Wave Optics Electromagnetic Waves
Question 1. The intensity ratio of the maxima and minima in an interference pattern produced by two identical coherent sources of light is 9: 1. The intensities of the used light sources are in the ratio
- 3:1
- 4:1
- 9:1
- 10:1
Answer: 2. 4:1
For maximum intensity, phase difference = Φ = 0 and for minimum intensity, Φ =180°.
Resultant intensity,
⇒ \(I=I_1+I_2+2 \sqrt{I_1 I_2} \cos \phi\)
⇒ \(I_{\max }=I_1+I_2+2 \sqrt{I_1 I_2}=\left(\sqrt{I_1}+\sqrt{I_2}\right)^2\),
and \(I_{\min }=I_1+I_2-2 \sqrt{I_1 I_2}=\left(\sqrt{I_1}-\sqrt{I_2}\right)^2\)
Given that \(\frac{I_{\max }}{I_{\min }}=\frac{9}{1}=\left[\frac{\sqrt{I_1}+\sqrt{I_2}}{\sqrt{I_1}-\sqrt{I_2}}\right]^2\)
⇒ \(\frac{\sqrt{I_1}+\sqrt{I_2}}{\sqrt{I_1}-\sqrt{I_2}}=\frac{3}{1} \Rightarrow \sqrt{\frac{I_1}{I_2}}=\frac{2}{1}\)
Hence, \(\frac{I_1}{I_2}=4: 1\).

Question 2. In an interference pattern, the intensities of two interfering waves are I and 4I respectively. They produce intensities at two points A and B with phase differences of TC/2 and π respectively. The difference in their intensities is
- I
- 2I
- 4I
- 5I
Answer: 3. 4I
Given that I1 = 1 and I2 = 4I.
Intensity at A is
⇒ \(I_{\mathrm{A}}=I_1+I_2+2 \sqrt{I_1 I_2} \cos \frac{\pi}{2}=I+4 I=5 I\)
Intensity at B is
⇒ \(I_{\mathrm{B}}=I_1+I_2+2 \sqrt{I_1 I_2} \cos \pi=I+4 I-2 \sqrt{I \cdot 4 I}=I\)
Difference in intensities = IA – IB = 5I – I = 4I.
Read And Learn Also NEET Physics Multiple Choice Question and Answers
Question 3. In Young’s double-slit experiment, the two slits act as coherent sources of equal amplitude a and of wavelength λ. In another experiment with the same set-up, the two slits are sources of equal amplitude a and wavelength λ but are incoherent. The ratio of intensities of light at the midpoint of the screen in the first case to that in the second case is
- 2:1
- 1:2
- 3:4
- 4:3
Answer: 1. 2:1
When the sources are coherent, the intensity at the central maxima (where phase difference Φ = 0), I1 = k(a + a)2– k4a2.
When the sources are incoherent, there is no phase relationship, hence no sustained interference. The net intensity, I2 = k(a2 + a2 ) = k2a2.
∴ \(\text { ratio }=\frac{I_1}{I_2}=\frac{k\left(4 a^2\right)}{k\left(2 a^2\right)}=2: 1\)
wave optics mcqs
Question 4. What is the path difference for destructive interference?
- n
- \(\frac{(n+1)}{2} \lambda\)
- n(λ + 1)
- \(\left(\frac{2 n+1}{2}\right) \lambda\)
Answer: 4. \(\left(\frac{2 n+1}{2}\right) \lambda\)
For destructive interference,
∴ path difference A = odd multiple of \(\frac{\lambda}{2}=(2 n+1) \frac{\lambda}{2}\)
Question 5. A monochromatic beam of light is used for the formation of fringes on the screen by illuminating the two slits in Young’s double-slit experiment. When a thin film of mica is interposed in the path of one of the interfering beams then
- Die fringe width increases
- The fringe width decreases
- The fringe width remains the same but the pattern shifts
- The fringe pattern disappears
Answer: 3. The fringe width remains the same but the pattern shifts
When a thin film of mica sheet covers one of the two slits, a path difference Δ = (μ – l)f is introduced in one path of interfering waves. So, there will be no change in the fringe width but the central maximum with the whole fringe pattern will shift.
Question 6. A double-slit experiment is performed with a light of wavelength 500 nm. A thin film of thickness 2 μm and a refractive index of 1.5 is introduced in the path of the upper beam. The location of the central maximum will
- Remain unshifted
- Shift downward by nearly two fringes
- Shift upward by nearly two fringes
- Shift downward by five fringes
Answer: 3. Shift upward by nearly two fringes
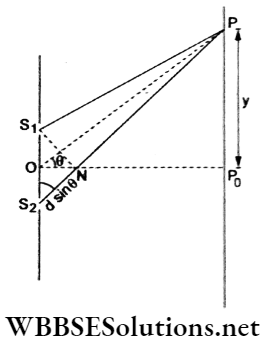
Given that λ = 500 nm, thickness t = 2 pm and μ =1.5.
The optical path introduced for wave originating from the upper slit is Δ = (μ-l)t.
Central maximum P0 will shift to P where the path difference is zero.
Thus, at P, S2P – S1P – (μ-l)t = 0
=> dsinθ =(μ-l)t
⇒ \(d \frac{y}{D}=(\mu-1) t\)
⇒ \(y=\frac{D}{d}(\mu+1) t=N \beta=N \frac{D \lambda}{d}\)
The Number of fringes shifted is
∴ \(N=\frac{(\mu-1) t}{\lambda}=\frac{(1.5-1)\left(2 \times 10^{-6} \mathrm{~m}\right)}{\left(500 \times 10^{-9} \mathrm{~m}\right)}=2\)
Two fringes will shift upward.
Question 7. Young’s double-slit experiment is first performed in air and then in a medium other than air. It is found that the 8th bright fringe in the medium lies where the 5th dark fringe lies in the air. The refractive index of the medium is nearly
- 1.25
- 1.50
- 1.69
- 1.78
Answer: 4. 1.78
When in the air, the position of the 5th dark fringe from the centre is
⇒ \(y=\left(4+\frac{1}{2}\right) \beta_{\mathrm{a}}=\frac{9}{2} \beta_{\mathrm{a}}=\frac{9}{2} \frac{D \lambda_{\mathrm{a}}}{d}\)
In the medium, the position of the 8th bright fringe is
⇒ \(y=8 \beta_m=\frac{8 D \lambda_m}{d}\)
⇒ \(\frac{9}{2} \frac{D}{d} \lambda_{\mathrm{a}}=\frac{8 D}{d} \lambda_{\mathrm{m}}\)
The refractive index of the medium is
∴ \(\mu_{\mathrm{m}}=\frac{\lambda_{\mathrm{a}}}{\lambda_{\mathrm{m}}}=\frac{8}{4.5}=\frac{16}{9}=1.78\)
Question 8. Two coherent monochromatic light beams of amplitudes 3 and 5 units are superposed. The ratio of the maximum and minimum intensities in the resulting interference pattern will be
- 4: 1
- 16:1
- 1:4
- 2:1
Answer: 2. 16:1
Given that the ratio of amplitudes of interfering waves is
⇒ \(\frac{A_1}{A_2}=\frac{5}{3}\)
Now, \(\frac{A_1+A_2}{A_1-A_2}=\frac{5+3}{5-3}=\frac{8}{2}=\frac{4}{1}\)
Hence, \(\frac{I_{\max }}{I_{\min }}=\frac{\left(A_1+A_2\right)^2}{\left(A_1-A_2\right)^2}=\left(\frac{4}{1}\right)^2=16: 1\)
wave optics questions
Question 9. The two coherent sources with intensity ratio β produce interference. The fringe visibility will be
- \(\frac{2 \sqrt{\beta}}{1+\beta}\)
- 2β
- \(\frac{2}{1+\beta}\)
- \(\frac{\sqrt{\beta}}{1+\beta}\)
Answer: 1. \(\frac{2 \sqrt{\beta}}{1+\beta}\)
Given that the intensity ratio of two coherent waves is
⇒ \(\frac{I_1}{I_2}=\beta\)
Fringe visibility is defined as
\(V=\frac{I_{\max }-I_{\min }}{I_{\max }+I_{\min }}\) → (1)
Now, \(I_{\max }=\left(\sqrt{I_1}+\sqrt{I_2}\right)^2 ; I_{\min }=\left(\sqrt{I_1}-\sqrt{I_2}\right)^2\)
Substituting these in (1),
∴ \(V=\frac{4 \sqrt{I_1 I_2}}{2\left(I_1+I_2\right)}=\frac{2 I_2 \sqrt{\beta}}{I_2(\beta+1)}=\frac{2 \sqrt{\beta}}{1+\beta}\).
Question 10. Two slits are separated by a distance of 0.5 mm and illuminated with a light of wavelength λ = 600 nm. If the screen is placed at 2.5 m from the slits, the distance of the third bright fringe from the centre will be
- 1.5 mm
- 3 mm
- 6 mm
- 9 mm
Answer: 4. 9 mm
Given that slit separation = d = 0.5 mm;
wavelength = λ, = 600 nm; D = 2.5 m.
Fringe width is
⇒ \(\beta=\frac{D \lambda}{d}=\frac{(2.5 \mathrm{~m})\left(600 \times 10^{-9} \mathrm{~m}\right)}{\left(0.5 \times 10^{-3} \mathrm{~m}\right)}=3000 \times 10^{-6} \mathrm{~m}=3 \mathrm{~mm}\)
Distance of the third bright fringe = 3β = 3 x 3 mm = 9 mm.
Question 11. In a double-slit experimental arrangement, interference fringes of width 1 mm each are observed when light of wavelength 500 nm is used. Keeping the set-up unaltered, if the light source is replaced by another of wavelength 600 nm, the fringe width will be
- 1.2 mm
- 0.5 mm
- 1 mm
- 1.5 mm
Answer: 1. 1.2 mm
Fringe width = \(\beta=\frac{D \lambda}{d} \Rightarrow \beta \propto \lambda\)
Ratio \(\frac{\beta_1}{\beta_2}=\frac{\lambda_1}{\lambda_2}=\frac{500 \mathrm{~nm}}{600 \mathrm{~nm}}=\frac{5}{6}\)
Given that \(\beta_1=1 \mathrm{~mm}, \text { hence } \beta_2=\frac{6}{5} \beta_1=\frac{6}{5}(1 \mathrm{~mm})=1.2 \mathrm{~mm}\)
Question 12. In Young’s double-slit experiment, the spacing between the slits is 0.3 mm and the screen is kept at a distance of 1.5 m. The second bright fringe is found at 6 mm from the central fringe. The wavelength of the light used in the experiment is
- 625 nm
- 600 nm
- 550 nm
- 500 nm
Answer: 2. 600 nm
Given that d = 0.3 mm, D =1.5 m, 2β = 6 mm.
Now, \(\beta=3 \mathrm{~mm}=\frac{D \lambda}{d}=\frac{(1.5 \mathrm{~m})(\lambda)}{(0.3 \mathrm{~mm})}=5 \times 10^3 \lambda\)
Wavelength \(\lambda=\frac{3 \mathrm{~mm}}{5 \times 10^3}=0.6 \times 10^{-6} \mathrm{~m}=600 \mathrm{~nm}\)
Question 13. In Young’s double-slit experiment if the fringe order is represented by m then the fringe width is
- Independent of m
- Directly proportional to m
- Directly proportional to (2m +1)
- Inversely proportional to (2m +1)
Answer: 1. Independent of m
In the double-slit experiment, all the interference fringes are of the same width \(\left(\beta=\frac{D \lambda}{d}\right)\) and are independent of the fringe order m.
Question 14. Which among the following cannot produce coherent sources?
- Fresnel biprism
- Lloyd’s mirror
- Young’s double-slit
- Prism
Answer: 4. Prism
A single prism can produce dispersion, deviation, spectrum, etc., but cannot produce coherent sources.
Question 15. In Young’s double-slit experiment if the separation between the two slits is made three times then the fringe width will become
- 9 times
- \(\frac{1}{9} \text { times }\)times
- 3 times
- \(\frac{1}{3} \text { times }\)times
Answer: 4. \(\frac{1}{3} \text { times }\)times
Fringe width = \(\beta=\frac{D \lambda}{d}\)
If D and X are constants, \(\beta \propto \frac{1}{d}\)
⇒ \(\frac{\beta_1}{\beta_2}=\frac{d_2}{d_1}\)
Given that d2 = 3d1
Hence, \(\beta_2=\frac{\beta_1}{3}\)
Question 16. When exposed to sunlight, a thin film of oil on water exhibits brilliant colours due to the phenomenon of
- Diffraction
- Interference
- Polarization
- Dispersion
Answer: 2. Interference
Sunlight incident on a thin film of oil spread over the surface of water undergoes multiple reflections and interference. Bright colours are obtained which satisfy the condition for maxima.
wave optics questions with answers pdf
Question 17. When a compact disc (CD) is illuminated by a source of white light, coloured lines are observed. This is due to
- Interference
- Dispersion
- Refraction
- Diffraction
Answer: 4. Diffraction
A compact disc (CD) consists of thousands of pits arranged in the form of spiral tracks. Visible light incident on the pits gets diffracted and constituent colours of the polychromatic light get separated and rainbow-like colours are observed.
Question 18. Young’s double-slit experiment is performed first in the air and then in a medium with a refractive index of 1.78. It is observed that the position of the nth bright fringe in the medium coincides with the 5th dark fringe in the air. The fringe order n has the value
- 5
- 7
- 8
- 10
Answer: 3. 8
Let y be the position of the 5th dark fringe in the air, so
⇒ \(y=\left(n+\frac{1}{2}\right) \beta=\left(4+\frac{1}{2}\right) \beta=\frac{9}{2} \frac{D \lambda_{\mathrm{a}}}{d}\)
In a medium of refractive index JI =1.78, wavelength = \(\lambda^{\prime}=\frac{\lambda_{\mathrm{a}}}{\mu}=\frac{\lambda_{\mathrm{a}}}{1.78}\)
the position of the nth bright fringe is
⇒ \(y^{\prime}=n \beta^{\prime}=n \frac{D \lambda^{\prime}}{d}=\frac{n D}{d} \frac{\lambda_{\mathrm{a}}}{1.78}\)
Given that \(y=y^{\prime}, \text { hence } \frac{9}{2} \frac{D \lambda_{\mathrm{a}}}{d}=\frac{n}{1.78} \frac{D \lambda_{\mathrm{a}}}{d}\)
∴ \(\frac{9}{2}=\frac{n}{1.78} \Rightarrow n=\frac{9 \times 1.78}{2}=8.01 \approx 8\)
Question 19. The intensity of the maximum in Young’s double-slit experiment is I0. The distance between the two slits is d = 5λ, where λ is the wavelength of light used in the experiment. What will be the intensity in front of one of the slits on the screen placed at a distance D =10d?
- \(\frac{I_0}{4}\)
- \(\frac{3}{4} I_0\)
- \(\frac{I_0}{2}\)
- I0
Answer: 3. \(\frac{I_0}{2}\)
Let I be the intensity of the two slits. At the maximum intensity,
⇒ \(I_0=I_1+I_2+2 \sqrt{I_1 I_2} \cos \phi\)
⇒ I + I + 2Icos 0° = 4I
\(I=\frac{I_0}{4}\) → (1)
Path difference at point P (in front of slit S1) is
⇒ \(\Delta=S_2 P-S_1 P=\sqrt{D^2+d^2}-D\)
⇒ \(D\left(1+\frac{d^2}{D^2}\right)^{1 / 2}-D=D+\frac{d^2}{2 D}-D=\frac{d^2}{2 D}\)
Given that d = 5λ and D = l0d =10(5λ) = 50λ.
path difference = \(\Delta=\frac{25 \lambda^2}{2(50 \lambda)}=\frac{\lambda}{4}\)
and phase difference = \(\phi=\frac{2 \pi}{\lambda} \Delta=\frac{\pi}{2}\)
Hence, the intensity at P is
∴ \(I_{\mathrm{p}}=I+I+2 \sqrt{I I} \cos \frac{\pi}{2}=2 I=2\left(\frac{I_0}{4}\right)=\frac{I_0}{2}\) [From (1)]
Question 20. An interference pattern is obtained with two coherent light sources of intensity ratio n. In the interference pattern, the ratio \(\frac{I_{\max }-I_{\min }}{I_{\max }+I_{\min }}\) will be
- \(\frac{\sqrt{n}}{n+1}\)
- \(\frac{2 \sqrt{n}}{n+1}\)
- \(\frac{\sqrt{n}}{(n+1)^2}\)
- \(\frac{2 \sqrt{n}}{(n+1)^2}\)
Answer: 2. \(\frac{2 \sqrt{n}}{n+1}\)
Given that = \(\frac{I_1}{I_2}=n=\frac{a_1^2}{a_2^2}\), where a1 and a2 are the amplitudes of the coherent sources.
Hence, \(\frac{a_1}{a_2}=\frac{\sqrt{n}}{1}\).
⇒ \(\frac{\left(a_1+a_2\right)}{\left(a_1-a_2\right)}=\frac{\sqrt{n}+1}{\sqrt{n}-1} \Rightarrow \frac{\left(a_1+a_2\right)^2}{\left(a_1-a_2\right)^2}=\frac{(\sqrt{n}+1)^2}{(\sqrt{n}-1)^2} \Rightarrow \frac{I_{\max }}{I_{\min }}=\frac{(\sqrt{n}+1)^2}{(\sqrt{n}-1)^2}\)
∴ \(\frac{I_{\max }-I_{\min }}{I_{\max }+I_{\min }}=\frac{(\sqrt{n}+1)^2-(\sqrt{n}-1)^2}{(\sqrt{n}+1)^2+(\sqrt{n}-1)^2}=\frac{2 \sqrt{n}}{n+1}\)
wave optics questions with answers pdf
Question 21. In a double-slit experiment, the two slits are 1 mm apart and the screen is placed 1 m away. A monochromatic light of wavelength 500 run is used. What will be the width of each slit for obtaining ten maxima of double-slit within the principal maxima of a single-slit pattern?
- 0.2 mm
- 0.1mm
- 0.5 mm
- 0.02 mm
Answer: 1. 0.2 mm
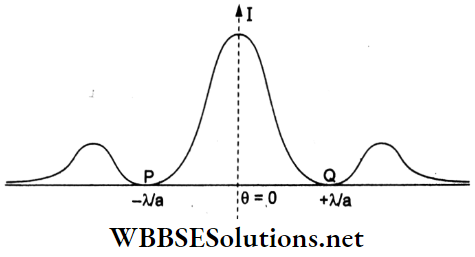
In the given figure, intensity distribution due to a single-slit diffraction pattern is shown. The angular width of the principal maxima is \(\frac{2 \lambda}{a}\), where a = width of each slit.
The linear width is PQ = D (angular width), where D is the distance between the screen and the slits.
\(P Q=\frac{2 \lambda}{a} \cdot D\) → (1)
Given that 10 double-slit fringes are within PQ, we have
\(P Q=10 \beta=10 \frac{D \lambda}{d}\) → (2)
Equating (1) and (2)
⇒ \(\frac{2 \lambda}{a} D=\frac{10 D \lambda}{d}\)
∴ \(a=\frac{d}{5}=\frac{1 \mathrm{~mm}}{5}=0.2 \mathrm{~mm}\)
Question 22. Two slits in Young’s experiment have widths in the ratio 1: 25. The ratio of intensity at the maxima to the minima in the interference pattern \(\left(\frac{I_{\max }}{I_{\min }}\right)\) is
- \(\frac{9}{4}\)
- \(\frac{121}{49}\)
- \(\frac{49}{121}\)
- \(\frac{4}{9}\)
Answer: 1. \(\frac{9}{4}\)
Assuming that slit width is proportional to the intensity of light, we have
⇒ \(\frac{I_1}{I_2}=\frac{25}{1}=\left(\frac{a_1}{a_2}\right)^2, \text { where } \frac{a_1}{a_2}\) is the ratio of amplitudes.
⇒ \(\frac{a_1}{a_2}=\frac{5}{1} \Rightarrow \frac{a_1+a_2}{a_1-a_2}=\frac{5+1}{5-1}=\frac{3}{2}\)
Hence, \(\frac{I_{\max }}{I_{\min }}=\frac{\left(a_1+a_2\right)^2}{\left(a_1-a_2\right)^2}=\frac{9}{4}\)
Question 23. In Young’s double-slit experiment, the intensity of light at a point on the screen where the path difference is λ (λ being the wavelength of the light used) is k. The intensity at a point where the path difference is λ/A will be
- k
- \(\frac{k}{2}\)
- \(\frac{k}{4}\)
- Zero
Answer: 2. \(\frac{k}{2}\)
Let I be the intensity of the component of the interfering sources.
For path difference Δ = λ, phase difference = 2π
and intensity k = I + I + 2√II cos 2π = 4I
⇒ \(I=\frac{k}{4}\)
For path difference = \(\Delta=\frac{\lambda}{4}, \text { phase difference }=\phi=\frac{2 \pi}{\lambda} \frac{\lambda}{4}=\frac{\pi}{2}\).
∴ intensity = \(I^{\prime}=I+I+2 \sqrt{I I} \cos \frac{\pi}{2}=2 I=2\left(\frac{k}{4}\right)=\frac{k}{2}\).
Question 24. In Young’s double-slit experiment, the slits are 2 mm apart and are illuminated by photons of two wavelengths λ1 = 1200 run and λ2 = 1000 nm. At what minimum distance from the common central bright fringe on the screen, 2 m away from the slits will a bright fringe from one interference pattern coincide with a bright fringe from the other?
- 8 mm
- 6 mm
- 4 mm
- 3 mm
Answer: 2. 6 mm
Given that slit separation d = 2 mm, λ1 =1200 run, λ2 =1000 run and D = 2 m.
Fringe width = \(\beta=\frac{D \lambda}{d}\)
Since λ1 > λ2, so β1 > β2.
Let nth bright fringe of λ1 coincide with (n + 1)th bright fringe of λ2.
So, \(n \frac{D \lambda_1}{d}=(n+1) \frac{D \lambda_2}{d}\)
⇒ n(1200 run) = (n +1)(1000 run)
⇒ n = 5.
Hence, the minimum distance from the common centre is
∴ \(5 \beta_1=\frac{5 D \lambda_1}{d}=\frac{5(2 \mathrm{~m})\left(1200 \times 10^{-9} \mathrm{~m}\right)}{\left(2 \times 10^{-3} \mathrm{~m}\right)}=6 \mathrm{~mm}\).
wave optics questions with answers pdf
Question 25. Interference is possible in
- Light waves only
- Sound waves only
- Both light and sound waves
- Neither light nor sound waves
Answer: 3. Both light and sound waves
Interference is a wave phenomenon, which is observed in longitudinal (sound waves) as well as in transverse waves (light waves).
Question 26. If a yellow light emitted by a sodium lamp in Young’s double-slit experiment is replaced by a monochromatic blue light of the same intensity,
- Fringe width will decrease
- Fringe width will increase
- Fringe width will remain unchanged
- Fringes will become less intense
Answer: 1. Fringe width will decrease
Fringe width = \(\beta=\frac{D \lambda}{d}\)
λyellow > λblue.
Hence, βyellow > βblue.
Fringe width will decrease.
Question 27. In Young’s experiment, two coherent sources are placed 0.90 mm apart and a fringe pattern is observed one metre away. If it produces a second dark fringe at a distance of 1 mm from the central fringe, the wavelength of monochromatic light used is
- 60 x 10-4 cm
- 10 x 10-4 cm
- 10 x 10-5 cm
- 6 x 10-5 cm
Answer: 4. 6 x 10-5 cm
Given that d = 0.90 mm and D =1.0 m.
The distance of the 2nd dark fringe from the central fringe is
⇒ \(\beta+\frac{\beta}{2}=\frac{3}{2} \beta=1 \mathrm{~mm}\)
∴ \(\frac{3}{2} \frac{D \lambda}{d}=1 \mathrm{~mm}\)
⇒ \(\lambda=\frac{2 d}{3 D} \times 1 \mathrm{~mm}=\frac{2}{3}\left(\frac{9 \times 10^{-4} \mathrm{~m}}{1.0 \mathrm{~m}}\right)(1 \mathrm{~mm})=6 \times 10^{-7} \mathrm{~m}=6 \times 10^{-5} \mathrm{~cm}\).
Question 28. In Young’s double-slit experiment, the fringe width is found to be 0.4 mm. If the whole set-up is immersed in water of refractive index \(\frac{4}{3}\) without any change in the set-up, the new fringe width would be
- 0.30 mm
- 0.40 mm
- 0.53 mm
- 450 microns
Answer: 1. 0.30 mm
Given that β = 0.4 mm (in air). Let it be β’ in water.
∴ \(\frac{\beta^{\prime}}{\beta}=\frac{D \lambda^{\prime} / d}{D \lambda / d}=\frac{\lambda^{\prime}}{\lambda}=\frac{f \lambda^{\prime}}{f \lambda}=\frac{c}{c_0}=\frac{1}{\mu}\)
∴ \(\beta^{\prime}=\frac{\beta}{\mu}=\frac{0.4 \mathrm{~mm}}{4 / 3}=0.3 \mathrm{~mm}\)
wave optics questions with answers pdf
Question 29. A Young’s double-slit experiment is performed with blue and green lights of wavelengths 4360 A and 5460 A respectively. If X be the distance of the 4th maxima from the central one then
- X (blue) = X (green)
- X (blue) > X (green)
- X (blue) < X (green)
- \(\frac{X \text { (blue) }}{X \text { (green) }}=\frac{5460}{4360}\)
Answer: 3. X (blue) < X (green)
Given that λblue = 4360 Å and λgreen = 5460 Å.
Since fringe width = \(\beta=\frac{D \lambda}{d}\)
⇒ \(\beta_{\text {blue }}<\beta_{\text {green }}\)
⇒ \(4 \beta_{\text {blue }}<4 \beta_{\text {green }}\)
∴ \(X_{\text {blue }}<X_{\text {green }}\)
Question 30. An achromatic combination of lenses consists of
- Two convex lenses in contact
- Two concave lenses in contact
- One convex and one concave lens in contact
- One convex and one plane mirror in contact
Answer: 3. One convex and one concave lens in contact
An achromatic combination of lenses constitutes a combination of two lenses of different materials satisfying the condition \(\frac{\omega_1}{f_1}+\frac{\omega_2}{f_2}=0\).
As f1 and f2 are of opposite signs, one is convex and the other concave.
| Class 11 Physics | Class 12 Maths | Class 11 Chemistry |
| NEET Foundation | Class 12 Physics | NEET Physics |
Question 31. The focal length of a convex lens will be maximum for
- Blue light
- Yellow light
- Greenlight
- Red light
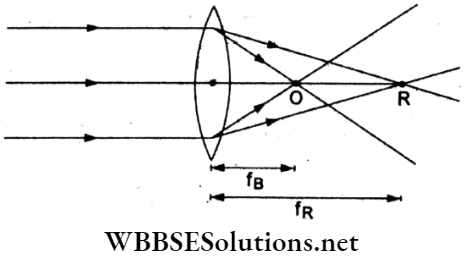
Answer: 4. Red light
Sunlight emerging from a convex lens undergoes dispersion, and blue light converges most and red light the least as shown in the given.
Hence, fred is the maximum.
This can also be seen in the relation
⇒ \(\frac{1}{f}=(\mu-1)\left(\frac{1}{R_1}-\frac{1}{R_2}\right)\)
Now, \(\mu_{\text {blue }}>\mu_{\text {green }}>\mu_{\text {yellow }}>\mu_{\text {red }}\)
∵ μ is least for red, \(\frac{1}{f_{\text {red }}}\) is least, hence is maximum.
Question 32. Cauchy’s dispersion formula is
- \(\mu=A+B \lambda^{-2}+C \lambda^{-4}\)
- \(\mu=A+B \lambda^{-2}+C \lambda^4\)
- \(\mu=A+B \lambda^2+C \lambda^{-4}\)
- \(\mu=A+B \lambda^2+C \lambda^4\)
Answer: 1. \(\mu=A+B \lambda^{-2}+C \lambda^{-4}\)
Cauchy’s dispersion formula is an empirical relationship between the refractive index and the wavelength of light for a particular transparent material. It is expressed as
⇒ \(\mu=A+\frac{B}{\lambda^2}+\frac{C}{\lambda^4}\)
where μ = refractive index and A, B, and C are constants.
Question 33. If the red light is replaced by a white light then
- The width of the diffraction pattern will increase
- The width of the diffraction pattern will decrease
- A central white band will be obtained
- No effect will be observed
Answer: 3. A central white band will be obtained
White light is a combination of seven colours. When diffracted through a single slit, the principal maxima will be white where all the components superpose in the same phase
Question 34. Greater accuracy in the determination of the position of a particle with an optical microscope can be had if the beam of light used
- Has higher wavelength
- Is polarized
- Has higher frequency
- Has greater intensity
Answer: 3. Has higher frequency
For a microscope, resolving power = \(\frac{2 \mu \sin \theta}{1.22 \lambda}\)
For higher resolution, λ should be small which means that a light of higher frequency should be used.
Question 35. The ratio of the resolving power of an optical microscope for two wavelengths λ1 = 400 nm and λ2 = 600 nm is
- 9:4
- 3:2
- 16: 81
- 8: 27
Answer: 2. 3:2
Resolving power of an optical instrument \(\propto \frac{1}{\lambda}\)
⇒ \(\frac{R_1}{R_2}=\frac{\lambda_2}{\lambda_1}=\frac{600 \mathrm{~nm}}{400 \mathrm{~nm}}=\frac{3}{2}\).
Question 36. A linear aperture whose width is 0.20 mm is placed immediately in front of a lens of focal length 60 cm. The aperture is illuminated normally by a parallel beam of monochromatic light of wavelength 500 nm. The distance of the first dark band of the diffraction pattern from the centre of the screen is
- 0.10 cm
- 0.25 cm
- 0.20 cm
- 0.15 cm
Answer: 4. 0.15 cm
Given that slit width a = 0.2 mm, λ = 500 nm and f = D = 60 cm.
Diffracted rays will be focused by a convex lens at its focal plane, so D = f.
The angular position of the first dark band is \(\theta_1=\frac{\lambda}{a}\) and its distance from the centre of the screen is
∴ \(y=D \theta=D \frac{\lambda}{a}=\frac{(60 \mathrm{~cm})(500 \mathrm{~nm})}{(0.2 \mathrm{~mm})}=0.15 \mathrm{~cm}\)
Question 37. In a diffraction pattern due to a single slit of width a, the first minimum is observed at an angle of 30° when light of wavelength 500 nm is incident on the slit. The first secondary maximum is observed at an angle of
- \(\sin ^{-1}\left(\frac{1}{2}\right)\)
- \(\sin ^{-1}\left(\frac{3}{4}\right)\)
- \(\sin ^{-1}\left(\frac{2}{3}\right)\)
- \(\sin ^{-1}\left(\frac{1}{4}\right)\)
Answer: 2. \(\sin ^{-1}\left(\frac{3}{4}\right)\)
The angular position of the first minimum = θ1
where sin \(\theta_1=\frac{\lambda}{a}=\sin 30^{\circ} \Rightarrow \frac{\lambda}{a}=\frac{1}{2}\)
Let the angular position of the first maximum be 01:
⇒ \(\sin \theta_1^{\prime}=\frac{3}{2} \frac{\lambda}{a}=\frac{3}{2}\left(\frac{1}{2}\right)=\frac{3}{4}\)
∴ \(\theta_1^{\prime}=\sin ^{-1}\left(\frac{3}{4}\right)\).
Question 38. At the first minimum adjacent to the central maximum of a single-slit diffraction pattern, the phase difference between the Huygens wavelet from the edge of the slit and the wavelet from the midpoint of the slit is
- π radian
- \(\frac{\pi}{2}\) radian
- \(\frac{\pi}{4}\) radian
- \(\frac{\pi}{8}\) radian
Answer: 1. π radian
While explaining the diffraction pattern due to a single slit, the unobstructed wavefront through the slit divided into 2, 3, and 4, parts take the path difference \(\Delta=a \sin \theta \text { as } \lambda, \frac{3}{2} \lambda, 2 \lambda\),…, etc. For the first minimum, the Huygens wavelets originating from the edge of the slit and from the midpoint of the slit are in opposite phases (phase difference = π) so that all cancel out leading to minimum intensity.
Question 39. For a parallel beam of monochromatic light of wavelength λ, diffraction is produced by a single slit whose width a is of the order of the wavelength of light. If D is the distance of the screen from the slit, the width of the central maxima will be
- \(\frac{D a}{\lambda}\)
- \(\frac{2 D a}{\lambda}\)
- \(\frac{2 D \lambda}{a}\)
- \(\frac{D \lambda}{a}\)
Answer: 3. \(\frac{2 D \lambda}{a}\)
The angular width of the central maxima is
⇒ \(2 \theta_1=2\left(\frac{\lambda}{a}\right)\)
So, the linear width of the central maxima is
∴ \(y=D\left(2 \theta_1\right)=2 \frac{D \lambda}{a}\)
Question 40. A beam of light of wavelength 600 nm from a distant source falls on a single slit 1 mm wide. The resulting diffraction pattern is observed on a screen 2 m away. The distance between the first dark fringes on either side of the central bright fringe is
- 1.2 cm
- 2.4 cm
- 1.2 mm
- 2.4 mm
Answer: 4. 2.4 mm
Given that λ, = 600 nm, slit width = a = 1 mm and D = 2 m.
The distance between the first minima on either side of the central bright is the width of the principal maxima which is
⇒ \(y=D\left(2 \theta_1\right)=2 \frac{D \lambda}{a}\)
⇒ \(\frac{2(2 \mathrm{~m})\left(600 \times 10^{-9} \mathrm{~m}\right)}{\left(1 \times 10^{-3} \mathrm{~m}\right)}=2.4 \mathrm{~mm}\).
Question 41. A parallel beam of fast-moving electrons is incident normally in a narrow slit. A fluorescent screen is placed at a large distance from the slit. If the speed of the electrons is increased, which of the following statements is true?
- The angular width of the central maximum will decrease.
- The angular width of the central maximum of the diffraction pattern will increase.
- The angular width of the central maximum will remain unaffected.
- Diffraction patterns will not be observed on the screen in the case of electrons.
Answer: 1. The angular width of the central maximum will decrease.
The de Broglie wavelength of fast-moving electrons is \(\lambda=\frac{h}{m v}\), which decreases with the increase in speed v. Hence, the angular width of the central maximum, \(2 \theta=\frac{2 \lambda}{a}\) will also decrease.
Question 42. A beam of monochromatic light of wavelength λ is incident normally on a narrow slit. A diffraction pattern is formed screen placed perpendicular to the direction of the incident beam. At the second minimum of the diffraction pattern, the phase difference between the rays coming from the two edges of the slit is
- 2π
- 3π
- 4π
- πλ
Answer: 3. 4π
In order to describe the pattern of diffraction for the 2nd minima, we divide the unobstructed wavefront through the slit into four equal segments so that the 1st and 2nd as well as the 3rd and 4th cancel out. The path difference for wavelets originating from the extreme edges is 2A, and the equivalent phase difference is \(\phi=\frac{2 \pi}{\lambda}(2 \lambda)=4 \pi\).
Question 43. The angular resolution of a 10-cm-diameter telescope at a wavelength of 600 nm is of the order of
- 106 rad
- 10-2 rad
- 10-4 rad
- l0-6 rad
Answer: 4. l0-6 rad
Angular resolution = \(\Delta \theta=1.22 \frac{\lambda}{D}\),
Where = wave length = 600 nm and D = diameter = 10cm.
∴ \(\Delta \theta=\frac{1.22\left(600 \times 10^{-9} \mathrm{~m}\right)}{\left(10 \times 10^{-2} \mathrm{~m}\right)}=7.32 \times 10^{-6} \mathrm{rad} \approx 10^{-6} \mathrm{rad}\).
Question 44. A telescope has an objective lens of 10 cm diameter and is situated at a distance of one kilometre from two objects. The minimum distance between these two objects which can be resolved by the telescope when the mean wavelength of light is 500 nm, is of the order of
- 0.5 m
- 5 m
- 5 mm
- 5 cm
Answer: 3. 5 mm
According to the Rayleigh criterion, two objects can be resolved if the principal maxima of one falls on the first minima of the other in the diffraction pattern.
Hence, the limit of resolution is
⇒ \(\theta=1.22 \frac{\lambda}{d}=\tan \theta=\frac{y}{D}\)
where y is the minimum distance and D = distance of the objects from the lens.
⇒ \(y=1.22 \frac{\lambda}{d} \times D=1.22 \times \frac{\left(500 \times 10^{-9} \mathrm{~m}\right)(1000 \mathrm{~m})}{\left(10 \times 10^{-2} \mathrm{~m}\right)}\)
∴ 6.10 x 10-3 m = 6 mm = 5 mm.
Question 45. The diameter of the human eye lens is 2 mm. What will be the minimum separation between two points to resolve them, which are situated at a distance of 50 m from the eye? The wavelength of light is 500 nm.
- 2.32 m
- 4.28 m
- 1.52 cm
- 12.48 cm
Answer: 3. 1.52 cm
The required minimum separation is
⇒ \(y=D \tan \theta=D \theta=D\left(1.22 \frac{\lambda}{d}\right)\)
Given that d = 2 mm, D = 50 m and A = 500 nm.
⇒ \(y=\frac{50 \mathrm{~m}(1.22)\left(500 \times 10^{-9} \mathrm{~m}\right)}{\left(2 \times 10^{-3} \mathrm{~m}\right)}=15.25 \times 10^{-3} \mathrm{~m}=1.52 \mathrm{~cm}\)
Question 46. An astronaut is looking down on the earth’s surface from a space shuttle at an altitude of 400 km. Assuming that the astronaut’s pupil diameter is 5 mm and the wavelength of visible light is 500 nm, the astronaut will be able to resolve linear objects of size about
- 0.5 m
- 5 m
- 50 m
- 500 m
Answer: 3. 50 m
As done in the preceding question, the minimum size to be resolved,
∴ \(y=1.22 \frac{D \lambda}{d}=\frac{1.22(400 \mathrm{~km})(500 \mathrm{~nm})}{(5 \mathrm{~mm})}\)
⇒ \(\frac{1.22\left(400 \times 10^3 \mathrm{~m}\right)\left(500 \times 10^{-9} \mathrm{~m}\right)}{\left(5 \times 10^{-3} \mathrm{~m}\right)}=4.88 \times 10 \mathrm{~m} \approx 50 \mathrm{~m}\).
Question 47. A beam of light is incident normally on a diffraction grating through which the first diffraction is seen at 32°. In this case, there will be the 2nd-order diffraction?
- At 80°
- At 64°
- At 48°
- There will be no second-order diffraction
Answer: 4. There will be no second-order diffraction
In a diffraction grating, the nth-order diffraction angle (θn) is given by dsin 0n = nλ.
For the lst-order diffraction (n = 1),
d sin θ1 = X
⇒ d sin 32° = X.
For the 2nd-order diffraction (n = 2),
d sin θ2 = 2X = 2(d sin 32°)
⇒ sin θ2 = 2 x sin 32° = 2(0.53) =.1.06.
Since sin θ2 cannot be greater than one, there will be no second-order diffraction.
Question 48. At what angle of incidence will the light reflected from glass (μ = 1.5) be completely polarized?
- 56.3°
- 40.3°
- 72.8°
- 51.6°
Answer: 1. 56.3°
According to Brewster’s law, refractive index μ = tan ip, where ip = angle of polarization.
∴ ip = tan-1(1.5) = 56.3°
Question 49. Two polaroids P1 and P2 are placed with their pass axes perpendicular to each other. Unpolarized light of intensity I0 is incident on P2. A third polaroid P3 is kept in between P1 and P2 such that its pass axis makes an angle of 45° with that of P1. The intensity of transmitted light through P2 is
- \(\frac{I_0}{4}\)
- \(\frac{I_0}{8}\)
- \(\frac{I_0}{16}\)
- \(\frac{I_0}{2}\)
Answer: 2. \(\frac{I_0}{8}\)
The intensity of the light transmitted through polaroid P1 is I0/2 which is plane polarized. Since the pass axes of P1 and P2 are inclined at 45°, the intensity of the light transmitted through P3 (according to Malus’s law)
⇒ \(\left(\frac{I_0}{2}\right) \cos ^2 45^{\circ}=\frac{I_0}{4}\)
Pass axes of P3 and P2 are also inclined at 45°, so the final intensity of light transmitted through P2 will be
∴ \(\frac{I_0}{4} \cos ^2 45^{\circ}=\frac{I_0}{8}\)
Question 50. In the case of linearly polarized light, the magnitude of the electric field vector
- Does not change with time
- Varies periodically with time
- Increases and decreases linearly with time
- Is parallel to the direction of propagation
Answer: 2. Varies periodically with time
Light is an electromagnetic wave in which electric and magnetic fields are mutually perpendicular and perpendicular to the direction of propagation. In the case of linearly (or plane-) polarized light, \(\vec{E}\) -vector varies periodically with time
Question 51. Which of the following diagrams represents the variation of the electric field vector with time for a circularly polarized light?
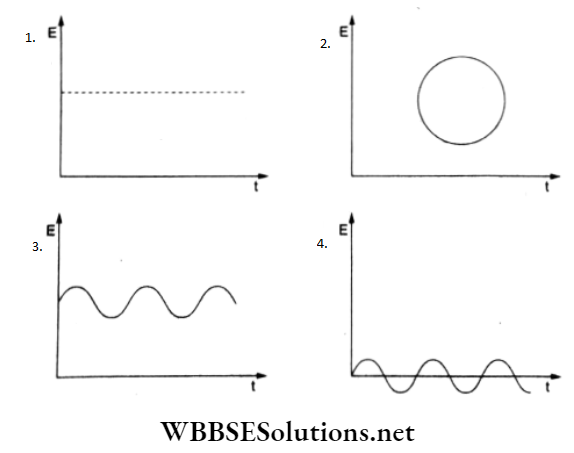
Answer: 2.
Circular polarization of an electromagnetic wave describes a polarization state in which at each point, the electric field of the wave has a constant magnitude but its direction rotates with time at a steady rate in a plane perpendicular to the direction of the wave. The tip of the electric field vector at any point in space describes a circle as time passes.
Question 52. In a YDSE, a light of wavelength 500 nm is used. The distance between the slits and the screen is 1.8 m and the slit separation is 0.4 mm. If the screen is moved parallel to itself with a speed of 4 m s-1, with what speed will the first maxima move?
- 2 mm s-1
- 3 mm s-1
- l mm s-1
- 5 mm s-1
Answer: 4. 5 mm s-1
When the screen is displaced parallel to itself, the position of the central maxima remains unchanged but the fringe width increases.
Now, \(\beta=\frac{D \lambda}{d}\)
⇒ \(\frac{d \beta}{d t}=\frac{\lambda}{d} \frac{d D}{d t}=\frac{500 \times 10^{-9} \mathrm{~m}}{0.4 \times 10^{-3} \mathrm{~m}} \times v_{\text {screen }}\)
⇒ \(\left(\frac{5}{4} \times 10^{-3}\right)\left(4 \mathrm{~m} \mathrm{~s}^{-1}\right)=5 \mathrm{~mm} \mathrm{~s}^{-1}\)
This is the speed with which the first maxima will move.
Question 53. Find the resolving power of a microscope whose objective lens has focal length f0 = 5 cm, and diameter = 2 cm. (Wavelength of light used, λ = 6000 A.)
- 11.9 x l05 m-1
- 10.9 x 105 m-1
- 10.9 x l04 m-1
- 10.9 x l03 m-1
Answer: 2. 10.9 x 105 m-1
Given that f0 = 5 cm and sin \(\theta \approx \tan \theta=\frac{D}{f}=\frac{2}{5}\)
The resolving power of the microscope is
∴ \(\frac{2 \mu \sin \theta}{1.22 \lambda}=\frac{2(1)(2 / 5)}{1.22\left(600 \times 10^{-9} \mathrm{~m}\right)}=10.9 \times 10^5 \mathrm{~m}^{-1}\)
Question 54. In a double-slit experiment, when light of wavelength 400 nm was used, the angular width of the first minima formed on a screen placed 1 m away was found to be 0.2°. What will be the angular width of the first minima if the entire set-up is immersed in water? \(\left(\mu_{\text {water }}=\frac{4}{3}\right)\).
- 0.26°
- 0.15°
- 0.05°
- 0.1°
Answer: 2. 0.15°
The angular width of a fringe is
⇒ \(\theta=\frac{\beta}{D}=\frac{D \lambda}{d D}=\frac{\lambda}{d}\)
Now, \(\frac{\theta_{\text {water }}}{\theta_{\text {air }}}=\frac{\lambda_\omega}{\lambda_{\mathrm{a}}}=\frac{C_\omega / v}{C_{\mathrm{a}} / v}=\frac{1}{\mu_\omega}=\frac{3}{4}\)
Hence, \(\theta_{\text {water }}=\frac{3}{4}\left(\theta_{\text {air }}\right)=(0.75)\left(0.2^{\circ}\right)=0.15^{\circ}\).
Question 55. In a YDSE, the ratio of the slit widths is 4: 1. The ratio of the intensity of the maxima to that of the minima in the fringe pattern close to the central fringe on the screen will be
- 3: 16
- 2: 9
- 9: 1
- 4: 1
Answer: 3. 9: 1
Assuming that the slit width is proportional to the intensity of light transmitted,
⇒ \(\frac{I_1}{I_2}=\frac{4}{1}=\frac{a_1^2}{a_2^2}\),
where a1 and a2 represent the corresponding amplitudes.
∴ \(\frac{a_1}{a_2}=\frac{2}{1} \Rightarrow \frac{a_1+a_2}{a_1-a_2}=\frac{3}{1}\)
Hence, \(\frac{I_{\max }}{I_{\min }}=\frac{\left(a_1+a_2\right)^2}{\left(a_1-a_2\right)^2}=\frac{9}{1}\).
Question 56. In a YDSE, when a thin film of thickness t having refractive index μ is introduced in front of one of the slits, the maximum at the centre of the fringe system shifts by one fringe width. The value of t in terms of λ (the wavelength used) is
- \(\frac{\lambda}{(2 \mu-1)}\)
- \(\frac{2 \lambda}{(\mu-1)}\)
- \(\frac{\lambda}{(\mu-1)}\)
- \(\frac{\lambda}{2(\mu-1)}\)
Answer: 3. \(\frac{\lambda}{(\mu-1)}\)
Insertion of a thin film in the path of rays coming from one slit will introduce an additional optical path Δ = (μ- l)t, where 4 is the refractive index of the film and t is its thickness. Since one fringe shift corresponds to an additional path μ, therefore
∴ \((\mu-1) t=\lambda \Rightarrow t=\frac{\lambda}{(\mu-1)}\).
Question 57. In an interference experiment, the ratio of amplitudes of coherent waves is \(\frac{a_1}{a_2}=\frac{1}{3}\). The ratio of maximum and minimum intensities of fringes will be
- 4
- 2
- 9
- 18
Answer: 1. 4
Given that \(\frac{a_1}{a_2}=\frac{1}{3} \Rightarrow \frac{a_2+a_1}{a_2-a_1}=\frac{4}{2}=\frac{2}{1}\)
∴ \(\frac{I_{\max }}{I_{\min }}=\left(\frac{a_2+a_1}{a_2-a_1}\right)^2=\left(\frac{2}{1}\right)^2=4\).
Question 58. Two coherent sources produce waves of different intensities which interfere. After interference, the ratio of the maximum intensity to the minimum intensity is 16. The intensities of the component waves are in the ratio
- 16:9
- 5:3
- 25:9
- 4:1
Answer: 3. 25:9
Given that \(\frac{I_{\max }}{I_{\min }}=\frac{16}{1}=\left(\frac{a_1+a_2}{a_1-a_2}\right)^2\)
⇒ \(\left(\frac{a_1+a_2}{a_1-a_2}\right)=\frac{4}{1} \Rightarrow \frac{a_1}{a_2}=\frac{5}{3} \Rightarrow \frac{I_1}{I_2}=\frac{a_1^2}{a_2^2}=\frac{25}{9}\).
Question 59. In Young’s double-slit experiment with slit separation 0.1 mm, one observes a bright fringe at an angle (1/40) radian by using a light of wavelength λ1. When a light of wavelength λ2 is used, a bright fringe is seen at the same angle in the same set-up. Given that λ1 and λ2 are in the visible range (380 nm-740 nm), their values are
- 380 nm and 525 nm
- 400 nm and 500 nm
- 380 nm and 500 nm
- 625 nm and 500 nm
Answer: 4. 625 nm and 500 nm
The angular position of the nth bright fringe at P„ is
⇒ \(\theta=\frac{P_0 P_n}{O P_0}=\frac{y}{D}\)
If this region contains n bright fringes,
⇒ \(y=n \beta=n \frac{D \lambda}{d}\); hence
⇒ \(\theta=\frac{1}{D}\left(\frac{n D \lambda}{d}\right)=\frac{n \lambda}{d}\)
⇒ \(n \lambda=\theta \cdot d=\frac{1}{40} \times(0.1 \mathrm{~mm})=\frac{10^{-4} \mathrm{~m}}{40}=\frac{10000}{4} \mathrm{~nm}=2500 \mathrm{~nm}\)
Thus, \(\lambda=\frac{2500}{n} \mathrm{~nm} \text {, where } n=1,2,3\),….
For k to lie within the visible range (380 nm-740 nm),
⇒ \(\lambda=\frac{2500 \mathrm{~nm}}{5}, \frac{2500 \mathrm{~nm}}{4}\),…
Hence, λ1= 500 nm and λ2 = 625 nm.
Question 60. In a YDSE, the slit separation is 0.320 mm and light of wavelength λ = 500 nm is incident on the slits. The total number of bright fringes that are observed in the angular range -30° < 0 < 30° is
- 320
- 321
- 640
- 641
Answer: 4. 641
Given that slit separation = d = 0.32 mm and wavelength = λ = 500 nm.
If N bright fringes are contained within the angular range 0 then the path difference is
A = dsinθ = Nλ,
⇒ \(N=\frac{d \sin \theta}{\lambda}=\frac{\left(0.32 \times 10^{-3} \mathrm{~m}\right)(0.5)}{\left(500 \times 10^{-9} \mathrm{~m}\right)}=320\).
Same number of fringes (N = 320) will be contained in the range θ = 0 to θ = -30°.
Thus, the total number of bright fringes contained will be 2N + central bright fringe = 641.
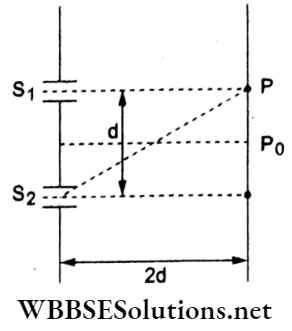
Question 61. Consider a YDSE set-up as shown in the figure. What should be the slit-separation d in terms of wavelength X such that the first minima occurs directly in front of the slit S1?
- \(\frac{\lambda}{2(5-\sqrt{2})}\)
- \(\frac{\lambda}{(5-\sqrt{2})}\)
- \(\frac{\lambda}{2(\sqrt{5}-2)}\)
- \(\frac{\lambda}{(\sqrt{5}-2)}\)
Answer: 3. \(\frac{\lambda}{2(\sqrt{5}-2)}\)
Since the position of the first dark fringe P is directly in front of slit Sl, the path difference
at P will be \(\Delta=\frac{\lambda}{2}\)
or \(\frac{\lambda}{2}=\Delta=\mathrm{S}_2 \mathrm{P}-\mathrm{S}_1 \mathrm{P}=\sqrt{4 d^2+d^2}-2 d\)
⇒ (√5 – 2)d
⇒ \(d=\frac{\lambda}{2(\sqrt{5}-2)}\)
Question 62. In a YDSE, the path difference at a certain point on the screen between two interfering waves is \(\frac{1}{8}\) of the wavelength. The ratio of the intensity at this point to that at the centre of a bright fringe will be close to
- 0.94
- 0.74
- 0.85
- 0.80
Answer: 3. 0.85
Let I be the intensity of each of the coherent waves. The phase difference is
⇒ \(\phi=\frac{2 \pi}{\lambda} \Delta=\frac{2 \pi}{\lambda} \cdot \frac{\lambda}{8}=\frac{\pi}{4}\)
Intensity at point P is
⇒ \(I_{\mathrm{P}}=I+I+2 \sqrt{I} \sqrt{I} \cos \frac{\pi}{4}=2 I\left(1+\frac{1}{\sqrt{2}}\right)\)
Intensity at the central bright fringe,
⇒ \(I_0=I+I+2 \sqrt{I} \sqrt{I} \cos 0^{\circ}=4 I\)
∴ \(\frac{I_{\mathrm{P}}}{I_0}=\frac{\sqrt{2}+1}{2 \sqrt{2}}=0.85\)
Question 63. In a YDSE, green light (A. = 5303 A) falls on the slits having a slit separation of 19.44 |j,m and a width of 4.05 |im. The number of bright fringes between the first and the second diffraction minima is
- 4
- 10
- 9
- 5
Answer: 4. 5
Angular positions of the first and the second minima are \(\theta_1=\frac{\lambda}{a} \text { and } \theta_2=\frac{2 \lambda}{a}\)
where a is the width of each slit. The linear separation between the two minima is
⇒ \(D \theta=\left(\theta_2-\theta_1\right) D=\frac{D \lambda}{a}=N \beta\)
where N is the number of bright fringes and j} = fringe width = \(\frac{D \lambda}{d}\)
⇒ \(N\left(\frac{D \lambda}{d}\right)=\frac{D \lambda}{a}\)
∴ \(N=\frac{d}{a}=\frac{19.44 \mu \mathrm{m}}{4.05 \mu \mathrm{m}}=4.8 \approx 5\)
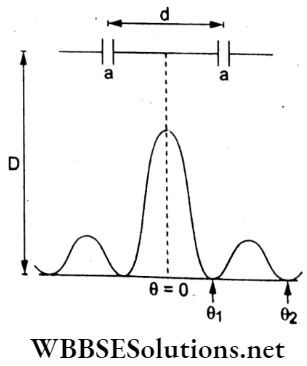
Question 64. The value of the numerical aperture of the objective lens of a microscope is 1.25. If light of wavelength 500 nm is used, the minimum separation between two points to be seen distinctly will be
- 0.38 μm
- 0.48 μm
- 0.24 μm
- 0.12 μm
Answer: 3. 0.24 μm
The minimum separation between two objects that can be resolved by a microscope is given by
⇒ \(d_{\min }=\frac{1.22 \lambda}{2 \mu \sin \theta}=\frac{1.22 \lambda}{2(N A)}\)
where numerical aperture = 1.25 and λ = 500 run.
∴ \(d_{\min }=\frac{1.22\left(500 \times 10^{-9} \mathrm{~m}\right)}{2(1.25)}=2.44 \times 10^{-7} \mathrm{~m}=0.24 \mu \mathrm{m}\)
Question 65. Calculate the limit of resolution of a telescope objective having a diameter of 200 cm, if it has to detect a light of wavelength 500 nm coming from a star.
- 305 x 10-9 radian
- 152.5 x l0-9 radian
- 610 x 10-9 radian
- 457.5 x 10-9 radian
Answer: 3. 610 x 10-9 radian
The limit of resolution (angular) for a telescope is
⇒ \(\Delta \theta=1.22 \frac{\lambda}{\mathrm{D}}=\frac{1.22\left(500 \times 10^{-9} \mathrm{~m}\right)}{2 \mathrm{~m}} \mathrm{rad}\)
∴ 610 x 10-9 rad.
Question 66. The diameter of the objective lens of a telescope is 250 cm. For a light of wavelength 600 nm coming from a distant object, the limit of resolution of the telescope is close to
- 3.0 x 10-7 rad
- 2.0 x 10-7 rad
- 1.5 x 10-7 rad
- 4.5 x 10-7 rad
Answer: 1. 3.0 x 10-7 rad
Limit of resolution = \(1.22 \frac{\lambda}{D}=1.22 \frac{\left(600 \times 10^{-9} \mathrm{~m}\right)}{250 \times 10^{-2} \mathrm{~m}}=2.9 \times 10^{-7} \mathrm{rad}\)
∴ 3.0 x 10-7 rad.
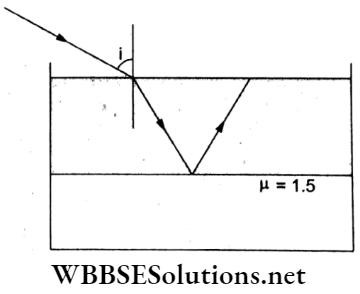
Question 67. Consider a tank made of glass (refractive index 1.5) with a thick bottom. It is filled with a liquid of refractive index μ. A student finds that irrespective of what the incident angle i is (as shown in the figure), a beam of light entering the liquid-glass interface is never completely polarized. For this to happen, the tire minimum value of p should be
- \(\frac{3}{\sqrt{5}}\)
- \(\frac{5}{\sqrt{3}}\)
- \(\frac{4}{3}\)
- \(\sqrt{\frac{5}{3}}\)
Answer: 1. \(\frac{3}{\sqrt{5}}\)
Under the given situation, light will be transmitted from liquid to air if r ≤ θC, where θC is the critical angle for the liquid.
Further, transmitted light in the air will never be completely polarized if the polarizing angle 0p>θC.
Thus, tan 0p > tan θC.
⇒ \(\frac{\mu_{\mathrm{g}}}{\mu_l}>\frac{\sin \theta_{\mathrm{c}}}{\cos \theta_{\mathrm{c}}} \Rightarrow \frac{\mu_{\mathrm{g}}}{\mu_l}>\frac{1 / \mu_l}{\sqrt{1-\frac{1}{\mu_l^2}}}\)
⇒ \(2 \mu_c->\frac{1}{\sqrt{\mu_l^2-1}} \Rightarrow 9\left(\mu_l^2-1\right)>4 \mu_l^2\)
∴ Hence, \(\mu_l>\frac{3}{\sqrt{5}}\).
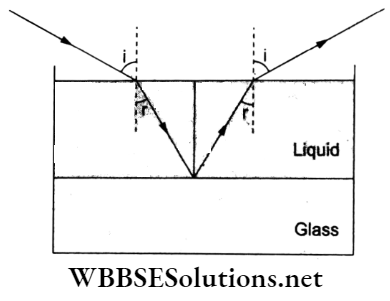
Question 68. A system of three polaroids P1, P2 and P3 is set up such that the pass axis of P3 is crossed with respect to that of P1. The pass axis of P2 is inclined at 30° to that of P1. When a beam of unpolarized light of intensity I0 is incident on P1 the intensity of light transmitted by the three polaroids is I. The ratio I0/I is equal to (nearly)
- 5.33
- 16.00
- 10.67
- 1.80
Answer: 3.
Pass axes of I1 and I2 are inclined at 30°, so according to Malus’s law,
⇒ \(I_2=I_1 \cos ^2 30^{\circ}=\frac{3 I_1}{4}\)
But \(I_1=\frac{I_0}{2}, \text { so } I_2=\frac{3}{4}\left(\frac{I_0}{2}\right)=\frac{3 I_0}{8}\)
Similarly, P2 and P3 are inclined at 60°, so
⇒ \(I=I_2 \cos ^2 60^{\circ}=\frac{3}{8} I_0 \times \frac{1}{4}=\frac{3}{32} I_0\)
∴ \(\frac{I_0}{I}=\frac{32}{3}=10.67\)
Question 69. An electromagnetic wave is represented by the electric field \(\vec{E}=E_0 \sin (\omega t+6 y-8 z) \hat{n}\). Then, the direction of propagation of the wave is
- \(\frac{-3 \hat{j}-4 \hat{k}}{5}\)
- \(\frac{-3 \hat{j}+4 \hat{k}}{5}\)
- \(\frac{-3 \hat{i}-4 \hat{k}}{5}\)
- \(\frac{3 \hat{i}-4 \hat{j}}{5}\)
Answer: 2. \(\frac{-3 \hat{j}+4 \hat{k}}{5}\)
From the given wave equation, it is clear that the direction of propagation of the wave is along the negative direction of \(6 \hat{j}-8 \hat{k}\). The unit vector along the direction of propagation will be
∴ \(-\frac{6 \hat{j}-8 \hat{k}}{\sqrt{36+64}}=\frac{-6 \hat{j}+8 \hat{k}}{10}=\frac{-3 \hat{j}+4 \hat{k}}{5}\).
Question 70. A plane electromagnetic wave travels in free space along the electric field component of the wave at a particular point of space and time is E = 6 V m-1 along the y-direction. Its corresponding magnetic field component B would be
- 6 x 10-8 T along the z-direction
- 6 x 10-8 T along
- 2 x 10-8 T along z-direction
- 2 x 10-8 T along y-direction
Answer: 3. 2 x 10-8 T along z-direction
Since \(\frac{E}{B}=c\), hence magnitude of the magnetic field is
⇒ \(B=\frac{E}{c}=\frac{6 \mathrm{Vm}^{-1}}{3 \times 10^8 \mathrm{~m} \mathrm{~s}^{-1}}=2 \times 10^{-8} \mathrm{~T}\)
But \(\vec{E}=E_0 \hat{i}\) (given) and the wave travels along the x-direction, \(\hat{E} \times \hat{B}=\hat{i}\), gives the direction along the z-direction, \(\vec{B}=\left(2 \times 10^{-8} \mathrm{~T}\right) \hat{k}\).
Question 71. A plane electromagnetic wave of frequency 50 MHz travels in free space along the positive x-direction. At a particular point in space at time t, \(\vec{E} \text { is }\left(6.3 \mathrm{Vm}^{-1}\right) \hat{j}\). The corresponding magnetic field \(\vec{B}\) a that point will be
- \(\left(18.9 \times 10^8 \mathrm{~T}\right) \hat{k}\)
- \(\left(6.3 \times 10^{-8} \mathrm{~T}\right) \hat{k}\)
- \(\left(18.9 \times 10^{-8} \mathrm{~T}\right) \hat{k}\)
- \(\left(2.1 \times 10^{-8} \mathrm{~T}\right) \hat{k}\)
Answer: 4. \(\left(2.1 \times 10^{-8} \mathrm{~T}\right) \hat{k}\)
Given that \(\vec{E}=\left(6.3 \cdot \mathrm{Vm}^{-1}\right) \hat{j}\). The direction of propagation of the electromagnetic wave is along the x-direction. Thus,
⇒ \(\frac{E}{B}=c \text { and } \hat{E} \times \hat{B}=\hat{i} \Rightarrow \hat{j} \times \hat{B}=\hat{i}\)
Thus, \(B=\frac{E}{c}=\frac{6.3}{3 \times 10^8} \mathrm{~T}=2.1 \times 10^{-8} \mathrm{~T}\)
and \(\hat{B}=\hat{k}\)
∴ \(\vec{B}=B \hat{B}=\left(2.1 \times 10^{-8} \mathrm{~T}\right) \hat{k}\).
Question 72. If the magnetic field of a plane electromagnetic wave is given by \(B=\left(100 \times 10^{-6} \mathrm{~T}\right) \sin \left[2 \pi \times 2 \times 10^{15}\left(t-\frac{x}{c}\right)\right]\) then the maximum electric field associated with the wave is
- 6 x 104 N C-1
- 4 x 104 N C-1
- 3 x 104 N C-1
- 4.5 x 104 N C-1
Answer: 3. 3 x 104 N C-1
The amplitude of the magnetic field B0 = 100 x10-6 T, hence the corresponding amplitude of the electric field is
E0 = B0 x c = (100 x10-6 T)(3 x108 m s-1 ) = 3 x104 N C-1.
Question 73. The intensity of a plane-polarized light is 3.3 W m-2. A polarizer of area 3 x 10-4 m2 rotates at a constant angular frequency of 10jc rad s-1. The total amount of energy transmitted in one complete revolution is
- 6.95 x 10-4 J
- 1.0 x 10-4 J
- 3.95 x 10-4J
- 2.95 x 10-4J
Answer: 2. 1.0 x 10-4 J
According to Malus’s law, the intensity of a transmitted light is
I = I0 Cos2θ.
The total energy transmitted in one revolution is
⇒ \(E=\int I A d t=\int I_0 \cos ^2 \theta A d t\)
⇒ \(I_0 A \int \cos ^2 \omega t d t=\frac{I_0 A^{2 \pi}}{\omega} \int_0^{2 \pi} \cos ^2 \omega t d(\omega t)\)
∴ \(\frac{I_0 A \pi}{\omega}=\frac{\left(3.3 \mathrm{~W} \mathrm{~m}^{-2}\right)\left(3 \times 10^{-4} \mathrm{~m}^2\right) \pi}{(10 \pi) \mathrm{rad} \mathrm{s}^{-1}}\)
= 0.99 x 10-4 J ≈1.0 x 10-4 J
Question 74. A single slit of width 0.6 x10-4 m is illuminated by a light of wavelength 600 nm. The highest order of minima on both sides of the central maxima is
- 200
- 100
- 20
- 10
Answer: 2. 100
For a single-slit diffraction pattern, the position for the minima is d sin0 = nX
⇒ \(\sin \theta=\frac{n \lambda}{d}<1\)
Hence, \(n \leq \frac{d}{\lambda}\)
Here, d = slit width = 0.6 x 10-4m and λ = 600 x 10-9 m.
∴ \(n \leq \frac{6 \times 10^{-5} \mathrm{~m}}{6 \times 10^{-7} \mathrm{~m}}=100\)
Question 75. In the given figure, P and Q are two equally intense coherent sources emitting radiation of the same wavelength 20 m. The separation between P and Q is 5 m and the phase of P is ahead of Q by 90°. A, B and C are three points each at the same distance from the midpoint O of PQ. The intensities observed at A, B and C will be in the ratio
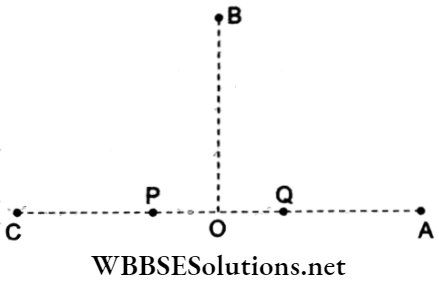
- 0:1:4
- 0:1:2
- 4:1:0
- 2:1:0
Answer: 2. 0:1:2
Resultant intensity due to superposition is
I = 2I0(1 + cos π), where Φ= phase difference.
At point A: path difference = \(\Delta=P A-Q A=P Q=5 \mathrm{~m}=\frac{20 \mathrm{~m}}{4}=\frac{\lambda}{4}\)
and phase difference = \(\frac{2 \pi}{4}=\frac{\pi}{2}\)
Since P is ahead of Q by \(\frac{\pi}{2}\), hence net phase difference Φ = π.
IA = 2I0(l + cos π) = 0.
At point B: B is equidistant from both P and Q, hence path difference = 0.
But P is ahead in phase by \(\frac{\pi}{2}, \text { so } \phi=\frac{\pi}{2}\)
⇒ \(I_{\mathrm{B}}=2 I_0\left(1+\cos \frac{\pi}{2}\right)=2 I_0\)
At point C: path difference = \(\Delta=Q C-P C=\frac{\lambda}{4}\)
But P leads by \(\frac{\pi}{2}\left(\equiv \frac{\lambda}{4}\right)\), hence Φ = 0.
∴ Ic = 2I0(1 + cos 0°) = 4I0.
∴ Hence, IA : IB : Ic = 0 : 2I0 : 4I0 = 0 : 1 : 2.
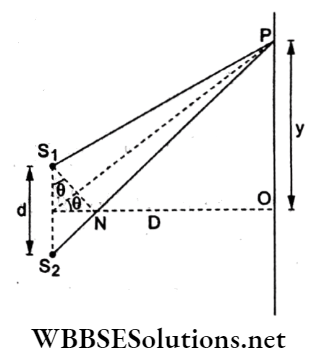
Question 76. In a YDSE with a slit separation of 1 mm and distance of the screen from the slit 1 m, the wavelength of the light used is 650 nm. At a distance y =1.270 mm from the central fringe where a bright fringe is observed, the path difference is
- 1.27 μm
- 2.45 μm
- 2.27 μm
- 0.27 μm
Answer: 1. 1.27 μm
Path difference at P due to sources S1 and S2 is
Δ = S2P-S1P ≈ S2N = S1S2sin θ.
For θ to be small, \(\sin \theta \rightarrow \tan \theta=\frac{y}{D}\)
⇒ \(\Delta=d\left(\frac{y}{D}\right)=(1 \mathrm{~mm}) \frac{(1.27 \mathrm{~mm})}{1 \mathrm{~m}}\)
= 1.27 μm.
Question 77. In a YDSE, the total number of fringes observed between points O and P with λ = 700 nm was 16. With λ’ = 400 nm, the total number of fringes in the same region OP will be
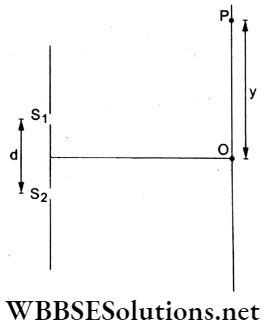
- 7
- 12
- 28
- 14
Answer: 3. 28
y = N1p1 = N2P2
⇒ \(16 \cdot \frac{D(700 \mathrm{~nm})}{d}=N_2 \cdot \frac{D(400 \mathrm{~nm})}{d}\)
∴ Hence, \(N_2=\frac{16 \times 700}{400}=28\).
Question 78. In a YDSE, the intensity at a point where the path difference is λ is k units. At another point where the path difference is \(\frac{\lambda}{6}\), the intensity is\(\frac{n}{12}\) K. The value of n is
- 7
- 12
- 6
- 9
Answer: 4. 9
Intensity, \(I=2 I_0(1+\cos \phi)=2 I_0\left(1+\cos \frac{2 \pi \Delta}{\lambda}\right)\).
For \(\Delta=\lambda, I=4 I_0=k ; \text { for } \Delta=\frac{\lambda}{6} ; \phi=\frac{\pi}{3}\), so
⇒ \(I^{\prime}=2 I_0\left(1+\cos \frac{\pi}{3}\right)=2 I_0 \cdot \frac{3}{2}=3 I_0=\frac{3 k}{4}=9\left(\frac{k}{12}\right)\)
∴ n = 9.
Question 79. Two light waves having the same wavelength λ in vacuum are in phase initially. If the first wave travels a path L1 through a medium of refractive index μ1 while the second wave travels a path of length L2 through a medium of refractive index μ2 then the phase difference between the two is
- \(\frac{2 \pi}{\lambda}\left(\frac{L_1}{\mu_1}-\frac{L_2}{\mu_2}\right)\)
- \(\frac{2 \pi}{\lambda}\left(\mu_1 L_1-\mu_2 L_2\right)\)
- \(\frac{2 \pi}{\lambda}\left(\frac{L_1}{\mu_2}-\frac{L_2}{\mu_1}\right)\)
- 0
Answer: 2.
Optical path = μL.
Path difference = Δ = (μ1L1 – μ2L2).
Phase difference = \(\phi=\frac{2 \pi}{\lambda} \Delta=\frac{2 \pi}{\lambda}\left(\mu_1 L_1-\mu_2 L_2\right)\)
Question 80. In a YDSE, if the separation between coherent sources is halved and the distance of the screen from the coherent sources is doubled then the fringe width becomes
- Half
- Four times
- One fourth
- Double
Answer: 2. Four times
Fringe width = \(\beta=\frac{D \lambda}{d}\)
Given that d is changed to \(\frac{d}{2}\) and D is changed to 2D
∴ Hence, \(\beta^{\prime}=\frac{D^{\prime} \lambda}{d^{\prime}}=\frac{2 D \lambda}{d / 2}=4 \frac{D \lambda}{d}=4 \beta\)
Question 81. The Brewster angle ip for an interface should be
- \(30^{\circ}<i_p<45^{\circ}\)
- \(45^{\circ}<i_{\mathrm{p}}<90^{\circ}\)
- \(i_p=90^{\circ}\)
- \(0^{\circ}<i_{\mathrm{p}}<30^{\circ}\)
Answer: 2. \(45^{\circ}<i_{\mathrm{p}}<90^{\circ}\)
Polarization from reflection is
ip + r = 90° ⇒ = 90°- r.
Thus, ip <90°.
For ip = r; 2ip = 90° ⇒ ip = 45°
⇒ 45° < ip <90°.
Question 82. Assume that light of wavelength 600 nm is coming from a star. The limit of resolution of a telescope whose objective lens has a diameter of 2 m is
- 1.83 x l0-7 rad
- 7.32 x l0-7 rad
- 6.00 x l0-7 rad
- 3.66 x l0-7 rad
Answer: 4. 3.66 x l0-7 rad
According to the Rayleigh criterion, the angular limit of resolution is
⇒\(\theta=1.22 \frac{\lambda}{D}=1.22 \times \frac{600 \times 10^{-9} \mathrm{~m}}{(2 \mathrm{~m})}\)
= 1.22 x 300 x l0-9 rad
= 3.66 x l0-7 rad.