Class 11 Chemistry Chemical Bonding And Molecular Structure Long Answer Type Questions
Question 1. What are valence electrons?
Answer: The electrons in the ultimate (or outermost) and in some cases, the penultimate shell of an atom that is responsible for chemical bonding are known as valence electrons
Question 2. Give the Lewis symbols of—(1) Br (2) N (3) O2- (4) S2- (5) N3-
Answer:
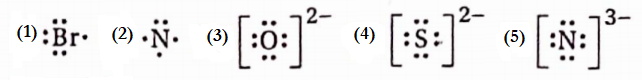
Question 3. How is crystalline NaCl formed from constituent elements?
Answer: When a Na-atom combines with a Cl-atom, the electron lost by die electropositive Na-atom is gained by the electronegative Cl-atom, resulting in the formation of Na+ and Cl– ions respectively, each having inert gas configuration. The oppositely charged ions are bound by a strong electrostatic force of attraction to form an ionic, crystalline solid, NaCl.
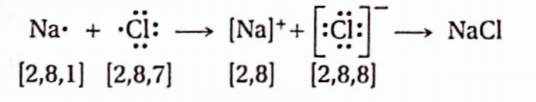
In the crystal of NaCl, each Nation is surrounded by 6 Cl- ions, and each Cl- ion is surrounded by 6 Na+ ions. This results in the formation of a three-dimensional crystal, where the lattice sites are alternately occupied by Na+ and Cl– ions.
Question 4. The elements belonging to which group(s) of the periodic table combine to form electrovalent compounds and why?
Answer: Elements of groups 1 and 2 form electrovalent compounds because they are highly electropositive.
Question 5. Which elements exhibit variable electrovalency and why?
Answer: Transition elements. This is because the outermost electron of the ns -subshell and the penultimate (n-1)d subshell are involved in bonding.
Question 6. Why the ionic compounds do not exhibit isomerism?
Answer: Electrostatic force in an ionic compound is distributed uniformly in all directions. Thus, each compound holds a definite number of oppositely charged ions. Hence there are no discrete molecules in ionic compounds. Since ionic bonds are non-directional, ionic compounds do not exhibit isomerism.
Question 7. Which type of ionic compounds exhibit isomorphism?
Answer: Isoelectronic ionic compounds
Question 8. What is solvation energy solvation enthalpy?
Answer: The process of orientation of polar solvent molecules around the ions of the polar solute molecules is called solvation and the energy released in this process is called solvation energy.
Question 9. What is the condition for dissolution of an ionic compound in a particular solvent?
Answer: An ionic compound is soluble (dissolves) in a particular solvent only if the solvation energy exceeds the lattice energy of the crystal (ionic compound).
Question 10. The ionic compounds are soluble in polar solvents but insoluble in non-polar solvents—why?
Answer: In the case of polar solvents, the solvation energy of ionic compounds is greater than lattice enthalpy. So, ionic compounds are soluble in polar solvents, but they are insoluble in non-polar solvents because solvation by nonpolar solvents is not possible for ionic compounds.
Question 11. Why do ionic compounds conduct electricity only in a solution or molten state and not in a solid state?
Answer: Ionic compounds are good conductors of electricity in solution or in the molten state as in these states, their ions are free to move. As the ions are charged, they are attracted towards electrodes and thus act as carriers of electric current.
Question 12. Draw Iewis dot structures of H3PO4 and CO2-3.
Answer:
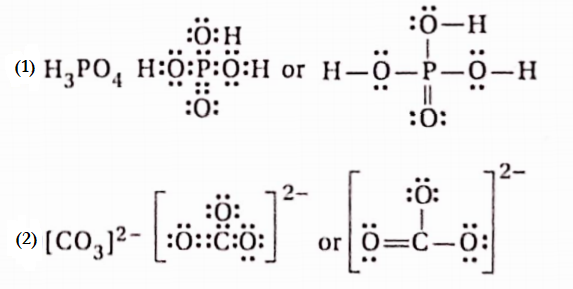
Question 13. Calculate the formal charge on N-atom in the HNO3 molecule
Answer: Formal charge on N-atom in HNO3
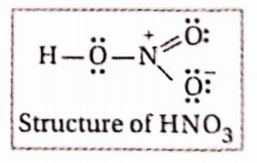
No. of valence electrons of Natom] – [No. of unshared electrons \(-\frac{1}{2} x\) [No. of shared electrons] \(=5-0-\frac{1}{2} \times 8=5-4=+1\)
Question 14. In which of the given molecules, the central atom does not obey the octet rule? ClF3, SF2, OsFg, BCl3, NH3, NO2
Answer:
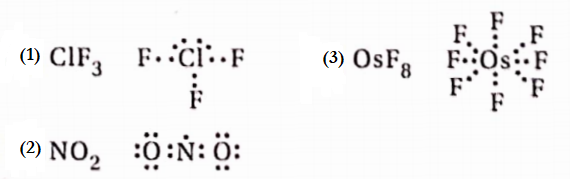
Question 15. The melting point of MgBr2 is 700°C while that of AlBr3 is only 97°C. Give reason.
Answer: According to Fajan’s rule, only the potential (phi) of the cations increases with an increase in cationic charge and a decrease in cationic radii. Consequently, the covalent character increases and the melting point of the corresponding salts decreases.
- In case of MgBr2 and AlBr3,
- The charge of Mg2+ is less than the charge of Al3+.
- Radius of Mg2+ is greater than the radius of Al3+.
- Thus, the melting point of AlBr3 is less than that of MgBr2
Question 16. What CuCl is more covalent than NaCl?
Answer: If the charge and size of the cations remain constant, the cation with pseudo noble gas (18 electrons) configuration, as in the case of Cu+ (3s² 3p6 3d10) causes larger polarisation on the electron cloud of the anion than a cation with noble gas (8 electrons) configuration, as in case of Na+(2s22p6) because, (n-1) p electrons are more effective in shielding the outer electrons compared to the (n-1)d electrons.
As a result, an appreciable increase in electron charge cloud density between the two nuclei takes place, leading to an increase in the covalent character of the bond. Hence, CuCl is more covalent than NaCl.
Question 17. Arrange in increasing order according to the given properties and explain the order: MgCl2, AlCl3, NaCl, SiCl4 (melting point); LiBr, NaBr, KBr (melting point); (HI) MgCO3, CaCO3, BeCO3 (thermal stability); Hgl2, HgCl2 (intensity of color).
Answer: SiCl4 < AlCl3 < MgCl2 < NaCl; [Ionic potential increases with either increase in charge on cation or cationic radius. As a result, the covalent character and melting point of the compounds formed increases.]
The order of melting point of the given bromides should be: LiBr < NaBr < KBr. Due to the increase in the size of the cation from Li+ to K+, the value of p increases.
So, the covalent character of the compounds increases. However, due to a decrease in lattice enthalpy from NaBr to KBr, the melting point decreases. Therefore, the correct order of melting point is LiBr < NaBr > KBr.
Question 18. Explain why AgCl is white whereas Agl is yellow, If the degree of polarization of the anion is higher, then the electrovalent compound becomes colored (Example Pbl2 yellow) but if it is lower, then the compound is either white or colorless (Example PbCl2 is white)—why?
Answer: The larger the anionic radius, the greater its tendency to get polarized. The higher polarisability of 1- ion, owing to its larger radius, facilitates the transition of electron (from anion to metal-ion) in the visible range, imparting a yellow color to Agl. On the other hand, the lower polarisability of the Cl– ion, facilitates the transition of electrons in the UV range. Hence, AgCl appears white
Question 19. LiCl is soluble in organic solvents while the chlorides of other alkali metals are not. Explain.
Answer: As we move down a group, the cationic radius increases, which decreases the polarising power of the cation, which ultimately decreases the covalent character of the compound. Since LiCl is the most covalent compound among all the alkali metal chlorides, it is soluble in organic (non-polar) solvents while the rest are not.
Question 20. Give reasons: SnCl2 is solid at room temperature while SnCl4 is liquid. Fel3 cannot be prepared. What is a coordinate covalent bond or coordinate bond?
Answer: Sn4+ has a higher positive charge than Sn4+ liana greater polarising power than Sn2, Hence the covalency of the corresponding chlorides increases from SnCI2 to SnCI4, which results In a decrease In the melting point from SnCl2 to SnC2. Therefore SnCl2 Is a liquid while SnCl2 Is a solid at room temperature
Question 21. Aluminium chloride exists as a dimer—Explain.
Answer:
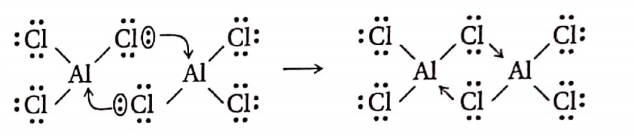
In AlCl3, the Al-atom has only 6 electrons in its valence shell. It requires two more electrons to complete Its octet. So it accepts a lone pair of electrons from the Cl-atom of another AlCl3 molecule as shown above. Thus, AlCl3 exists as a dimer.
Question 22. Give examples of two compounds in which there exists electrovalency, covalency and coordinate covalency.
Answer: Ammonium chloride (NH4Cl) Sodium fluoroborate (NaBF4)
Question 23. AlCIg forms a dimer but BC13 cannot—Explain What do you understand by 1 bond length, 2 bond dissociation enthalpy, and 3 bond angle?
Answer: The size of Al is much larger than that of B. Hence Al can easily accommodate 4 Cl-atoms around it. In AlCl3, as there are 6 electrons around the Al-atom, it completes its octet by accepting a lone pair of electrons from the Cl-atom of an adjacent molecule. As a result, AlCl3 exists as a chlorine-bridged dimer forming Al2Cl6.
On the other hand, B is comparatively smaller in size. Though it has an incomplete octet in BCl3, it cannot accommodate the fourth chlorine atom around it, owing to the large size of the Cl-atom. Thus, BCl3 does not exist as a dimer.
Question 24. Arrange the following compounds in increasing order of carbon-carbon bond strength and explain the order. CH2=CH2, CH3-CH3, HC=CH
Answer: The increasing order of C—C bond strength is given by: C—C < C=C < C=C i.e., CH3—CH3 < CH2=CH2 < CH=CH Greater the bond multiplicity, the greater the bond dissociation enthal. In CH3—CH3, there is only an or -bond between the C-atoms whereas, CH2=CH2 and, CH=CH contain one and two n -bonds respectively, in addition to the cr -bond. So the energy required to break the carbon-carbon bonds increases in the order C—C < C—C < C=C.
Question 25. In water, the first and second 0 —H bond dissociation enthalpies are SO2 and 427kJ mol-1 respectively. Determine the value of bond enthalpy of the O —H bond.
Answer: The bond enthalpy of water is given by the average of bond dissociation enthalpies of the two Q —H bonds.
Bond enthalpy \(=\frac{502+427}{2}=464.5 \mathrm{~kJ} \cdot \mathrm{mol}^{-1}\)
Question 26. Arrange the given compounds in order of their Increasing bond length: HCl, HI, HBr, HF. Explain the order.
Answer: For halogen acids, the bond length increases, with an increase in the size of the halogen atom. Since the size of the halogen atoms increases in the order F < Cl < Br <I, the bond length increases in the order HF < HCl < HBr < HI.
Question 27. Why does the value of the bond angle increase with the increased electronegativity of the central atom in the ABV type of molecule?
Answer: As the electronegativity of the central atom of a molecule of ABx type increases, the electron pair responsible for covalent bond formation will be attracted towards the central atom. Consequently, bp-bp repulsion increases leading to an increase in bond angle.
Question 28. Bond angles in Pbr3(101.5°), PCl3 (100°), and PF3(97°) decrease with an increase in the electronegativities of the surrounding atoms. However, bond angles in BF2, BCl2, and BBr3 do not change with a change in electronegativities of the surrounding atoms. Explain with reason.
Answer: PX2 has a trigonal pyramidal geometry. With the increase in electronegativity of the surrounding halogen (X) atoms, bond pairs are oriented more towards halogen atoms, resulting in a decrease in bond pair-bond pair repulsions. Hence X—P—X bond angles decrease in the order: Pbr3(101.5°) > PCl(100°) > PF3(97°)
BX3 has a trigonal planar geometry, where 3 halogen atoms are located at 3 corners of an equilateral triangle. With the increase in the electronegativity of the halogen atom, bond pairs tend to concentrate more towards the halogen atoms resulting in a decrease in bond pair- bond pair repulsion. Since all 3 halogen atoms lie on the same plane, forming 3 equivalent B—X bonds, there is no change in the X—B —X bond angle (120°).
Question 29. The bond angle of H2O is greater than that of H2S —explain.
Answer:
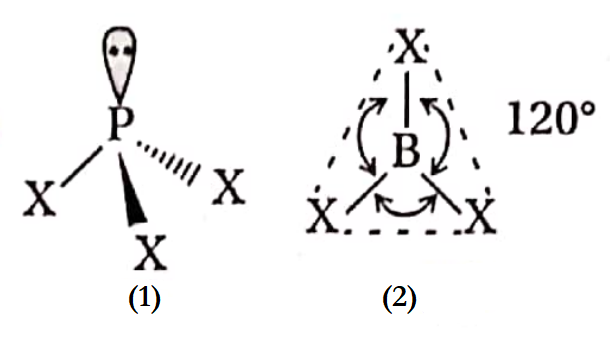
Both H2O and H2S have a tetrahedral geometry. Since Ip-bp repulsions are greater than bp-bp repulsions, these molecules attain a distorted tetrahedral geometry, where H —X—H (X = O or S) bond angles are less than the normal tetrahedral angle of 109°28.
Due to the higher electronegativity of the central O-atom than the S-atom, bp-bp repulsion is greater in the case of the O—H bond. Hence, the bond angle of H2O is greater than H2S.
Question 30. Arrange the following molecules/ions in order of decreasing —N —H bond angle and explain the order: NH3, NH+, NH2-
Answer: 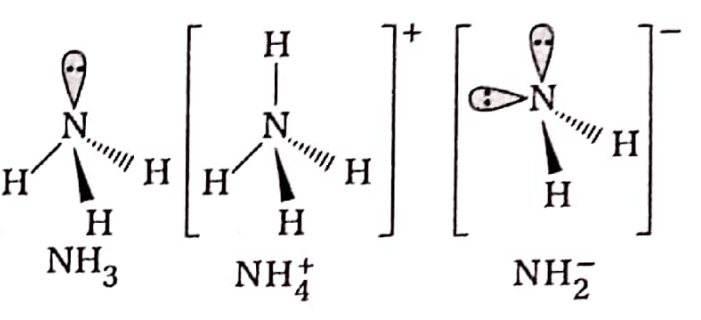
NH3 has one lone pair and 3 bond pairs, NH2 has four bond pairs and NH2 has two lone pairs and two bond pairs.
According to VSEPR theory since the repulsions follow the order: Ip -Ip > Ip- bp > bp – bp, the bond angles (H—N—H) decrease in the order: NH+> NH3 > NH2-.
Question 31. Why are the n -bonds weaker and more reactive than the cr -bonds?
Answer: The extent of axial overlapping is greater as compared to sideways overlapping. Hence n -bonds are weaker and more reactive than cr -bonds.
Question 32. Explain why the formation of a n-bond is not possible between a py and a pz-orbital.
Answer: px and py orbitals are mutually perpendicular, n -bonds are formed by the lateral overlap of two parallel p -orbitals. Since lateral overlap is not possible between two mutually perpendicular orbitals, a n -bond is not possible between a py and a pz orbital.
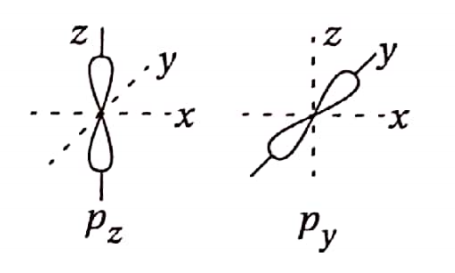
Question 33. Which symmetry element presents a n -bond? What is meant by the hybridization of atomic orbitals?
Answer: A pi-bond possesses a plane of symmetry, which is also known as a nodal plane.
Question 34. What will be the spatial distribution of sp³, sp², and sp hybrid orbitals?
Answer:
- sp3 — In this case, each of the hybrid orbitals is directed toward the four corners of the tetrahedron.
- sp2— In this case, each of the hybrid orbitals is. directed towards the three corners of a triangle.
- sp—In this case, the two hybrid orbitals are linearly arranged.
Question 35. Is hybridization possible in an isolated atom?
Answer: Hybridisation is not possible for isolated atoms. It occurs only when the atom takes part in bond formation.
Question 36. Predict the state of hybridization of the central atom and the shape of each of the following species:
Answer:
Question 37. Name the type of hybridization of the central atom that leads to each of the following geometries:
Answer:
- Square planar -dsp2
- Planar triangular -sp2
- Tetrahedral -sp3
- Linear-sp2
- Octahedral -sp3d2
- Trigonal bipyramidal-sp3d2
Question 38. Identify the state of hybridization of each carbon in:
- CH2=CH—CH=CH2
- CH2=C=CH2
- CH2=CH—CHO
- CH3—C= CH
- HCEEC—CHO
Answer: \(\stackrel{1}{\mathrm{C}} \mathrm{H}_2=\stackrel{2}{\mathrm{C}} \mathrm{H}-\stackrel{3}{\mathrm{C}} \mathrm{H}=\stackrel{4}{\mathrm{C}} \mathrm{H}_2 ; \mathrm{C}_1, \mathrm{C}_2, \mathrm{C}_3, \mathrm{C}_4-\text { all } s p^2hybridised.\)
Question 39. Arrange ethane, ethylene, and acetylene in order of their decreasing C—H bond length. Explain the order.
Answer: The 1 s -orbital of hydrogen overlaps with the sp3, sp2, and sp -hybrid orbitals of carbon in ethane, ethylene, and acetylene respectively to form C —H cr -bonds.
Since the size of these hybrid orbitals decreases in the order: sp3 > sp2 > sp2 the C —H bond length decreases in the order: C—H(C2H6) > C—H(C2H4) > C—H(C, H2)
Question 40. What are the possible geometrical shapes of covalent molecules of the general formula, AX2 and AX3 (X = a monovalent atom) when the central atom A has No lone pair of electrons, one lone pair of electrons, and two lone pairs of electrons?
Answer: AX2 (l) shape — linear, Example BeCl2
- shape — angular, Example CCl2
- shape — V-shaped, Example H2O
- AX3 shape — trigonal planar, Example BF3
- shape —pyramidal, Example NH3
- shape — T-shaped, Example ClF3
Question 41. Why are the P —Cl bonds in PCl5 not the same length?
Answer: In PCl5, two axial P—Cl bonds and three equatorial P—Cl bonds are present. An axial bond pair is repelled by three equatorial bond pairs at 90° and one axial bond pair at 180°.
Similarly, an equatorial bond pair is repelled by two axial bond pairs at 90° and two equatorial bond pairs at 120°. Thus, an axial bond pair is repelled by three electron pairs while an equatorial bond pair is repelled by two electron pairs. Thus, the axial bond pair suffers greater repulsion & hence slightly longer than equatorial bonds
Question 42. Which of the molecules Orions are iso-structural and why? BF3, NH+, CO2-, BF4, NO2, CH3+, CCl4 All the C-0 bond lengths – are not equal— explain.
Answer: BF3, CH+3 — trigonal planar geometry. The central atom undergoes sp2-hybridisation forming 3cr bonds with the neighbouring atoms. H4, CCl4, BF4 — tetrahedral geometry. The central atom undergoes sp3-hybridisation forming 4σ bonds with tire neighbouring atoms. NO3-, CO3+2 — trigonal planar geometry. The central atom is sp2 -hybridized forming σ bonds and pi bonds.
Question 43. Which is the most electronegative element according to Pauling’s scale of electronegativity?
Answer: According to the Pauling scale of electronegativity, fluorine is the most electronegative element with electronegativity = 4
Question 44. Which one among the following pairs is more electronegative and why?
- Csp or
- Carbon in CHl3 orcarbon in CHCl3,
- Na or Cl
- Carbon in C2H4 or C2H2
Answer: CS is more electronegative than Csp³ because, for hybrid orbitals, electronegativity increases with an increase in the s -the character of the hybrid orbital. 1
C in CHCl3 is more electronegative than C in CH3 because the electronegativity of an atom increases with an increase in the electronegativity of the atom bonded to it.
Chlorine (Cl) is more electronegative than sodium (Na) because, as we move from left to right in a given period, atomic size decreases, and effective nuclear charge increases. Hence electronegativity increases.
In C2H4, C is sp² hybridised while in C2H2 C is sp hybridised. Since electronegativity increases with an increase in the s -s-character of the hybrid orbitals, C in C2H2 is more electronegative than C in C2H4.
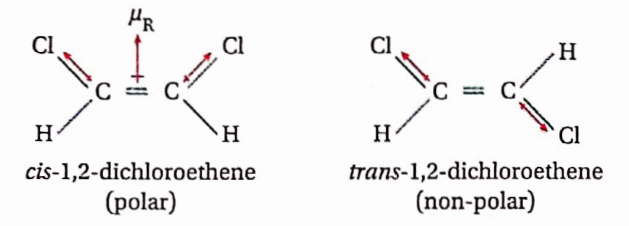
Question 45. How will you distinguish between the two geometrical isomers of l, 2-dichloroethane from their boiling points?
Answer: The 2 geometrical isomers of 1,2 dichloroethene are cis- 1,2 dichloroethene and trans-1,2 dichloroethene. cis-1,2 dichloroethene has a definite dipole moment (μ≠ 0) whereas the dipole moment of trans-1,2 dichloroethene is found to be zero (μ= 0).
The ct’s-isomer is highly polar indicating strong dipole-dipole attractive forces among the molecules. Hence a large amount of energy is required to separate the molecules from each other. Therefore boiling point of cis-1,2 dichloroethene is higher than the trans-isomer.
Question 46. Explain why the following molecules are non-polar:
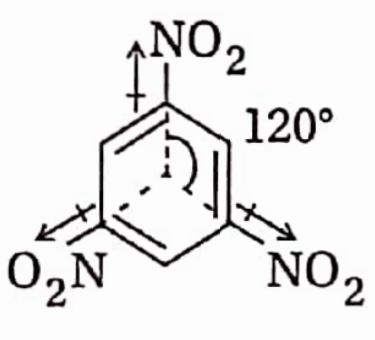
Answer: 1,3,5-trinitrobenzene, the three NO2 groups are bonded to 3 alternate sp2 hybridized C-atom of the benzene ring. The three C-NO2 bond moments act at an angle of 120° to each other. Therefore, the net dipole moment of the molecule is zero (p = 0) and the molecule is non-polar trans-2,3-dichlorobut-2-ene.
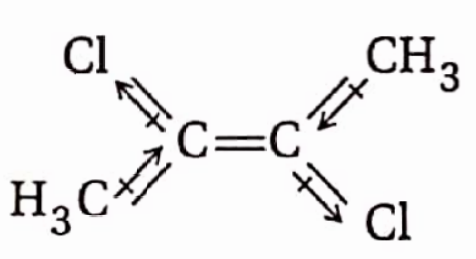
In trans-2,3-dichlorobut-2-ene, the two C —Cl and the two C —CH3 bond moments act in H3C opposite directions to balance each other, Because of this, the molecule possesses no net dipole moment. Hence, tram-2,3 dichloro but-2- ene is non-polar
Question 47. Predict the dipole moment of a molecule of the type, AB4 having square-planar geometry, a molecule of the type, AB2 having trigonal bipyramidal geometry, a molecule of the type, ABg having octahedral geometry, a molecule of the type, AB7 having pentagonal bipyramidal geometry.
Answer: All the four A—B bond moments act at an angle of 90° with each other. Therefore the net dipole moment of AB4 is zero (p = 0).
Due to the symmetrical structure of the molecule, the equatorial bond moments cancel each other. Similarly, the axial bond moments cancel each other. Therefore the resultant dipole moment is zero (p = 0).
Question 48. Which one of each pair has a higher dipole moment and why?
- CS2 and CO2;
- NH3 and NF3;
- CH3CH2Cl and CH2=CHCl;
- 1,3,5- tribromobenzeneand 1,3-dibromobenzene.
Answer: \(\text { (1) } \begin{array}{ll}
\mathrm{S} \equiv \mathrm{C} \equiv \mathrm{S} & \mathrm{O}=\mathrm{C} \\
\mathrm{CS}_2(\mu=0) & \mathrm{CoS}(\mu \neq 0)
\end{array}\)
Question 49. Explain why the dipole moment of CD3F (1.858D) is higher than thatofCH3F (1.847D)
Answer: D is more electron releasing than H. Difference in electronegativity between C and F in CD3F is much higher than that between C and F in CH3F. Hence, CD3F is more polar than CH3F. Therefore, the dipole moment of CD3F is higher than that of CH3F.
Question 50. NH3 molecules remain associated through intermolecular hydrogen bonding but there is no such association among HC1 molecules even though electronegativities of N and Cl are the same. Explain.
Answer: Although the electronegativities of nitrogen and chlorine are the same, nitrogen can form hydrogen bonds but Cl cannot. This is because the N-atom is much smaller than the CIatom.
Due to the large size of the Cl-atom, the electrostatic attraction between the Cl-atom of one molecule and the Hatom of another molecule becomes weak. Hence, Cl does not form hydrogen bonds while NH3 molecules undergo association by intermolecular hydrogen bonds.
Question 51. At normal temperature, o-hydroxybenzaldehyde is a liquid but p-hydroxybenzaldehyde is a solid. Give reason.
Answer: In o-hydroxybenzaldehyde, the —OH and — CHO groups are situated at two adjacent C-atoms of the benzene ring and are involved in intramolecular hydrogen bonding. These molecules exist as discrete molecules and have a lower melting point. On the other hand, in p -hydroxybenzaldehyde, the —OH and — CHO groups are situated away from each other. Hence, intramolecular hydrogen bonding does not exist.
These molecules remain associated through intermolecular hydrogen bonding and hence have a high melting point. Thus the ortho-isomer exists as a liquid while the para-isomer exists as a solid.
Question 52. Hydrogen bonds are usually longer than covalent bonds. Give an example where covalent and hydrogen bonds are equal in length. Explain.
Answer: In fluoride ion (HF2), the covalent bond and the hydrogen bond are equal in length and this is because the structure of this ion is a resonance hybrid of structures 1 and 2.
Question 53. Arrange the following species in order of increasing stability and give reasons: Li2, Li+2, Li-2 are as follows [li (z=3)]:
Answer: \begin{aligned}
& \mathrm{Li}_2-\mathrm{KK}\left(\sigma_{2 \mathrm{~s}}\right)^2 ; \text { B.O. }=\frac{2-0}{2}=1 \\
& \mathrm{Li}_2^{+}-\mathrm{KK}\left(\sigma_{2 \mathrm{~s}}\right)^1 ; \text { B.O. }=\frac{1-0}{2}=0.5 \\
& \mathrm{Li}_2^{-}-\mathrm{KK}\left(\sigma_{2 \mathrm{~s}}\right)^2\left(\sigma_{2 \mathrm{~s}}^{+}\right)^1 ; \text { B.O. }=\frac{2-1}{2}=0.5
\end{aligned}
The greater the bond order, the greater the bond dissociation enthalpy and hence greater the stability. Again stability decreases when excess electrons are present in a nonconjugate shell. Therefore the order of increasing stability of the given species is as follows:
⇒ \(\mathrm{Li}_2^{-}<\mathrm{Li}_2^{+}<\mathrm{Li}_2\)
Question 54. Inert gases are monoatomic. Explain in terms of MO theory.
Answer: The molecular orbital energy level diagram for inert gases shows that the number of electrons in bonding molecular orbitals is equal to those in the antibonding molecular orbitals i.e., bond order (of inert gases) \(=\left(\frac{N_b-N_a}{2}\right)=0\) Therefore, all inert gases are monoatomic. For Example He (2); Electronic configuration of He2 is:
⇒\(\mathrm{He}_2-\left(\sigma_{1 \mathrm{~s}}\right)^2\left(\sigma_{1 \mathrm{~s}}^*\right)^2\)
Bond Order \(=\frac{2-2}{2}=0\)
Question 55. A homonuclear diatomic molecule contains 8 electrons. Predict whether the molecule will exist or not.
⇒ \(\left(\sigma_{1 s}\right)^2\left(\sigma_{1 s}^*\right)^2\left(\sigma_{2 s}\right)^2\left(\sigma_{2 s}^*\right)^2\)
Nb = 4, Na = 4
Bond order \(=\frac{N_b-N_a}{2}=\frac{4-4}{2}=0\)
Hence, the molecule does not exist.
Question 108. Compare the stabilities of O2 and N2+ ions and comment on their magnetic nature.
Answer: Bond orders of N2 and O2 are 2.5 and 1.5 respectively. Hence, the N2+ ion is more stable. Both the ions contain unpaired electrons and hence are paramagnetic.
Question 56. if the electronic configuration of A atoms 1 s², comment
on the stability of the A2 molecule and A2+ ion.
Answer: \(\mathrm{A}_2:\left(\sigma_{1 s}\right)^2\left(\sigma_{1 s}^*\right)^2 \quad \mathrm{~A}_2^{+}:\left(\sigma_{1 s}\right)^2\left(\sigma_{1 s}^*\right)^1\)
The higher the bond order, the higher the bond dissociation enthalpy and the greater the stability. Hence stability of A2+ > A2. Thus, A2 will have no existence.
Question 57. The ionic frond between sodium and chloride ions is stronger than that between potassium and chloride ions. Explain.
Answer: Since the atomic number of K (Z = 19)) Is higher Ilian that of (Z = 11), K+ ion Is larger than Na+ Ion. According to Fajan’s rule, KCl should be more Ionic than NaCl. However, due to the smaller size of, Na+ ion, the charge density in Na– ion is higher than that of K+ ton.
As a consequence, the coulomblc forces of attraction between Na+ and Cl– ions (the lattice energy) are more titan than between K+ and Cl– ions. Therefore the ionic bond between Na+ and Cl– ions is stronger than that between K+ and Cl– ions.
Question 58. Silicon tetrachloride readily undergoes hydrolysis but carbon tetrachloride does not undergo hydrolysis under normal conditions. Explain.
Answer: Since carbon (of the second period) has no vacant d -d-orbital, its maximum covalency is 4. On the other hand, silicon (of the third period) has vacant d -d-orbitals, and its maximum covalency is 6. As the Si -atom can extend its covalency to 6, SiCl4 undergoes ready hydrolysis to yield SiO2.
A lone pair of electrons from the O- atom of H2O is donated to the empty d -orbitals of Si, forming a coordinate intermediate that has a trigonal bipyramidal structure. The intermediate [SiCl4(H2O)] loses a molecule of HCl to form SiCl3(OH). In the same way, the other 3Cl -atoms are replaced by 3-OH groups to form orthosilicic acid [Si(OH)4] which finally loses 2 molecules of water to give SiO2.
![]()
C-atom having no d -d-orbitals in its valence shell cannot extend its covalency beyond 4 so it does not undergo hydrolysis under normal conditions
Question 59. Covalent bonds have definite orientations but electrovalent bonds have no definite orientations — explain
Answer: Covalent bonds arc formed by the overlap of atomic orbitals having definite orientations. Consequently, covalent bonds have specific orientations. On the other hand, electrovalent bonds have no definite orientations because oppositely charged Ions attract each other from all possible directions by electrostatic forces.
Question 60. The second ionization enthalpy of Mg Is sufficiently high while the second electron affinity or electron gain enthalpy of oxygen is low (actually this value is positive), yet Mg2+ and O2- ions form the Ionic compound, MgO. Explain with reasons.
Answer: The sufficiently high second ionization enthalpy of Mg indicates that a large amount of energy is required to remove the second electron from the Mg -atom, Le., to convert Mg+ to Mg2+ ion. The second electron gain enthalpy of oxygen is positive indicating that the energy should be supplied to convert the O -atom into an O2- ion.
Since both processes are endothermic, MgO is not expected to be produced through the formation of anionic bonds. But actually, it is produced and this is because of its high lattice energy (mainly due to comparable sizes of Mg2+ and O2- ions.
Question 61. Both sodium and hydrogen are electropositive elements. Sodium reacts with chlorine to form an electrovalent compound but hydrogen reacts with chlorine to form a covalent compound —explain.
Answer: The ionization enthalpy of smaller H -atoms is sufficiently higher than that of larger Na -atoms.
Because of lower ionization enthalpy, sodium reacts with chlorine through the formation of Na+ ion to form the electrovalent compound, NaCl.
On the other hand, because of the much higher ionization enthalpy, hydrogen does not react with chlorine through the formation of H+. Instead both H+ and Cl– atoms donate one electron each to form an electron pair and produce the covalent compound, HCl by sharing the electron pair equally.
Question 62. The Melting Point Of Cal2 Is Much Lower (575°C) That Of caf2 (1392°C) explained with reasons.
Answer: According to Fajan’s rule, the tendency of a large-sized anion to be polarised is greater than that of a small-sized anion. So a compound containing a large-sized anion exhibits more covalent character than that with a small-sized anion.
Hence, Cal2 containing larger I- ion possesses a higher covalent character and melts at a relatively low temperature. On the other hand, CaF2 containing smaller F- ions possesses a much lower covalent character and melts at very high temperatures.
Question 63. The B — F bondin BF3 is shorter in length than the B — F bond in BFÿ — explain with reasons.
Answer: The outermost shell of the central B-atom of the BF3 molecule contains the electrons. Since the B-atom has an incomplete octet, it participates in resonance with the F-atoms to complete its octet.
As a result, the B — F bonds acquire partial double bond character. On the other hand, the B -atom in the BF2 ion has a filled octet, and so it does not participate in resonance. Therefore, B — F bonds do not assume a double bond character. Hence, the B — F bonds in the BF3 molecule are shorter in length than those in the BF4 ion.
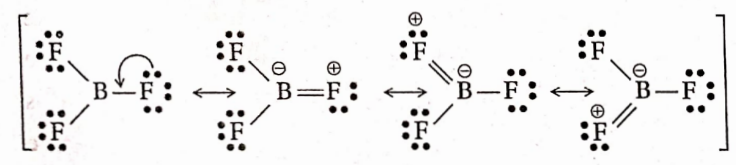
Question 64. The electronegativity of Br is less than that of F, yet BF3 is a weaker Lewis acid than BBr3
Answer: B and F atoms are elements of the same period (second period), having comparable sizes. In BF3, the octet of B-atom is not filled up. To fulfill the octet, the B-atom participates in the resonance (n -n-backbonding) with the F- F-atoms. This resonance involving orbitals of comparable sizes (2p- 2p overlap) is very effective.
As a result, electron density on B-atom increases, and the tendency of BF3 to behave as a Lewis acid decreases. On the other hand, Bris is an element of the fourth period. In BBr3, effective n-back bonding involving orbitals of dissimilar sizes (2p- 4p overlap) does not take place. Hence, the electron density on B does not increase and therefore, BBr3 behaves as a stronger Lewis acid than BF3.
Question 65. Acetylene dissolves in acetone but not in water. explain the observation.
Answer: Because of the considerable electronegativity of the sp -sp-hybridized C-atom of acetylene, the acetylenic hydrogen gets involved in intermolecular H-bonding with the O-atom of acetone. As a consequence, acetylene dissolves in acetone.
Since the energy of the H-bond formed is greater than the weak van der Waal’s attractive forces acting among the acetylene molecules and dipole-dipole attractive forces operating among the acetone molecules, the process of dissolution occurs easily.
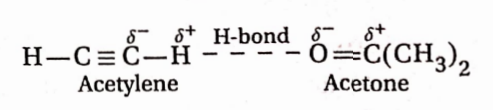
On the other hand, the intermolecular H-bonding between water molecules is stronger than the intermolecular H -H-H-bonding between water and acetylene molecules, if formed. So, acetylene shows no tendency to form H -bonds with water. Hence, acetylene does not dissolve in water.
Question 66. Arrange nitrogen dioxide molecule (NO2), nitronium ion (NO+2 ), and nitrite ion (NO-2) in increasing order of bond angle and explain the order.
Answer: NO2- ion: Total number of electrons in the valence shell of the N -atom of the ion= [5 valence electrons of N -atom + 2 electrons of O -atom linked by a double bond + 1 electron of O -atom linked by a single bond] = [8 electrons or 4 electron pairs] = [2 cr -bond-pairs + 1 lone pair+ 1 n -bondpair].
Since the n-bond pair plays no role in determining the shape of the molecule, according to VSEPR theory, the three electron pairs will be oriented towards the comers of an equilateral triangle, and the shape of the ion having one lone pair is angular.
In this case, the lone pair-bond pair repulsion is greater than the die repulsion between two bond pairs of the two bonds having partial double bond character due to resonance. As a result, the O —N —O bond angle (115°) is less than the expected regular trigonal shape with a greater O—N—O bond angle (120°).
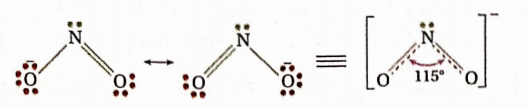
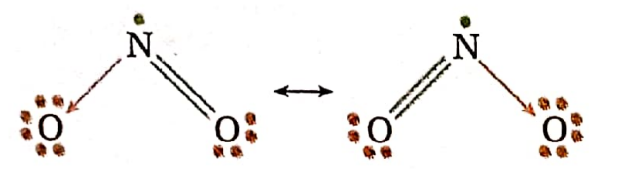
NO2 molecule: Total number of electrons in the valence shell of the N -atom of the molecule = [5 valence electron of N-atom + 2 electrons of O-atom linked by a double bond + zero electrons of the O-atom linked by an o-ordinate covalent bond] = 7 electrons =[3 electron pairs + 1 odd electron] = [l(r-bond pair + 1 coordinate cr-bond pair + In’ -bond pair + 1 odd electron].
According to VSEPR theory, two bond pairs and the odd electron are arranged trigonally in a plane.
So, the shape of the NO2 molecule having an odd electron is angular. In this case, the repulsive force between the bond pairs of two bonds having partial double bond character is greater than the repulsive force acting between the bond pairs and the odd electron. As a result, the value of the O—N—0 bond angle (135°) is greater than that of the regular planar trigonal shape (120°).
NO2 ion: Total number of electrons in the valence shell of the N -atom of the ion = 5 electrons of N -atom +4 electrons of two O -atoms linked by two double bonds -1 electron for the positive charge = 8 electrons = 4 electron pairs = 2 cr -bond pairs + 2a- -bond pairs.
The two n-bond pairs have no contribution toward the shape of the ion. According to VSEPER theory, the two bond pairs are oriented in opposite directions. Hence, the shape of the NO2 ion is linear and the value of the O —N —O bond angle is 180°. Therefore, the increasing order of the O —N —0 bond angle of the given species is: NO-2 < NO2 < NO+2.
Question 67. H2O is liquid while H2S is gas, though oxygen and sulfur, both belong to the same group of the periodic table
Answer: The oxygen atom is smaller and more electronegative than sulfur. Hence, in the H2O molecule, the O atom forms strong intermolecular H-bonds. However, in H2S molecules, S-atom, owing to its larger size and lesser electronegativity than Oatom, cannot form H-bonds.
Strong intermolecular H-bonds bind H2O molecules in an associated state, while molecules of H2S are held by weak van der Waals forces. Therefore H2O is a liquid while H2S is a gas at room temperature.
Question 68. Why does PClg form PCl3 and Cl2, on strong heating?
Answer: PCl5 has 2 axial and 3 equatorial bonds. When PCl5 is heated, the weaker axial bonds break, forming PCl3
⇒ \(\mathrm{PCl}_5 \xrightarrow{\text { Heat }} \mathrm{PCl}_3+\mathrm{Cl}_2\)
Question 69. Arrange methanol, water, and dimethyl etherin in order of increasing boiling points and explain the order.
Answer: The more molecules of the compound remain associated, the greater will be the boiling point of the compound. Water molecules having two —OH groups remain more associated by intermolecular H-bonding than methanol (CH3OH) molecules having only one —OH group.
Dimethyl ether (CH3OCH3) having no —OH group does not remain associated through H-bonding. Therefore, the boiling points of these liquids follow the order: of dimethyl ether < methanol < water.
Question 70. Explain why diamond melts at a very high temperature even though it is composed of covalently linked carbon atoms.
Answer: In the crystal diamond, each sp3 -hybridized C -atom is bonded to four others by single covalent bonds (bond length 1.54A), and a large number of tetrahedral units are linked together to form a three-dimensional giant molecule. Strong covalent bonds extend in all directions. Due to this compact structure involving strong bonds, diamond is very hard and has a very high melting point.
Question 71. Which out of 1-butyne and 1-butene has a larger dipole moment and why?
Answer: The structures of1-butyne and1-butene are as follows:
⇒ \(\begin{array}{cc}
\mathrm{H}_3 \mathrm{C}-\mathrm{CH}_2-\mathrm{C} \equiv \mathrm{CH} & \mathrm{H}_3 \mathrm{C}-\mathrm{CH}_2-\mathrm{CH}=\mathrm{CH}_2 \\
\text { 1-butyne } & \text { 1-butene }
\end{array}\)
As the sp hybridized terminal C-atom in 1-butyne is more electronegative than the sp² hybridized C-atom in 1-butene, the latter has a larger dipole moment than the forme.
Question 72. Hydrogen bonding between an F atom is stronger than that between H and O atoms. However, H2O is more viscous and its bp is greater than that of HF. Explain.
Answer: Hydrogen bonding between H F is much stronger than that between H→O because F is more electronegative than O. However boiling point of H2O is much higher than that of HF because a single molecule of water can form four Hbonds with four other HaO molecules, while one H —F molecule can form only two H-bonds with HF molecule.
Because of this, H2O is more viscous than HF and its boiling point is higher.
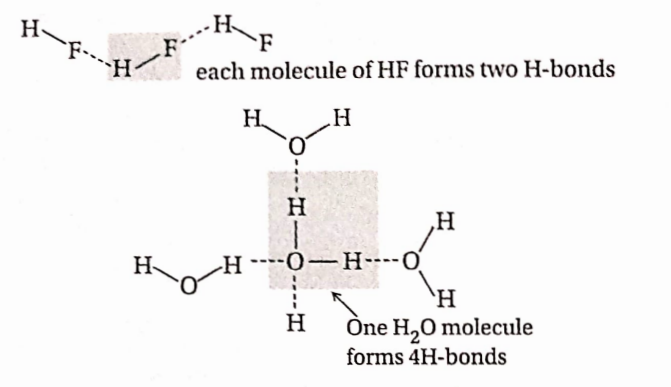
Question 73. Why viscosity and boiling point of concentrated H2SO4 very high?
Answer: The structure of sulphuric acid is as follows:
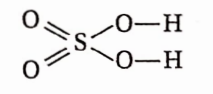
Each molecule of H2SO4 contains two OH groups and two doubly bonded oxygen atoms. Thus, each molecule of H2SO4 forms four H-bonds with other molecules. This causes extensive association among the H2SO4 molecules, increasing to boiling point.
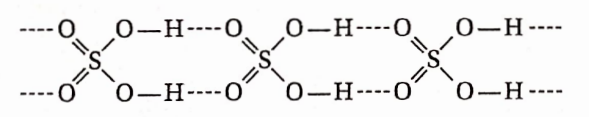
The extensive intermolecular H-bonding enhances the intermolecular attraction among the different layers of the liquid, leading to an increase in viscosity.
Question 74. In the SF4 molecule, the lone pair of electrons occupies an equatorial position rather than an axial position, in the overall trigonal bipyramidal arrangement. Why?
Answer: Depending on the position occupied by the lone pair, two structures of SF4 are possible:
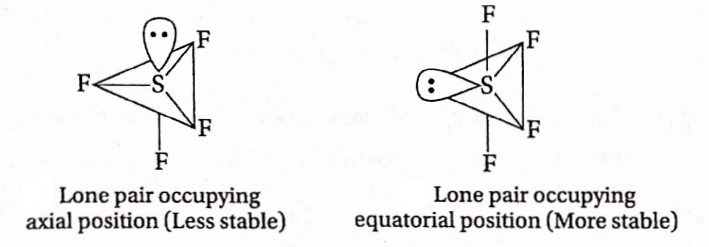
In (1), there are three lone-pair-bond pair repulsions at 90° whereas in (2) there are only two lone pair-bond pair expulsions at 90°. lienee (2) Is more stable than (1), lienee the lone pair occupies the equatorial position In the SF4 molecule.
Question 75. Explains the shape of the Ion.
Answer: The outer shell electronic configuration (In the ground state) of the central atom Is \(5 s^2 5 p_x^2 5 p_y^2 5 p_z^1 5 d^0\). It undergoes sp³d -hybridization. Out of the five sp³d hybrid orbitals, one is half-filled, one is empty and the remaining three arcs are filled.
The half-filled orbital forms a covalent bond with the iodine atom. The vacant orbital accepts an electron pair for the I- ion to form a coordinate bond. The remaining three fully-filled orbitals occupy equatorial positions. Thus, the geometry of three lone pairs and two bond pairs is trigonal bipyramidal and the shape of the I3 ion is linear.
Question 76. Give the structure of (CH3)3 N and [(CH3)3 Si]3N. Are they isostructural?
Answer: (CH3)3N is trimethyl amine. It has a pyramidal structure, while [(CH3)3Si]3N is planar.
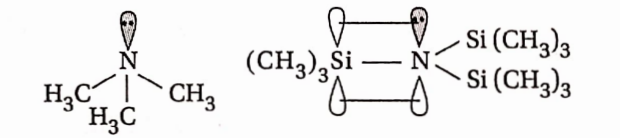
Thus, the two species are not isostructural. In (CH3)3N, N-atom is sp³ hybridised while in [(CH3)3Si]3N, the N-atom is sp² hybridised.
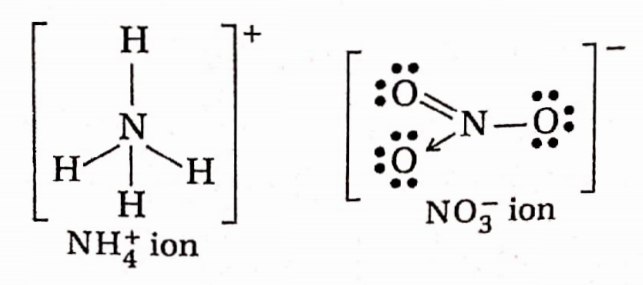
Question 77. Indicate the type of bonds present in NH4NO3 and state the mode of hybridization of two N-atoms.
Answer: NH4NO3 is an ionic compound in which the NH4+ ion is the cationic and NO3 is the anionic species. NH+ ion is formed by the combination of NH3 molecule and H+ ions through the dative bond.
N in NH3 ion is sp³ hybridized and has a tetrahedral geometry, while in NO3 ion, N is sp² hybridized and has a planar geometry. Thus three types of bonds, viz., ionic, covalent, and coordinate bonds are present in NH4NO3.
Question 78. ClF3 exists, but FCI2 does not.
Answer: The cl atom has empty d-orbitals. During horn) formation, the electrons from 3p -orbitals are promoted to 3d -orbitals
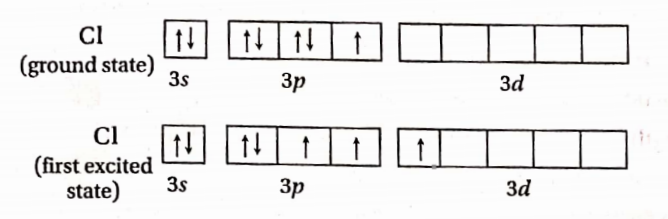
In the first excited state, Cl-atom can exhibit a covalency of three. Hence CH3 is possible. F-atom cannot expand Its octet due to the absence of empty cl -orbitals in the 2nd energy level. Hence cannot exhibit covalency more than. Therefore FCl3 is not possible.
Question 79. BaSG4 Is insoluble in water, even though it Is an ionic compound. Why?
Answer: The lattice energy (i.e., the energy required to break its crystal lattice, by separating Ba2+ and SO2- ions) of BaSO4 is greater than its solvation energy (i.e., the energy released due to solvation of Ba2+ and SO2- ions by water molecules). Hence, BaSO4 is insoluble in water.
Question 80. The dipole moment of CH3Cl (p = 1.87D) is greater than that of CH3F (μ = 1.81D) even though the C — F bond is more polar than the C — Cl bond. Explain with reasons
Answer: The dipole moment of a molecule, n = ex cl, where e = partial positive or negative charge developed on the bonded atoms and d = distance between the two charge centers. Because of the greater electronegativity of F compared to that of Cl, the charge developed in CH3F is higher than that in CH3Cl.
However, because of the larger size of the Cl -atom, the C—Cl bond length is greater than the C —F bond length, and consequently, the value of d in the case of CH3Cl is higher than that in the case of CH3F.In practice is found that the value of (ex d) for CH3F is lower than that for the CH3Cl molecule. Hence, CH3Cl has a greater dipole moment (p) than that of CH3F.
Question 81. Show by calculation that (lie dipole moment of methane of zero.
Answer: Methane molecule is tetrahedral.
⇒ \(\begin{aligned}
& \mu_{\mathrm{CH}_3}=3 \mu_{\mathrm{C}-\mathrm{H}} \cos \left(180^{\circ}-109^{\circ} 28^{\prime}\right) \\
& =3 \mu_{C-H^{\prime}} \cos \left(70^{\circ} 32^{\prime}\right) \\
&
\end{aligned}\)
⇒ \(\begin{aligned}
& =\frac{3 \mu_{\mathrm{C}-\mathrm{H}}}{3}=\mu_{\mathrm{C}-\mathrm{H}} \\
\mu_{\mathrm{CH}_3} & =\mu_{\mathrm{C}-\mathrm{H}}=\mu_1 \text { (say) and } \theta=180^{\circ}
\end{aligned}\)
The resultant dipole moment
⇒ \(=\sqrt{\mu^2 \mathrm{C}-\mathrm{H}+\mu^2 \mathrm{CH}_3+2 \mu_{\mathrm{C}-\mathrm{H}} \cdot \mu_{\mathrm{CH}_3} \cos 180^{\circ}}\)
⇒ \(=\sqrt{\mu^2 \mathrm{C}-\mathrm{H}+\mu^2 \mathrm{CH}_3+2 \mu_{\mathrm{C}-\mathrm{H}} \cdot \mu_{\mathrm{CH}_3} \cos 180^{\circ}}\)
Alternative method: For convenience, the four H-atoms of methane are designated as H1, H2, H3, And H4. The resultant moments of H1—C and H2—C bonds will be along the bisector of the H1—C—H2 angle, towards the carbon atom.
Similarly, the resultant of H3 —C and H4—C bond moments are along the bisector of the H3—C—H4 angle, towards the C-atom. These two resultants are equal in magnitude and opposite in direction. Hence, the molecule of methane has no resultant dipole moment.
Question 82. The boiling point of hydrogen fluoride is maximum among aU the halogen acids—explain.
Answer: Fluorine is the smallest and the most electronegative element Hence F atom in the HF molecule forms strong intermolecular hydrogen bonds. On the other hand, since Cl, Br, and atoms are larger and less electronegative than F, their molecules are held by weak van der Waals forces.
As the hydrogen bond is stronger than the van der Waals forces, more energy is required to break the intermolecular hydrogen bonds of the associated HF molecules. Therefore the boiling point of HF is much higher than those of the other halogen acids. The boiling point of halogen acids follows the order: HF >HI > HBr > HCl
Question 83. Both CO2 and N2O are linear. However, N2O is polar while CO2 is non-polar—explain.
Answer: The structures of CO2 and N2O are:
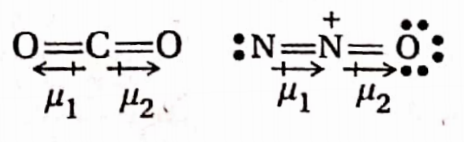
The CO2 molecule is linear with a C atom at the center. The CO2 molecule contains 2 polar C—O bonds, as oxygen is more electronegative than carbon. in CO2 molecule, the two bond dipoles (Cδ+—Oδ-) =pl, actin opposite directions and cancel each other. As a result, the resultant dipole moment becomes zero. Thus CO2 molecule is non-polar.
On the other hand, the N2O molecule, though linear, contains a polar coordinate bond (N→O) at one end and a lone pair of electrons on the N-atom at the other end. Moment due to N— o bond and that due to lone pair acting in opposition but they do not cancel each other as they are not equal in magnitude. Hence N2O has a resultant dipole moment although it has a low value. Therefore N2O is a polar molecule.
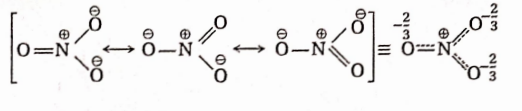
Question 84. Explain why all three nitrogen-oxygen bonds in the NO-3 ion are equal in length.
Answer: The nitrate ion (NO-3) can be represented as a resonance hybrid of the following three equivalent resonance structures or canonical forms: Since the hybrid structure is an average structure, so all N —O bond lengths are equal as shown above.
Question 85. Although H2, Li2, and B2 molecules have the same bond order 1), they are not equally stable. Explain this observation and arrange them in order of decreasing stability.
Answer: This observation can be explained as follows. The Li atom is much larger than the H atom. Hence, the Li—Li bond is much longer than the H —H bond (Li —Li bond length = 265 pm, H—H bond length = 74 pm).
Moreover, the Li2 molecule has two electrons in the antibonding σ*1s orbital while H2 has no electron in the antibonding orbitaL For these reasons, Li2 is less stable than H2 (bond energy of Li2 = H2O kJ. mol-1 while that of H2 = 438kJ. mol-1).
B atom is smaller in size than the Li-atom but larger than the H-atom. Hence the bond length of B2 is in-between (159 pm). Moreover, the B2 molecule has two electrons more in the bonding molecular orbitals [n(2px) and (2py)] Therefore, B2 is more stable than Li2 but less stable than H2 (bond energy of B2 = 290kJ – mol-1). Hence, the order of decreasing stability of these three molecules is H2 > B2 > Li2.
Chemical Bonding And Molecular Structure Very Short Type Questions
Question 1. What will be the nature of the compound formed between the metallic elements of groups 1 and 2 and non-metals of groups 6 or 7 of the periodic table?
Answer: Ionic compound;
Question 2. In terms of ionization enthalpy and electron gain enthalpy, which type of atoms combine to form an ionic compound?
Answer: A metal atom with low ionization enthalpy and a non-metal atom with high electron-gain enthalpy;
Question 3. Write the Lewis symbols of magnesium and aluminum.
Answer: Mg, Al2;
Question 4. Write the structure of an anion which is isostructural with BF3 and a cation which is isostructural with CH4
Answer: NO3 (triangular planar), NH+ (tetrahedral)
Question 5. Give an example of a compound in which electrovalent, covalent, and coordinate covalent bonds are present.
Answer: NH4Cl
Question 6. Which one of CHCI3 and CCl4 is regular tetrahedral in shape?
Answer: CCl4
Question 7. How many cr and n -bonds are present in CH2=CH—CH=CH2?
Answer: No. of bonds = 9 and no. of n -bonds = 2
Question 8. What is the hybrid state of the central atom in each of the following? BF-, NO2, PF5, CO-2
Answer: sp3, sp2 sp3d and sp respectively;
Question 9. Predict the shapes using VSEPR theory: IF?, C1F3, SF6, BeCl2.
Answer: IF2 Pentagonal bipyramidal; ClF3 – T-shaped; SF6 – Octahedral; BeCl2 – linear;
Question 10. How many resonance structures can be written for SO4-?
Answer: 6
Question 11. Arrange the halogen hydracids in order of their decreasing boiling points.
Answer: HF > HCl > HBr > HI;
Question 12. Find out the non-polar molecules among CH3Cl, SF6, SO2, C2H64 and HO—<g>—OH.
Answer: SF6
Question 13. Which one is less viscous between HF and H2O?
Answer: HF; (Each HF molecule is involved in forming two H-bonds, whereas each H2O molecule is involved in forming four H-bonds.)
Question 14. Which one out of O2 and O2 exhibits the highest paramagnetism?
Answer: O2 (it has two unpaired electrons);
Question 15. How will you distinguish B2 from the following species having the same bond order: Li2, O2- and F2?
Answer: B2 is paramagnetic but Li2 O2- and F2 are diamagnetic;
Question 16. Is there any change in bond order if the electron is added to the bonding molecular orbital?
Answer: Bond order will increase.
Question 17. Thebond order of He+ ion is—\(-\frac{1}{2}, 1, \frac{3}{2}, \mathrm{O}\)
Answer: \(-\frac{1}{2}, 1, \frac{3}{2}, \mathrm{O}\)
Chemical Bonding And Molecular Structure Fill In The Blanks
Question 1. A _____________covalent bond is formed between two atoms having different electronegativities.
Answer: Polar
Question 2. Pi bonds are_____________than sigma bonds.
Answer: Weaker
Question 3. In different resonating structures, the arrangement remains the same.
Answer: Atomic
Question 4. When______________atomic_ whereas orbitals when overlapping head-on, overlap laterally, bond formed bond is formed is.
Answer: sigma bond; pi bond
Question 5. AlCl3 is_____________compound while PCl5 is compound in terms of the octet rule.
Answer: electron deficient, hypervalent
Question 6. The C.G.S unit of dipole moment is_____________whereas its SI unit is
Answer: Debye, Coulomb-metre (G-m)
Question 7. In general, larger is the bond length,_____________ bond energy.
Answer: Smaller
Question 8. The energy of-bond is
Answer: . 12.5 to 41.5 kJ mol-1
Question 9. The hybrid state of S in the SO3 molecule is
Answer: sp2
Question 10. The shape of the molecule contains 3 bond pairs and one lone pair around the central atom is
Answer: trigonal pyramids
Question 11. The bond order of CO molecule is ________whereas that of CO+ ion is
Answer: 3,3.5
Question 12. In ice, each O atom is surrounded by out of which, _____________H-atoms are bonded by covalent bonds, while the rest are bonded by bonds.
Answer: four, two, H-bonds
Question 13. The shape of sulfur hexafluoride molecule is whereas that of sulfur tetrafluoride is _________.
Answer: Regular octahedral, distorted tetrahedral
Question 14. There are_____________π bonds in a nitrogen molecule.
Answer: Two
Question 15. The strongest hydrogen bond is formed between ____________and hydrogen atom.
Answer: Fluorine
Question 16. A hydrogen bond is then a covalent bond.
Answer: Weaker
Question 17. The dipole moment of methyl alcohol Is ._____________ that of CH3SH.
Answer: Higher
Question 18. d2sp3 hybridisation represents
Answer: Octahedral
Question 19. Amongst the three isomers of nitrophenol, the one that is least soluble in water Is
Answer: Ortho-isomer
Question 20. Amongst N2O, SO2, 1+ and l2 , the linear species are and
Answer: N2O and I3-.

