Chapter 3 Atoms And Molecules Short Answer Type Question And Answers
Question 1. Calculate the molar mass of HNO3. [N = 14, O = 16, H = 1]
Answer.
Given:
Molar mass of HNO3.
H = 1 × 1 = 01
N = 14 × 1 = 14
O = 16 × 3 = 48
Total mass = 63 grams
Molar mass of HNO3 = 63 grams.

Question 2. Calculate the formula mass of CaCl2. [Ca = 40, Cl = 35.5]
Answer.
Given:
1(Ca) + 2(Cl) 40 + 2 × (35.5) = 111 u
The formula mass of CaCl2 is 111 u.
Question 3. A certain non-metal X forms two oxides 1 and 2. The mass percentage of oxygen in oxide 1 (X4O6) is 43.7, which is same as that of X in oxide 2. Find the formula of the second oxide.
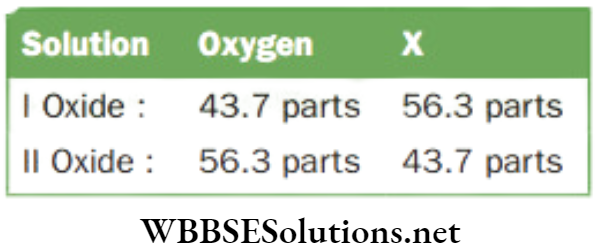
Answer.
Given
A certain non-metal X forms two oxides 1 and 2. The mass percentage of oxygen in oxide 1 (X4O6) is 43.7, which is same as that of X in oxide 2.
Now 43.7 parts of oxygen in I corresponds to = 6 oxygen atoms
Therefore, 56.3 parts of oxygen in II corresponds to
⇒ \(\frac{6 \times 56.3}{43.7}=7.730 \text { atoms }\)
Also 56.3 parts of X in 1 correspond to = 4 X atom
Therefore, 43.7 parts of X in 2 will correspond to
⇒ \(=\frac{4 \times 43.7}{56.3} \times 3.1 \times \text { atoms }\)
Now the atomic ration X : O in the second
Oxide = \(\frac{3.1}{3.1}: \frac{7.73}{3.1}\) or 1 : 25 or 2 : 5
The formula of the second oxide is X2O2.
Read And Learn More NEET Foundation Short Answer Questions
Question 4. Calculate the mass of 0.2 moles of water (O = 16, H = 1).
Answer.
Given:
Gram molecular weight of H2O = 2 × 1 + 16 = 18 g
1 mole of water weighs 18 g
Therefore, 0.2 moles of water weighs \(\frac{18}{1}\) × 0.2 = 3.6 g
Question 5. Calculate the volume of 7.1 g of chlorine (Cl = 35.5) at S.T.P.
Answer.
Given:
Gram Molecular Weight of Cl2 (one mole) = 35.5 × 2 = 71 g.
71 g of Cl2 at S.T.P occupies 22.4 litres
Therefore, 7.1 g of Cl2 at S.T.P occupies
⇒ \(\frac{22.4}{71} \times 7.1=2.24 \text { litres }\)
Question 6. The reaction between aluminium carbide and water takes place according to the following equation:
Al4C3 + 12H2O → 3CH4 + 4Al(OH)3
Calculate the volume of CH4 released from 14.4 g of Al4C3 by excess water at S.T.P. (C = 12, Al = 27)
Answer.
Given
CH4 released from 14.4 g of Al4C3 by excess water at S.T.P. (C = 12, Al = 27)
Molecular weight of Al4C3 is (27 × 4) + (12 × 3) = 144
144 g of Al4C3 produces 3 × 22.4 litres of CH4 at S.T.P
Therefore, 14.4 g Al4C3 produces \(\frac{3 \times 22.4}{144} \times\) 14.4
⇒ \(=\frac{967.7}{144}=6.72 \text { litres }\)
Question 7. How many litres of ammonia are present in 3.4 kg of it? (N = 14, H = 1)
Answer.
Given:
Gram molecular weight of NH3 = 14 + (1 × 3) = 17 g.
17 g of NH3 = 22.4 litres
Therefore, 3.4 × 103g of NH3 = \(\frac{22.4}{17} \times 3.4 \times 10^3\)
= \(\frac{76160}{17}\)
= 4480 litres.
4480 litres of ammonia are present in 3.4 kg of it
| Class 11 Physics | Class 12 Maths | Class 11 Chemistry |
| NEET Foundation | Class 12 Physics | NEET Physics |
Question 8. Define a mole.
Answer.
Mole: A mole is the amount of substance that contains as many elementary entities as there are atoms in 12 grams in carbon 12 isotopes .
Question 2. Find out the molar mass of sulphuric acid.
Answer.
Formula of sulphuric acid = H2SO4
No. of atoms:
H = 2
S = 1
O = 4
Atomic mass:
H = 1
S = 32
O = 16
Molar mass= (2 × 1) + (1 × 32) + (4 × 16) = 98 g/mol
Molar mass of sulphuric acid is 98 g/mol

Question 9. Calculate the number of moles and atoms in 240 grams of O (Oxygen) atom.
Answer.
Atomic Mass of O = 16 u
16 u of O = 1 atom of oxygen
16 g of O = 1 mole of oxygen
16 g of O = 6.022 × 1023 atom of O
240 g of O = 15 moles of O
240 g of O = 15 × 6.022 × 1023 atoms
Therefore, 240 g of O = 90.33 × 1023 atoms.
Question 10. Find out the weight of 120.44 × 1023 molecules of water.
Answer.
Molecular mass of H2O = 18 u [(1 × 2) H + (16 × 1)O]
So, 1 mole = 18 g H2O
6.022 × 1023 = 18 g
120.44 × 1023 = (120.44 × 1023/6.022 × 1023) × 18
= 20 × 18 = 360 g
The weight of 120.44 × 1023 molecules of water = 360 g
Question 11. 6.6 g of CaCO3on heating gave 2.98 g CaO and 3.62 g CO2. Prove that these observations agree with law of conservation of mass.
Answer.
Given:
6.6 g of CaCO3on heating gave 2.98 g CaO and 3.62 g CO2.
CaCO3 → CaO + CO2
6.6g → 2.88 g + 3.52 g
Mass of the reactant = 6.4 g
Mass of the product = (2.98 + 3.62) g = 6.6 g
These results agree with the law of conservation of mass as the mass of reactants is equal to the mass of products.
Question 12. An 80.0 g sample of an unknown compound contains 16.4 g of hydrogen. What is the percent by mass of hydrogen in the compound?
Answer.
Given:
An 80.0 g sample of an unknown compound contains 16.4 g of hydrogen.
Mass of the compound = 80 g
Mass of hydrogen in the compound = 16.4 g
Therefore, the mass fraction of hydrogen in the unknown compound = (16.4/80) x 100% = 20.5%
Question 13. What is the importance of law of conservation of mass in everyday life?
Answer.
Importance of law of conservation of mass in everyday life: Law of conservation of mass is important to study to produce chemical reactions. Chemists can predict the amount of products that will be produced in a chemical reaction if they know the amount and identities of the reactants.
Question 14. What is the limitation of law of definite proportions?
Answer.
Limitation of law of definite proportions: The law of definite proportions does not hold good for those elements who are also present in different isotopic forms in a compound.
