: 2px– 2pX;2px + 2py
Question 36. What is the change in bond order, if an electron is added to a bonding MO?
Answer: Bond order increases with the addition of an electron to a bonding molecular orbital.
Question 37. According to MO theory which of the following combinations between the orbitals are not possible?
- 2Pz > 2Pz
- 2s > 2Py
- ls, 2s
- 2Px, 2Px
Answer: 2s > 2Py ;1s,2s
Question 38. Out of O and O2, which one has a greater ionization enthalpy and why?
Answer: O has greater ionization enthalpy. In O2, the first electron has to be removed from the π2px orbital, which has higher energy than the 2p -orbital of the O-atom.
Question 39. Sodium chloride is a solid with having high melting point but carbon tetrachloride is a liquid—why?
Answer: NaCl is an ionic compound. In the crystal of NaCl, the oppositely charged Na+ and Cl– are held together by strong electrostatic forces of attraction. Hence a large amount of energy is required to bring the ions into the liquid state.
Hence, sodium chloride is a solid with having high melting point. Carbon tetrachloride (CCl4), on the other hand, consists of non-polar covalent molecules and the only force that operates among the molecules is the weak van der Waals force.
So, the molecules are weakly held together. Hence, carbon tetrachloride has a very low melting point and it is liquid at ordinary temperature.
Question 40. Sodium chloride is soluble in water but insoluble in benzene or hexane. Explain the observation.
Answer: Sodium chloride is an ionic solid and water is a polar solvent. Water molecules attract Na+ and Cl– ions by their negative and positive poles respectively. As a result, the ions get detached from the crystal lattice and undergo solvation with the evolution of solvation energy. In this case, since the solvation energy is greater than lattice t energy, NaCl dissolves in water.
On the other hand, benzene and hexane are non-polar organic solvents and they cannot solvate Na+ and Cl– ions. Hence, sodium chloride is’ insoluble in benzene or hexane.
Question 41. Nitrogen produces only NCI3 but phosphorus produces both PCl3 and PCl6. Give reasons
Answer: The valence shell electronic configurations of nitrogen and phosphorus are as follows:
Both nitrogen and phosphorus contain 3 unpaired electrons in their valence shells. Using these unpaired electrons, they can form three covalent bonds with chlorine. In this way, NCl3 and PCl3 are produced.
Due to the presence of a vacant 3d -orbital in phosphorus, one 3selectron can be promoted to 3d -orbital and the five unpaired electrons thus obtained can form five covalent bonds with five chlorine atoms to produce PCl5. On the other hand, nitrogen has no d -d-orbital in its second shell. Unlike phosphorus, it cannot extend its covalency to five and hence, it cannot produce NCl5.
Question 42. Aqueous solution of hydrogen chloride is strongly acidic but the solution of hydrogen chloride in benzene is not at all acidic —why?
Answer: Polar H —Cl molecules undergo complete ionization in water and produce H+ ions. Hence, aqueous solution of HCl becomes strongly acidic. On the other hand, HCl molecules do not undergo ionization in non-polar benzene. Because of this, HCl in benzene is not at all acidic.
⇒ \(\mathrm{H}_2 \ddot{\mathrm{O}}: \stackrel{\delta+}{+}+\overbrace{\mathrm{H}}^{\delta-} \mathrm{Cl} \longrightarrow \mathrm{H}_3 \mathrm{O}^{+}(a q)+\mathrm{Cl}^{-}(a q)\)
Question 43. MgO has a higher lattice energy than NaF. Why?
Answer: The lattice energy of an anionic compound increases with an increase in charge of the ions and decreases with the sum of the ionic radii. The charges on the two ions in MgO (Mg2+ and O2 ions) are twice those on the two ions in NaF ( Na+ and F ions). The sum of ionic radii of Mg2+ and O2 — ions (0.64 A+1.40 A = 2.04 A) is less than that of Na+ and F– ions (0.95A+ 1.36A = 2.31A). Therefore, MgO has a higher lattice energy than that of NaF.
Question 44. SnCl4 is a covalent compound whereas SnCl2 is an ionic compound —why
Answer: According to Fajan’s rule, a cation having a small size and high charge exerts a large polarising effect on the neighboring anion resulting in the development of a considerable amount of covalent character in the compound.
Sn4+ ions having a high charge and small size compared to those of Sn2+ ions polarise Cl– ion to a greater extent. Therefore, SnCl4 behaves as a covalent compound whereas SnCl2 is an ionic compound.
Question 45. The ionic radius of Na+ is less than the atomic radius of Na+ but the ionic radius of Cl— is greater than the atomic radius of Cl —why?
Answer: The Na+ ion has 11 protons in its nucleus but it has 10 extranuclear electrons. Since the number of electrons is less than that of protons, these electrons are attracted by the nucleus to a greater extent. Hence, the ionic radius of Na+ is smaller than that of having the same number of protons (11) and electrons (11).
On the other hand, the Cl– ion has 17 protons in its nucleus but has 18 extranuclear electrons. Since the number of protons is less than that of electrons, the electrons are not strongly attracted by the nucleus. To be relieved of the strain of the electron-electron repulsion, the electron cloud gets more diffused. Hence, the ionic radius of Cl– is greater than the atomic radius of Cl.
Question 46. Arrange Al3+, Na+, and Mg2+ ions in the decreasing order of their ionic radii and explain the order
Answer: The decreasing order of ionic radii of these three ions is: \(r_{\mathrm{Na}^{+}}>r_{\mathrm{Mg}^{2+}}>r_{\mathrm{Al}^{3+}}\) These three ions are isoelectronic and they contain 10 electrons each in their extra-nuclear shells. Since the magnitude of nuclear charge gradually increases from Na to Al, the nuclear attractive force for the same number of electrons increases. As a result, their ionic radii gradually decrease.
Question 47. Arrange O2+, N3, and F– ions in the decreasing order of their ionic radii and explain the order.
Answer: The ionic radii of these three ions decrease in the order:
⇒ \(r_{\mathrm{N}^{3-}}>r_{\mathrm{O}^{2-}}>r_{\mathrm{F}^{-}}\) ions 316 isoelectronic and they contain 10 electrons each in their extra-nuclear shells. As the number of protons goes on increasing from N3– to F– ions, a nuclear attractive force for the same number of electrons gradually increases and as a consequence, their ionic radii gradually decrease i.e., their ionic radii follow the above sequence.
Question 48. Explain the following order of thermal stability of the carbonates of the alkaline earth metals: \(\mathrm{BaCO}_3>\mathrm{SrCO}_3>\mathrm{CaCO}_3>\mathrm{MgCO}_3\)
Answer: As the ionic potential of the metal ion increases, its attraction for the electron of the O-atom of CO– ion increases. As a consequence, the tendency of thermal decomposition of the metal carbonate (MCO) to produce metal oxide (MO3) and carbon dioxide (CO2) increases. As the ionic potential increases progressively from Ba2+ to Mg2+, the thermal stability of the carbonates follows the given order.
Question 49. HCl is volatile but NaCl is not. Explain.
Answer: The volatility compound depends on its boiling point which in turn depends on the intermolecular attractive forces. The only attractive force that operates among the polar covalent HCl molecules is dipole-dipole attraction.
Since this attractive force is relatively weak, the boiling point of HO is low and it is volatile (at ordinary temperature, it is a gas). On the other hand, in the electrovalent compound NaCl, the oppositely charged Naÿ and Cl– ions are held together by strong electrostatic forces of attraction in the crystal lattice, and because of this, at ordinary temperature, NaO is a non-volatile solid.
Question 50. What type of bond is formed between two elements, A and B if both of them are highly electronegative or they have huge differences in their electronegativities? Give examples.
Answer: If both the elements A and B are highly electronegative, the bond formed between them would be covalent For example, the bond between N and O in the NCl3 molecule is covalent However, if the elements differ widely in their electronegativities, the bond formed between them would be ionic. For example, in NaCl, the bond between Na and Cl isionic.
Question 51. Out of AlF3 and AICl3, which one is more covalent and why?
Answer: The electron cloud of a large anion is easily distorted by small cations and this results in greater covalency in the compound. The larger Cl– ion is polarised to a greater extent than the smaller F– ion and because of this AICl3 is more covalently natural than AlF3.
Question 52. Indicate the nature of chemical bonding present in each of the following compounds:
- CH3OH,
- NH3
- CaC12
- CaH2
- K2O
- CO2
- Al2O2
- NH4Cl
- HCl
- Mg3N2
- Ci2O
- NaBH4
Answer:
- Covalent,
- Covalent,
- Electrovalent
- Electrovnt lent,
- Electrovalent
- Covalent,
- Electrovalont, electrovalent.
- Covalent and co-ordinate covalent, covalent.
- Electrovalent,
- Covalent,
- Electrovalent
- Colvent and coordinate covalent.
Question 53. Which one between p and sp –orbitals have more directional characteristics and why?
Answer: The directional nature of the sp-orbital is greater than that of the orbital. Because the two lobes of p -p-orbitals are similar in size and possess the same electron density while one of the two lobes of sp -orbitals is larger and has higher electron density
Question 54. In a certainpolar solvent, PCI- undergoes ionization as follows \(2 \mathrm{PCl}_5 \rightleftharpoons \mathrm{PCl}_4^{+}+\mathrm{PCl}_6^{-}\) Predict geometrical shapes of all the species involved.
Answer: The geometrical shapes of the molecules or ions can be predicted from the hybridization state of the central atom.
⇒ \(\text { For } \mathbf{P C l}_5: H=\frac{1}{2}[5+5-0+0]=\frac{10}{2}=5\)
Therefore, the central P-atom is sp3d-hybridized. Consequently, the molecule is a trigonal bipyramidal shape.
⇒ \(\text { For } \mathbf{P C l}_4^{+}: H=\frac{1}{2}[5+4-1+0]=\frac{8}{2}=4 \text {. }\)
Therefore, the central P-atom is sp3 -hybridized. Consequently, the geometrical shape of this ion is tetrahedral.
⇒ \(\text { For } \mathbf{P C l}_6^{-}: H=\frac{1}{2}[5+6-0+1]=\frac{12}{2}=6 \text {. }\)
Therefore, the central P-atom is sp3d2 hybridized. Consequently, the geometrical shape of the ion is octahedral.
Question 55. MgCl2 is linear but SnCl2 is angular—explain
Answer: For \(H=\frac{1}{2}[2+2-0+0]=\frac{4}{2}=2,\) i.e.,, the central Mg -atom is sp -hybridised. Therefore, the molecule is linear. On the other hand, for SnCl2,\(H=\frac{1}{2}[4+2-0+0]=\frac{6}{2}=3,\) i.e. the central Sn-atom is sp2 -hybridised. Therefore, the two bond pairs and one lone pair present in the molecule are directed toward the comers of an equilateral triangle. Hence, the SnCl2 molecule is angular.
Question 56. Arrange the following in the increasing order of their polarities and explain with reason: B — Cl, Ba — Cl, Br — Cl, Cl — Cl
Answer: The increasing order of polarity of these bonds is: Cl — Cl<Cl — Br < Cl — B < Cl — Ba. The greater the difference in electronegativity between the covalently bonded atoms, the greater the polarity of the bond. The electronegativities of B, Ba, Br, and Cl follow the order: of Cl > Br > B > Ba. Consequently, the polarity of the bonds formed by these atoms with Cl-atom progressively increases.
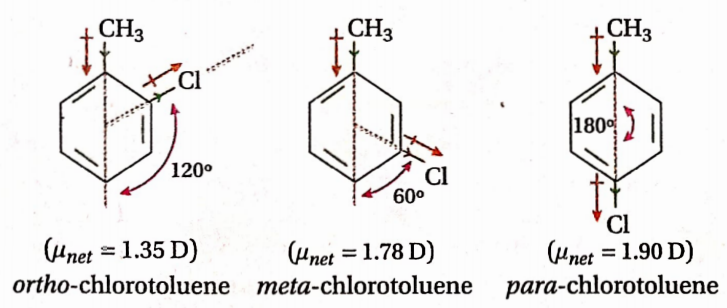
Question 57. Identify the three isomeric chlorotoluenes having dipole moments: 1.35D, 1.9D, and 1.78D.
Answer: Methyl group ( —CH3) can exert a +1 effect while Cl-atom exerts a -I effect. In p-chlorotoluene, -CH3 and -Cl group moments act linearly. So its dipole moment is equal to the sum of the two group moments. Hence, the dipole moment of this compound is maximum. -CH3 group moment is considered to act in the direction of the ring. Soin m-chlorotoluene, these two group moments act at an angle of 60°.
Hence, the dipole moment of m-chlorotoluene is somewhat less than that of its p-isomer. In o-chlorotoluene, these two group moments act at an angle of 120° and hence the dipole moment of this isomer is less than that of the m-isomer. Thus, dipole moments of 1.35D, 1.78D and 1.90D correspond to o-, m- and p-chlorotoluenes.
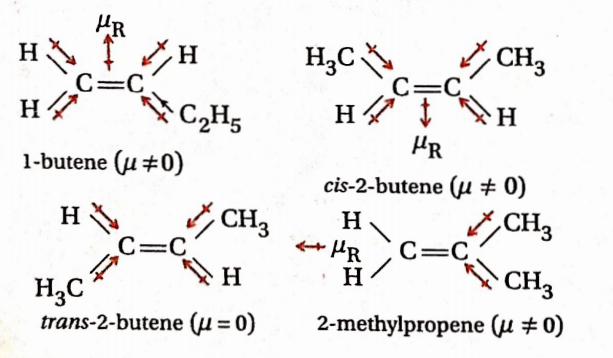
Question 58. Which has the least dipole moment— 1-butene, cis- 2-butene, draws-2-butene and 2-methylpropene?
Answer: The structure of the given compounds is as follows
Question 59. What do you mean by hydrogen bond donor and hydrogen bond acceptor? Define protic and aprotic solvents. Give examples.
Answer: H-bonding represented as X—H—Y denotes the interaction between a donor species and an acceptor species. X—H is considered as a donor and Y is an acceptor of H.
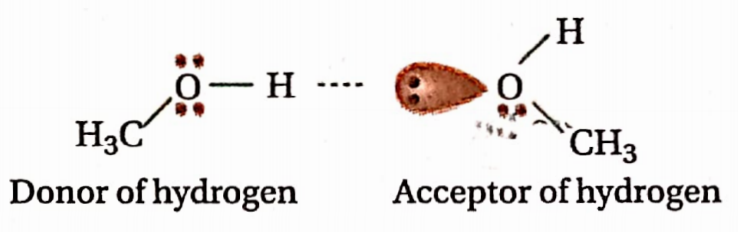
Protic solvent: The solvent whose molecules can act as hydrogen-bond donors are called protic solvents. Water (H2O), ethanol (G2H5OH), acetic acid (CH3COOH), etc., are some common examples ofprotic solvents.
Aprotic solvent: The solvent whose molecules can’t act as a bond donor is known as aprotic solvent. Diethyl ether (C2H5OC2H5), methylene chloride (CH2C12), hexane [CH3(CH2)4CH3], etc., are some examples of aprotic solvents.
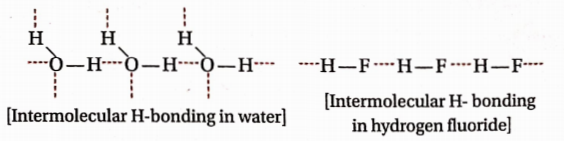
Question 60. The boiling point of water (100°C) is much higher than that of HF(19.5°C), even though they have similar molecular masses. Explain.
Answer: Each water molecule is involved in intermolecular H -bonding with four other water molecules but each HF molecule is involved in intermolecular H -bonding with two other HF molecules.
Therefore, the degree of molecular association in water is much higher than that in HF. Therefore, the boiling point of water is much higher than that of HF.
Question 61. Explain the following observations: Glycerol [HOCH2CH(OH)CH2OH] is a highly viscous liquid. When 30 mL of water is added to 30 mL of ethanol, the volume of the mixture becomes less than 60 ml.
Answer: A glycerol molecule contains three —OH groups. Hence glycerol molecules remain extensively associated through intermolecular H-bonding. This accounts for the high viscosity of glycerol.
The intermolecular H-bonding formed between ethanol and water is stronger than that formed in ethanol itself. When water is added to ethanol, the stronger intermolecular H-bonding between ethanol and water leads to a contraction of volume. So, when 30 mL of water is added to 30 mL of ethanol, the volume of the mixture becomes less than 60 mL.
Question 62. Arrange water, methanol, and dimethyl ether in increasing order of their viscosity and give reasons in favor of that order.
Answer: The greater the extent of intermolecular H-bonding, the greater the intermolecular attraction among different layers, and hence, the greater the viscosity of the compound.
Water molecules having two -OH groups have greater intermolecularH-bonding than methanol (CH3OH) molecules having only one -OH group. Dimethyl ether (CH3OCH3) having no -OH group does not remain associated through H-bonding. Therefore, the viscosity of these liquids follows the order: of dimethyl ether < methanol < water.
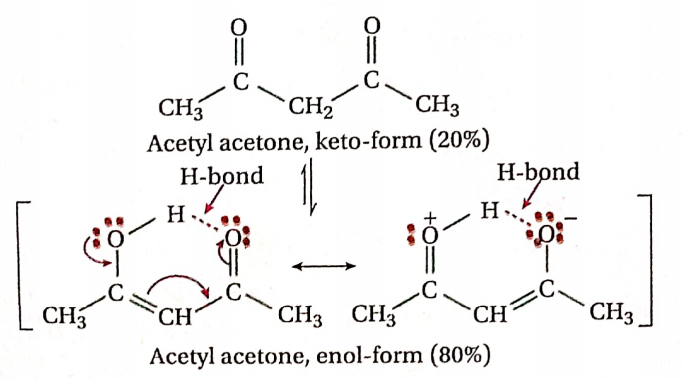
Question 63. At equilibrium, acetylacetone (CH3COCH2COCH3) exists mainly (80%) in enol form— explain.
Answer: The enol-form of acetylacetone is stabilized by resonance. Besides this, its stability is further increased by strong intramolecular bonding. Similar stability in the case of keto form is not possible. Hence, at equilibrium, acetylacetone exists mainly in the enol form.
Question 64. State the useful rule related to the solubility of a compound. Unlike ethane, ethanol dissolves in water. Explain and discuss in terms of energy change.
Answer: The useful rule relevant to the solubility of a compound is “like dissolves like” which means a polar solute dissolves in a polar solvent & a non-polar solute dissolves in a non-polar solvent Ethanol molecules get involved in intermolecular Hbonding with water molecules. So, ethanol readily dissolves in water.
On the other hand, non-polar ethane (CH3CH3) molecules are not capable of forming H -bonds with water molecules so ethane does not dissolve in water. Alternatively, it can be said that H-bonds between different ethanol and different water molecules are replaced by very similar H-bonds (in terms of energy) between water and ethanol molecules.
So, the dissolution of ethanol in water takes place readily. Since non-polar ethane molecules are not solvated by polar water molecules, no solvation energy is liberated. Thus, the energy required to separate the water molecules (associated with strong H -bonding) is not available, and so stronger H -bonds are not replaced by any other intermolecular forces. Hence, ethane does not dissolve in water.
Question 65. Inert gases do not generally participate in chemical reactions—explain with reason.
Answer: Because of the very stable electronic configuration of the outermost shell, inert gases tend to exhibit the least chemical reactivity. The two possible reasons that can explain the stable electronic configuration, as well as the poor reactivity of inert gases, are as follows:
Since there are no odd electrons in the valence shell of inert gases, they have the least tendency to form a covalent bond by forming an electron pair i.e., they produce no covalent compounds.
Since the s-and p -p-orbitals of the valence shell are filled with electrons, the ionization enthalpy of inert gases is very high while their electron gain enthalpy is negligibly small. Hence, they exhibit the least tendency to form ionic compounds.
Question 66. The bond dissociation enthalpy of N2 is higher than that of N2 but the bond dissociation enthalpy of is higher than that of O2. Explain.
Answer:
⇒ \(\begin{aligned}
& \mathrm{N}_2: K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\left(\sigma_{2 p_z}\right)^2 \\
& \mathrm{~N}_2^{+}: K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\left(\sigma_{2 p_z}\right)^1 \\
& \mathrm{O}_2: K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\sigma_{2 p_z}\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\left(\pi_{2 p_x}^*\right)^1\left(\pi_{2 p_y}^*\right)^1 \\
& \mathrm{O}_2^{+}: K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\sigma_{2 p_z}\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\left(\pi_{2 p_x}^*\right)^1
\end{aligned}\)
⇒ \(\begin{array}{|c|c|c|c|c|}
\hline \text { B.O. } & \mathbf{N}_2 & \mathbf{N}_2^{+} & \mathbf{O}_2 & \mathbf{O}_2^{+} \\
\hline \frac{\boldsymbol{N}_b-\boldsymbol{N}_a}{2} & \frac{8-2}{2}=3 & \frac{7-2}{2}=2.5 & \frac{8-4}{2}=2 & \frac{8-3}{2}=2.5 \\
\hline
\end{array}\)
The greater the value of bond order, the higher will be the value of bond dissociation enthalpy. Hence, the bond dissociation enthalpy of N is higher than that of N2 but the die bond dissociation enthalpy of O2 is lower than that of O2.
Question 67. Arrange the following species in order of their increasing bond lengths and explain: C2, C2, and C2-2.
Answer:
⇒ \(\begin{aligned}
& \mathrm{C}_2: K K^*\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2, \\
& \mathrm{C}_2^{-}: K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\left(\sigma_{2 p_z}\right)^1 \\
& \mathrm{C}_2^{2-}: K K\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\left(\sigma_{2 p_z}\right)^2
\end{aligned}\)
⇒ \(\begin{array}{|c|c|c|c|}
\hline \text { B.0. } & \mathrm{C}_2 & \mathrm{C}_2^{-} & \mathrm{C}_2^{2-} \\
\hline \frac{N_b-N_a}{2} & \frac{6-2}{2}=2 & \frac{7-2}{2}=2.5 & \frac{8-2}{2}=3 \\
\hline
\end{array}\)
Since bond length is inversely proportional to bond order, the increasing sequence of bond length of the given species: C2 < C2 < C2
Question 68. Explain the following order of bond dissociation enthalpies: F—F < Cl—Cl < 0=0 < N = N
Answer: Since the size of the F -atom is smaller than that of the Cl -atom, the F—F bond length is less than the Cl—Cl bond length. Therefore, the die F —F bond dissociation enthalpy is expected to be higher than the Cl—Cl bond dissociation enthalpy. But actually, the reverse is true. In the F2 molecule, each F -atom contains three lone pairs of electrons. Because of their proximity, diese unshared electron pairs exert strong repulsive forces towards each other.
Similar forces of repulsion caused by the same number of lone pairs are much less in the case of Cl2 because of the larger size of Cl. So despite the smaller bond length, the die bond dissociation enthalpy of the F —F bond is less than the Cl—Cl bond dissociation enthalpy.
In the O2 molecule, the two 0 -atoms are attached by a double bond. Due to the presence of two unshared pairs of electrons in each 0- atom, the force of repulsion is relatively lower. So, 0=0 bond dissociation enthalpy is higher than the Cl —Cl bond dissociation enthalpy.
In an N2 molecule, the two N -atoms are linked together by a triple bond. Each N -atom contains only one unshared electron pair which causes minimum force of repulsion. Thus, the bond dissociation enthalpy of the N = N bond is the highest. Hence, the bond dissociation enthalpies of the bonds present in die given molecules follow’ the given sequence.
Question 69. Determine the shapes of the given molecules or ions:
\(\text {(1) } \mathrm{POCl}_3\left(2) \mathrm{CH}_3^{-} \text {(3) } \mathrm{CH}_3 \text { (4) } \mathrm{PO}_4^{3-} 6 \text (5) \mathrm{~F}_2 \mathrm{O}\right.\)
Answer: The p-atom in POCl–, molecule is attached to the 0-atom by a double bond and to three Cl -atoms by three single bonds. The number of electrons in the valence shell of P-atom = 5 + 2 + 1 + 1 + 1 = 10. Out of these 10 electrons or 5 electron pairs,1 electron pair is involved in the formation of the bond which has no role in determining the shape of the molecule. According to VSEPR theory, four electron pairs are oriented tetrahedrally, i.e., the shape of the molecule is tetrahedral.
C-atom in CH3 ion is bonded to three H-atoms by three single bonds. The number of electrons in the valence shell of C-atom = 4 + 3 + 1 (for the negative charge) = 8. Out of these 8 electrons or 4 electron pairs, there are three bond pairs and one lone pair. According to VSEPR theory, the ion is pyramidal.
C-atom in the CH3 ion is bonded to three H -atoms by three single bonds. Thus, the number of electrons present in the valence shell of C =4 + 3-1 (for the positive charge) = 6. Because of the presence of 6 electrons or 3 electron pairs, the ion is trigonal planar.
The central P-atom in PO- ion is bonded to three O-atoms by three single bonds and one O-atom by a coordinate covalent bond. Thus, the number of electrons present in the valence shell of P-atom = 5 + 3 + 0=8 or 4 electron pairs (three covalent cr bond pairs and one coordinate cr bond pair). According to VSEPR theory, the four electron pairs are oriented tetrahedrally, i.e., the ion is tetrahedral.
The central O -atom in the F2O molecule is bonded to two F -atoms by two single bonds. The number of electrons in the valence shell of O -atom =6+1 + 1 =8. Out of these 8 electrons or 4 electron pairs, two are bond pairs and two are lone pairs. According to VSEPR theory, the shape of the molecule is angular or V-shaped.
Question 70. The molecule of any compound composed of two dissimilar elements Is always polar—justify the statement.
Answer: The statement is not always true. A diatomic molecule (A-B) consisting of two dissimilar elements of different electronegativities is always polar because there is no possibility of cancellation of the bond moment HCl, HF, etc., are examples of such molecules.
A poly-atomic molecule with two dissimilar elements may or may not be polar. The polarity of such a molecule depend of such a molecule depends on its geometrical shapes If the molecule is linear having a structure like A—B—A (i.e., the individual bond moments cancel out each other), it is polar and if not, it is polar.
The linear CO2 molecule, for example, is nonpolar because the two equal and opposite C2O bond moments cancel out each other. However, the angular H2O molecule is polar because the two O—H bond moments do not cancel out each other and there exists a resultant moment.
Question 71. Explain the following observations:
- H+2ion is more stable than H2 ion even though the bond orders of both ions are the same.
- When a magnet is dipped in a jar containing liquid oxygen, some oxygen molecules cling to it.
- O2 is more paramagnetic than O2.
- The molecular ion, HeH- does not exist
Answer: The antibonding,\(\sigma_{1 s}^*\) molecular orbital of the H2 ion contains one electron but the antibonding crÿs molecular orbital of the H2 ion contains no electron. Therefore, H2 ion is more stable than H2 ion, even though their bond orders are the same.
The electronic configuration oxygen molecule (O2) shows the presence of two unpaired electrons. This suggests that the molecule is paramagnetic. Thus, when a bar magnet is dipped in a jar of liquid oxygen, some molecules cling to
The electronic configurations of O2 and O2 show that the former contains two unpaired electrons (one in each \(\pi_{2 p_x}^*\) and \(\pi_{2 p_y}^*\) while the latter contains one unpaired electron in 7r2p. Therefore, O2 is more paramagnetic than O+2.
Total number of electrons in HeH- = 2 (for He) + 1 (for H ) + 1 (for the negative charge) = 4. Its electronic configuration is \(\left(\sigma_{1 s}\right)^2\left(\sigma_{1 s}^*\right)^2\) while the latter contains one unpaired electron in \(\pi_{2 p_x}^*\) Therefore, 02 is more paramagnetic than O+2.
Total number of electrons in HeH- = 2 (for He) + 1 (for H ) + 1 (for the negative charge) = 4. Its electronic configuration is \(\left(\sigma_{1 s}\right)^2\left(\sigma_{1 s}^*\right)^2\) So, bond
order \(=\frac{2-2}{2}=0\). hence, he- does not exist.
Question 72. Is it correct to say that bond order always increases with the loss of electrons? Explain your answer.
Answer: The statement is not always correct When an electron is expelled from a bonding MO, the bond order decreases, but when an electron is expelled from an antibonding MO, the bond order increases.
For example, loss of an electron from \(\mathrm{H}_2\left(\sigma_{1 s}\right)^2\left(\sigma_{1 s}^*\right)^0 \text { to from } \mathrm{H}_2^{+}\left(\sigma_{1 s}\right)^1\left(\sigma_{1 s}^*\right)^0\) results in decrease of bond order. However, loss of an electron from \(\mathrm{H}_2^{-}\left(\sigma_{1 s}\right)^2\left(\sigma_{1 s}^*\right)^1 \text { to form } \mathrm{H}_2\left(\sigma_{1 s}\right)^2\left(\sigma_{1 s}^*\right)^0\) results in increase of bond order. Bond order of \(H_2^{+}=\frac{1}{2}(1-0)=\frac{1}{2}\mathrm{H}_2=\frac{1}{2}(2-0)=1 ; \mathrm{H}_2^{-}=\frac{1}{2}(2-1)=\frac{1}{2} \text {. }\)
Question 73. Identify polar and non-polar molecules from the following: Cl2, CHCl3, NH3, and BCl3, The dipole moment of the NF-, molecule is less than that of the NH3 molecule. Explain.
Answer: Polar Molecules: CHCH3 NH3,Non-polar Molecules: Cl2, BCI3
Question 74. X is the central atom of the XO2 molecule. If the dipole moment of the molecule is zero, indicate the hybridization state of X.
Answer: \(s p[O=X=O]\)
Question 75. Arrange the following compounds in increasing order of boiling point: HF, H2O, NH3
Answer: \(\mathrm{NH}_3<\mathrm{HF}<\mathrm{H}_2 \mathrm{O}\)
Question 76. Arrange the following molecules in increasing order of the number of lone pairs of electrons. H2O, PCl3, H2O, BF3.
Answer: Considering the lone pair of the central atom: BF3(0/p) < PCl3(l/p) < H2O(2/p)
Considering the lone pairs of all the atoms present in the molecule:
⇒ \(\begin{aligned}
& \mathrm{H}_2 \mathrm{O}<\mathrm{BF}_3<\mathrm{PCl}_3 \\
& (2 l p) \quad(9 l p) \quad(10 l p)
\end{aligned}\)
Question 77. Mention the state of hybridization of the central atom of the following molecules/ions: CO2, PH4, ClO3, CS2 Or, Write the resonating structures of the ClO4 ion
Answer:
⇒ \(\begin{array}{|c|c|c|c|c|}
\hline \text { Molecule/Ion } & \mathrm{CO}_3^{2-} & \mathrm{PH}_4^{+} & \mathrm{ClO}_3^{-} & \mathrm{CS}_2 \\
\hline \begin{array}{c}
\text { Hybridisation of } \\
\text { central atom }
\end{array} & s p^2 & s p^3 & s p^3 & s p \\
\hline
\end{array}\)
Question 78. Mention the nature of bonding of the following molecules/ions: CaH2, BH4, Na2O2, SiH4
Answer:
⇒ \(\begin{array}{|c|c|c|c|c|}
\hline \begin{array}{c}
\text { Molecule/ } \\
\text { Ions }
\end{array} & \mathrm{CaH}_2 & \mathrm{BH}_4^{-} & \mathrm{Na}_2 \mathrm{O}_2 & \mathrm{SiH}_4 \\
\hline \begin{array}{c}
\text { Nature of } \\
\text { bonding }
\end{array} & \text { ionic } & \begin{array}{c}
\text { covalent and } \\
\text { coordinate }
\end{array} & \begin{array}{c}
\text { ionic } \\
\text { and } \\
\text { covalent }
\end{array} & \text { covalent } \\
\hline
\end{array}\)
Question 79. Between N2O2, and NO2 molecules, which one is more polar? Explain.
Answer:
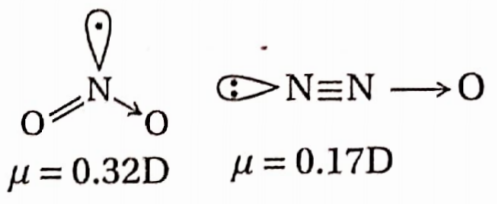
N2O is a linear molecule. In this molecule the N bond moment and the moment due to the unshared electron pair act in opposite directions which decreases the dipole moment making the molecule less polar. However, the resulting dipole moment is large in NO2 due to its angular nature.
Question 80. Arrange the following ions in the increasing order of their ionic radii. F–, Mg2+,Al3+, O2-
Answer: Al3+ < Mg2+ < F– < O2-
Question 81. Arrange the following molecules in increasing order of their dipole moments: NH3, NF2, CBr4
Answer: CBr4<NF3<NH3
Question 82. How many (cr) and (a) bonds are present in buta-1,3- diyne?
Anwer: H—C=C—C=C—H <x -bonds: 5; n -bonds: 4 Buta-l,3-diyne
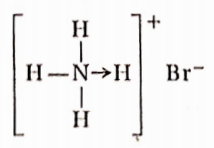
Question 83. What are the different types of bonds present in ammonium bromide molecules?
Answer: Ionic, covalent, and coordinate bonds.
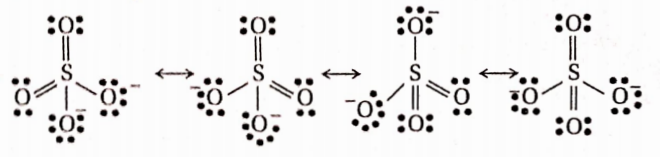
Question 84. Draw the resonating structures of sulfate ion
Answer:
Question 85. Which of the H2O or H2S molecules has a greater bond angle? Explain. Between o-nitrophenol and p-nitrophenol, which one has a greater boiling point? Explain.
Answer: As the electronegativity O-atom is higher than S, the bond angle of H2O is larger than that of H2S.

Question 86. Which of the following is not paramagnetic—
- N2+
- Li2
- O2
- H2+
Answer: \(\mathrm{Li}_2:\left(\sigma_{1 s}\right)^2\left(\sigma_{1 s}^*\right)^2\left(\sigma_{2 s}\right)^2\)
Question 87. Which bond among the following is the least ionic—
Answer: In F—F, there is no electronegativity difference
Question 88. When hydrogen combines with oxygen, a polar covalent product is formed—explain. What types of bonds are present in KHF2
Answer: Hydrogen reacts with oxygen to form H2O as a covalent compound. Due to angular structure the resultant of the two O —H bond moments and the moment contributed by the lone pair act in the same direction. Hence, the H2O molecule is polar.
K+[F…..H —F]- Ionic, covalent and hydrogen bond.
Question 89. Write canonicals of ClO4 ion.
Answer:
⇒ \(\begin{aligned}
& \mathrm{H}_2:\left(\sigma_{1 s}\right)^2, \mathrm{~B} \cdot \mathrm{O}=\frac{1}{2}(2-0)=1 \\
& \mathrm{He}_2:\left(\sigma_{1 s}\right)^2\left(\sigma_{1 s}^*\right)^2, \mathrm{~B} \cdot \mathrm{O}=\frac{1}{2}(2-2)=0
\end{aligned}\)
As the bond order is zero, the He2 molecule has no existence.
Question 90. In which of the following conversions there are changes of hybridization and shape
Answer: BF3 molecule exhibits trigonal planar geometry with each F—B—F bond angle 120°. B-atom is sp2 hybridised.
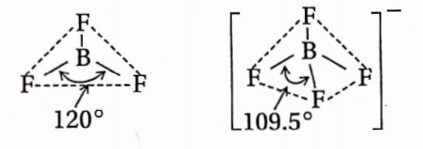
BF4 on the other hand exhibits tetrahedral geometry with each F —B —F bond angle 109.5°. B-atom is sp3 – hybridized.
Question 91. What are the types of hybridization of NH4, CO2-, H2S, and SO2? The C—0 bond is polar but CO2 does not have a dipole moment. Why?
Answer: In NH+4 and H2S the central atoms (N and S) undergoes sp3 hybridization. In CO2-3 the central atom C is sp2 hybridised. In SF6, the central atom S undergoes sp3d2 hybridization.
Question 92. The bond order of the He2+ ion is—
Answer: There is no electron present in the He2+ ion. Thus bond order = 0
Question 93. Which is not paramagnetic of the following— N+ CO O- NO
⇒ \(\left(\sigma_{1 s}\right)^2\left(\sigma_{1 s}^*\right)^2\left(\sigma_{2 s}\right)^2\left(\sigma_{2 p_z}\right)^2\left(\sigma_{2 s}^*\right)^2\left(\pi_{2 p_x}\right)^2\left(\pi_{2 p_y}\right)^2\)
Question 94. Arrange die following compounds according to their increase of melting point: NaCl, MgCl2, AlCl3 Between NH2 and NF3 which one is more polar and why?
Answer: AlCl3 < MgCl2 < NaCl
Question 95. Why does the PCl5 exist but NCl5 does not? Why is BaSO4 not soluble in water?
Answer: For a compound to be soluble in H2O, its lattice enthalpy should be low compared to its hydration enthalpy. Since both Ba2+ and SO -are large, they stabilize each other to form a strong lattice. This leads to the insolubility of BaSO4 in water.
Question 96. Which has the smallest bond length—
Answer: O+2. Its bond order is 2.5 (maximum among the given species) and we know bond-length \(\propto \frac{1}{\text { bond order }}\)
Question 97. What is the hybridization state of the central atom in 1-3
- sp3
- dsp2
- sp3d2
- sp3d
Answer: 4. sp3d
Question 98. Both Br(g) and NO2(g) are reddish-brown gaseous substances. How will you chemically distinguish between them? What will be the order of covalent character of the following compounds?
- LIP
- LiCl
- LiBr
- Lil
Answer: 1. LIP
Question 99. The state of hybridization of the central atom of which of the following is sp3d2 —
- SP4
- PCI5
- SP6
Answer: 3. SP6
Question 100. Which of the following is the correct order of repulsive interaction of lone pair (Ip) and bond pair (bp) of electrons —
- Ip- Ip > Ip- bp > bp- bp
- Ip- bp > Ip- Ip > bp -bp
- bp- bp > Ip-Ip >Ip- bp
- Ip- Ip > bp- bp > Ip -bp
Answer: 1. Ip- Ip > Ip- bp > bp- bp
Question 101. Draw the canonicals of CO3. Why is the boiling point of H2O greater than that of II2S?
Answer: Due to the presence of extensive intermolecular H bonding in H2O, it has a higher boiling point than H2S.
Question 102. Write Lewis dot symbols for atoms of the following elements: Mg, Na, B, O, N, Br.
Answer:
⇒ \(\begin{array}{|c|c|c|c|c|c|c|}
\hline \text { Element } & \mathbf{M g} & \mathbf{N a} & \mathbf{B} & \mathbf{O} & \mathbf{N} & \mathbf{B r} \\
\hline \begin{array}{c}
\text { Atomic } \\
\text { No. }
\end{array} & 12 & 11 & 5 & 8 & 7 & 35 \\
\hline \text { E.C. } & 2,8,2 & 2,8,1 & 2,3 & 2,6 & 2,5 & 2,8,18,7 \\
\hline \begin{array}{c}
\text { Lewis } \\
\text { dot } \\
\text { structure }
\end{array} & \ddot{\mathrm{Mg}} & \dot{\mathrm{Na}} & \cdot \dot{\mathrm{B}} \cdot & \mathbf{\text { Ö: }} & \mathbf{: \mathrm { N } :} & \mathbf{: \mathrm { Br } :} \\
\hline
\end{array}\)
Question 103. Write Lewis symbols for the following atoms and ions: S and S2-; Al and Al3+, H and H–
Answer:
⇒ \(\begin{array}{|c|c|c|c|}
\hline \text { Element } & 16^{\mathbf{S}} & { }^{13} \mathbf{A l} & \mathbf{1}^{\mathbf{H}} \\
\hline \text { E.C. } & 2,8,6 & 2,8,3 & 1 \\
\hline \text { Atom } & : \dot{\mathrm{s}}: & \cdot \dot{\mathrm{Al}} \cdot & \mathrm{H} \cdot \\
\hline \text { Ion } & {\left[\:_0\right]^{2-}} & {[\mathrm{Al}]^{3+}} & {[\mathrm{H}:]^{-}} \\
\hline
\end{array}\)
Question 104. Although the geometries of NH3 and H2O molecules are distorted tetrahedral, the bond angle in water is less than that of ammonia. Discuss.
Answer: The difference in the bond angles of water (H2O) and ammonia (NH3) is due to the difference in the number of lone pairs and bond pairs in these two species. In NH3, N has 1 lone pair and 3 bond pairs while in H2O, O has 2 lone pairs and 2 bond pairs. The lone pair-bond pair repulsion in H2O is much more than in NH3. Hence, the bond angle around the central atom in H2O is relatively smaller than that in NH3.
Question 105. Explain the important aspects of resonance concerning the CO2-3 ion
Answer: When a single structure cannot explain all the properties of a molecule, some probable hypothetical structures (canonical structures) are used. The actual structure is called the resonance hybrid which is an intermediate of the canonical structures. This is termed resonance.
Carbonate ion (CO2-3) can be represented by a combination of the following 3 resonating structures in which CO2-3 is the resonance hybrid of the three canonical structures 1,2 and 3.
Question 106. H3PO3 can be represented by structures 1 and 2. Can these two structures be taken as the canonical forms of the resonance hybrid representing H2PO3? If not give reasons for the same.
Answer: These cannot be taken as canonical forms, since the position of the atoms has been changed.
Question 107. Although both CO2 and H2O are triatomic molecules, the shape of the H2O molecule is bent while that of CO2 is linear. Explain this based on the dipole moment.
Answer: The bond moments of two C=0 bonds in CO2 cancel each other, indicating the linear structure of CO2. However, the H2O molecule has a net dipole moment (0). Thus, the bond moments of two O —H bonds do not cancel each other. As a result, H2O has a bent structure.
Question 108. Define electronegativity. How does it differ from electron gain enthalpy?
Answer: The electronegativity of an element is the tendency or ability of its atom to attract the shared pair of electrons towards itself in a covalent bond. On the other hand, electron gain enthalpy is the energy released, when one mole of gaseous atoms of the element accepts electrons from gaseous un negative ion.
Question 109. Arrange in order of increasing ionic character in the molecules: LiF, KaO, N2, SO2, and CIF3.
Answer: The ionic character of a molecule depends on the difference in electronegativities of the atoms involved in bond formation and also on the geometrical arrangements of the bonds. Therefore, the increasing order of ionic character is given by— N2 < SO2 < ClF3 < K2O < LiF.
Question 110. The skeletal structure of CH6COOH as shown below is 4.8.2, correct, but the sonic of the bonds is shown incorrectly. Write the correct Lewis structure for acetic acid.
Answer: The skeletal structure of CH3COOH is correct, but according to Lewis’s theory the arrangement of electrons is not perfect. The correct Lewis structure of CH3COOH
Question 111. Apart from tetrahedral geometry, another possible geometry for CH4 is square planar with the four H atoms at the corners of the square and the C atom at its center. Explain why CH4 is not square planar.
Answer: According to VSEPR theory, shared electron pairs around the central atom in a covalent molecule lie apart at the farthest possible distance to minimize the electrostatic forces of repulsion between them. For tetrahedral geometry, the bond angle is 109°28 while for square planar geometry, the bond angle is 90°.
In square planar geometry, repulsions between the bond pairs are greater than that in the tetrahedral geometry. Consequently, methane assumes tetrahedral geometry instead of square planar geometry. However, for square planar geometry, the required hybridization is dsp² which is not possible for carbon as it has no orbitals in the valence shell.
Question 112. Explain why the BeH2 molecule has a zero dipole moment although the Be —H bonds are polar.
Answer: The BeH2 molecule is linear (H —Be —H). Its bond angle is 180°. The two Be —H bond moments are equal and opposite and hence cancel out each other.
Question 113. Describe the change in hybridization (if any) of the AI in the following reaction. AlCl3 + Cl–→ AlCl-3
Answer: Using the equation \(H=\frac{1}{2}[V+X-C+A]\)
⇒ \(\text { In } \mathrm{AlCl}_3, \mathrm{H}=\frac{1}{2}(3+3-0+0)=3\)
∴ Hybrid state of Al = sp2
⇒ \(\text { In }\left[\mathrm{AlCl}_4\right]^{-}, \mathrm{H}=\frac{1}{2}(3+4-0+1)=4\)
∴ Hybrid State of Al= sp3
Question 114. Is there any change in the hybridization of B and N atoms as a result of the following reaction?
⇒ \(\mathrm{BF}_3+\mathrm{NH}_3 \rightarrow \mathrm{F}_3 \mathrm{~B} \cdot \mathrm{NH}_3\)
Answer: N in NH3 is the donor and B and BF3 are the acceptor. The hybrid state of B in BF3 is sp2 and that of Nin NH3 is sp3. In the compound F3B.NH3, both and B atoms are surrounded by 4 bond pairs. Thus both have a hybrid state of sp3. Hence, during combination, the hybrid state of B changes from sp2 to sp3 but that remains the same.
Question 115. Considering the x -x-axis as the intermolecular axis which out of the following will not form a sigma bond and why?
- Is, Is
- Is, 2px-,
- 2py, 2py
- Is, 2s.
Answer: σ -bond is formed between two atoms by the end-to-end (head-on) overlap of atomic orbitals. Hence form σ -bond. In the case of (3), if x -is considered as an intermolecular axis, then the n -bond will form due to lateral overlapping between py orbitals.
Question 116. Write the significance of a plus and a minus sign shown in representing the orbitals.
Answer: An orbital is a pictorial representation of wave function, where the ‘+’ and- ‘ sign represents the opposite phases of the wave.
The bonding molecular orbital is formed by a combination of ‘+’ with ‘+’ or ‘ with part of the electron waves whereas the antibonding molecular orbital is formed by the combination of ‘+’ with part.

