NEET Foundation Notes For Physics Chapter 1 Motion
Motion Definition Physics
In our day to day life, we find some objects are either moving or at rest. For instance, flying birds, crawling insects, moving cars and buses, walking people, running dogs etc., all are moving from one place to another. There are times when things seem to be static for some while moving for others. To understand it better, imagine you are travelling in a bus.
The bus is moving, in one direction and trees on the roadside are left behind. The passenger will feel as if the trees are moving back but literally the trees are at their own place.
Motion can be of different types. Sometimes you see an object moving straight, circular, rotating or vibrating or a combination of two or more types.
Read and Learn More: NEET Foundation Notes
This chapter will help you to learn the motion in various types. We will also learn motion with the help of graphs and equations.


NEET Foundation Notes For Physics Chapter 1 Motion
Movement of any object from one position to another position with respect to observer is called motion. As we have discussed, each object in the universe is always rotating. The location of an object is specified by the reference point which is normally called origin.
Position and Reference Point
Motion of any object is defined by its position with respect to the observer. Basically, position is the location of the object. Reference point is the point from which the location of object is measured. It is often called object. Any object can be located with the help of reference point and its direction. To understand this better, let us analyze the following example.
Example: Rahul’s school is 4 km south from his home. Here, the position of the school is specified with respect to Rahul’s home. Therefore, Rahul’s home is the origin.
To describe the position of an object, it is important to specify the origin.

We can choose this reference point according to our convenience. The origin is needed to specify the position of an object.
Motion can be of different types depending upon the type of path by which the object is going through.
- Circulatory motion/circular motion: In a circular path.
- Linear path: In a straight line path.
- Oscillatory and Vibratory motion: To and fro path with respect to origin.
Motion along a Straight Line
Motion is described as a change in position of an object with respect to time. The simplest type of motion is the motion along the straight line. For example, motion of lift.
When a body is moving in a straight path, it is known as motion in a straight line. It is also known as one dimensional motion or rectilinear motion. For example, stone falling down vertically, car moving in a straight path. The common thing among these examples is that, there is no movement of the object in lateral direction.
Representation of One Dimensional Motion
The path of one dimensional motion can be represented by a straight line parallel to X-axis, if X-axis is taken in the direction of motion. Each point on the straight line represents the position of a particle at different instants. The position of a particle at any instant t is expressed by specifying the x coordinate at that instant. Then as the particle moves, its x coordinate will change with time t.

Example: The position of a pebble falling freely and vertically downwards at different instants is given in the below table:

The motion of the pebble can be represented by choosing a proper scale for x on a straight line along X-axis. Here X-axis represents the vertically downward direction.

Types Of Motion Physics
Position in Straight Line Motion
- Positive sign shows position in right (position) direction
- Negative sign shows position in left (negative) direction
- Zero is usually considered as reference point or origin
Scalars and Vectors
Physical quantities that can be defined using magnitude only are known as scalar quantities. For example distance, speed, mass, density, temperature. Physical quantities that can be defined only if both its magnitude and direction are specified are called vector quantities. For example velocity, acceleration, force, torque.
Uniform Motion and Non-Uniform Motion
Uniform Motion
When an object covers equal distances in equal intervals of time, it is said to be in uniform motion. If a distance vs time graph is plot for uniform motion, it will be a straight line inclined to the time axis.
Non-uniform Motion
When an object covers unequal distances in equal intervals of time, it is said to be in non-uniform motion. Example: Car moving in a crowded street. If the distance vs time graph is plotted of non-uniform motion, it would be a curve inclined to time axis.
Difference between uniform and non-uniform motion



State of a Body
The state of the body is defined in context to the origin which can be chosen according to our convenience. It can be described in two ways:
- State of motion: When an object changes its position with respect to the origin in a time interval, it is said to be in a state of motion.
- State of rest: When an object does not changes its position with respect to the origin in a time interval, it is said to be in state of rest. Example 1 below depicts the state of rest of a stone.
State of rest and motion are relative to each other. Example 2 below shows how they are relative in nature.
Example 1: A stone lying on the ground is said to be at rest because it does not change its position with respect to the surrounding.
Example 2: A person sitting in a moving train is at state of rest with respect to its fellow passenger, whereas the same person is in state of motion with respect to the trees and buildings outside the train.
Distance and Displacement
The length of the path traversed by a body irrespective of its direction is called distance travelled by the object. The path is not necessarily straight. The shortest distance from the initial to the final position of the body is called displacement. It is in direction from the initial position to the final position. The SI unit of distance and displacement is same i.e., metre (m).
Example: In an object is moving from point A to point B. The total length from A to B is determined as the distance moved by the body while the length of straight line AB with respect to the direction from A to B (Shown by dotted line) is called displacement of the body.
The quantities like distance and displacement are known as physical quantities. The magnitude of a physical quantity depicts the size, length or amount of the physical quantity.

- Scalar quantity: The physical quantity that has only magnitude, i.e., the size is called scalar quantity. Scalar quantity has no direction.
- Vector quantity: The physical quantity that has magnitude as well as direction is called vector quantity.
Distinction Between Distance and Displacement
Distinguish features between distance and displacement

NEET Foundation Notes For Physics Chapter 1 Motion Measuring the Rate of Motion
Speed
The speed of an object is the rate of change of distance with time. Numerically, speed is the distance travelled by the body in 1 second. Each object takes different time to cover the same distance. The rate at which objects move may vary. The rate of motion of an object can be measured by finding out the distance travelled by the object in unit time which is called speed.
Speed with Distance
Speed is a scalar quantity because it is one dimensional measurement of a quantity. It is represented by the letter u or v, where u stands for initial speed and v stands for final speed. Speed of a body is calculated by dividing the distance travelled by the object by the time taken for it to complete that distance. Numerically, it is expressed as:
\(\text { Speed }=\frac{\text { Distance }}{\text { Time }}\)
If an object travels a distance s in time t, then speed v is equal to: v = s/t where, v = speed;
s = Distance travelled;
t = time taken.
Instantaneous speed is the speed of a particle at a given instant. It is defined as the ratio of the distance traveled in a extremely small interval of time tending to zero.
Unit of Speed
\(\text { Unit of speed }=\frac{\text { Unit of distance }}{\text { Unit of time }}=\frac{\mathrm{m}}{\mathrm{s}}=\mathrm{m} / \mathrm{s} \text { or } \mathrm{ms}^{-1}\)
Uniform Speed
An object is said to be moving with uniform speed if it covers equal distances in equal intervals of time through out its motion.
Example: The motion of a ball on a frictionless plane surface is with uniform speed.
Non-uniform Speed
An object is said to be moving with a non-uniform or variable speed if it covers unequal distances in equal intervals of time.
Example: The motion of a ball on a rough surface and motion of a car in a crowded street.
The speed of objects with non-uniform speed is calculated by their instantaneous speed and average speed.
Instantaneous Speed
When the speed of the object keeps on changing constantly with time, its speed at any point of time is called instantaneous speed.
\(\text { Instantaneous speed }=\frac{\text { Distance travelled in a short time interval }}{\text { Time interval }}\)
The unit of measurement of the instantaneous speed is meter per second [m/s]. The value of the instantaneous speed coincides with the magnitude of the instantaneous velocity at that point. Instantaneous speed is measured by speedometer.
Average Speed
The ratio of total distance travelled by the object to the total time of journey is called its average speed. Numerically, it is expressed as:
\(\text { Average Speed }=\frac{\text { Total distance travelled }}{\text { Total time taken }}\)
In case of an object moving with uniform speed, the instantaneous speed and the average speed are equal. average speed of some objects in m/s and in km/h.

Velocity
The velocity of an object is the distance travelled per second by the object in a specified direction.
Formula
\(\text { Velocity }=\frac{\text { distance travelled in a given direction }}{\text { time taken }}\)
- It is a vector quality.
- Symbol of velocity is u or v.
- Magnitude and direction should be known for velocity.
Unit
The S.I. unit of velocity is m/s and C.G.S. unit is cm/s.
Uniform Velocity
If an object travels equal distances in equal intervals of time along a particular direction, then the object is said to be moving with a uniform velocity. Since displacement is a vector, equal displacement implies the body is moving along a straight line path. Thus, a body moving with uniform velocity is in motion along a straight line path with a constant speed.
Example: Rain droplets touch the earth surface with uniform velocity.
If an object moves with a uniform velocity v , the displacement S of the object in a time interval t is given as
\(\vec{S}=\vec{v} t\vec{S}=\vec{v} t\)
Non-uniform Velocity
If an object travels unequal distances in a particular direction in equal intervals of time or it moves equal distances in equal intervals of time, but its direction of motion does not remain the same, then the velocity of the object is said to be non-uniform.
Example: Drop a ball from some height. Ball has non-uniform velocity as its speed keeps increasing.
Instantaneous Velocity
For an object with variable velocity, the velocity of the object at a certain instant is called its instantaneous velocity. The direction of instantaneous velocity is along the tangent drawn to the curve describing the path at that instant if the body undergoes curvilinear motion.
Formula
\(\text { Instantaneous velocity }=\frac{\text { distance travelled in small time interval }}{\text { time interval }}\)
Average Velocity
If the velocity of an object moving in a particular direction changes with time, the ratio of displacement to the time taken in completing a journey is called average velocity.
Formula
\(\text { Average velocity }=\frac{\text { displacement }}{\text { total time taken }}\)
Speed and Velocity are not Equal in Magnitude
When an object moves in a straight line the speed and velocity and their magnitudes are same.
But the magnitude changes when:
- Object does not move in a straight line; or
- Object changes its direction.
It has been illustrated below.

A boy goes to school from home by running in the east direction. The distance between home and school is 100 m. He reaches the school in 50 seconds.
\(\text { Speed }=\frac{\text { Distance }}{\text { Time }}\)
Therefore, \(\frac{100 \mathrm{~m}}{50 \mathrm{~s}}=2 \mathrm{~m} / \mathrm{s}\)
\(\text { Velocity }=\frac{\text { Displacement }}{\text { Time }}\)
= \(\frac{100 \mathrm{~m}}{50 \mathrm{~s}}=2 \mathrm{~m} / \mathrm{s}\)
In this example the magnitude of speed and velocity of the boy are same.
Let us take another example to illustrate when the magnitude of speed and velocity is different.
A boy goes to school from home in the morning and comes back in the evening. The distance between home and school is 100 m. He reaches the school in 50 seconds and comes back also in 50 seconds.

Distance travelled by the boy = 100 m + 100 m.
Time taken = 50 s + 50 s
\(\text { Speed }=\frac{\text { Distance }}{\text { Time }}\)
Speed = \(\frac{(100+100) \mathrm{m}}{(50+50) \mathrm{s}}\)
= \(\frac{200 \mathrm{~m}}{100 \mathrm{~s}}\)
= 2 m/s i.e. 2 ms-1
\(\text { Velocity }=\frac{\text { Displacement }}{\text { Time }}\)
The boy runs 100 m to east then 100 m to west,
Therefore, Displacement = 100 m – 100 m
Displacement = 0
Velocity = \(\frac{0}{100}=0 \mathrm{~m} / \mathrm{s}\)
Distinction between speed and velocity

NEET Foundation Notes For Physics Chapter 1 Motion Graphical Representation of Motion
Motion can be understood easily with the help of the graphs. In this section, we will try to understand different type of motion with the help of graphs. It gives a clear and easy way to understand the motion of an object. If an object moves in a straight line it is called linear motion.
Linear motion can be understood with the help of graphs in the following three ways: (1) Distance-Time graph (2) Velocity-Time graph (3) Acceleration-Time graph.
Distance–Time Graphs
The change in the position of the object with time can be represented on the distance – time graph. The distance-time graph convey us the following information:
- It describes the nature of the body i.e., whether its in rest, uniform motion or non-uniform motion.
- We can pin-point the position of the body at any instant of time.
- We can calculate the speed of the body through the slope of the curve.
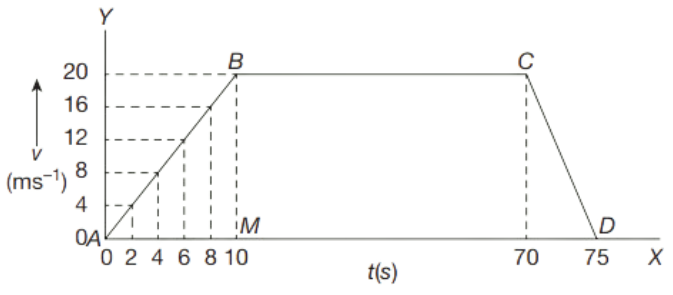
When an object is moving with a uniform velocity, the distance-time graph is always a straight line.

The above graph represents that the distance travelled by the object is directly proportional to the time taken. The diagonal line in the graph shows that the distance is increasing at a uniform rate. Distance–time graph is used to calculate the speed of an object.
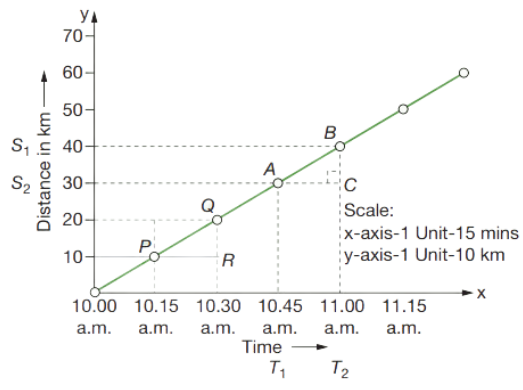
Distance–Time Graph for Uniform Speed
The distance- time graph or a straight line graph for a body moving at uniform speed is always a straight line as body in uniform motion, body moves equal distance in equal time interval.

In order to calculate the velocity, consider points A and B on the diagonal line. S1 and S2 are the points on Y axis where A and B points touch horizontally. T1 and T2 are the points on X axis where A and B touch vertically.
Distance = BC
= S2 − S1
Time = AC
= T2 – T1
\(\text { Slope }=\frac{\text { change in } y}{\text { change in } x}=\frac{B C}{A C}\)
v = \(\frac{\left(S_2-S_1\right)}{\left(T_2-T_1\right)}\)
Where v = velocity
(S2 – S1) = interval of distance
(T2 – T1) = interval of time
Therefore,
\(\text { Velocity }=\frac{\text { distance }}{\text { time }}\)
Distance–Time Graph for Non-uniform Speed
This is also known as curved graph. The distance – time graph is plotted for an object moving with non-uniform speed, the slope of graph will not be a straight line. The rising trend of slope shows the increasing trend of velocity.


The above graph is not a straight line because object is moving with an accelerated motion. The graph has a rising trend of slope which means the increasing trend of velocity.
The distance time graph is parallel to time axis when the body is at rest.
To calculate speed of body at any point say P, first draw two perpendiculars on time axis and distance axis say PA and PB respectively.
Speed of object = \(\frac{\mathrm{PA}}{\mathrm{PB}}\)
Here, PA represents distance travelled by body and PB represents time taken by body.
Velocity – Time Graph

In the above graph, time is represented along x-axis while velocity is represented along y-axis.
Velocity is a vector quantity.
- Positive velocity means object is moving in a direction away from the source.
- Negative velocity means object is moving towards the source.
Determination of Displacement from Velocity–time Graph
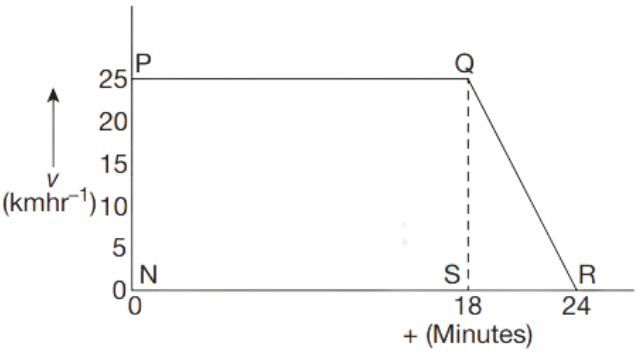
As we know, displacement is equal to the product of velocity and time, therefore, the area covered between velocity-time sketch and x-axis determines the displacement of the object.

In the above, the enclosed area that is above the x-axis is the positive displacement while the enclosed area below the x-axis is the negative displacement.
Total displacement is the sum of both positive and negative displacement.
Example: Consider x-axis shows time in seconds and y-axis shows velocity in m/s.
Area of trapezium above x axis = \(\frac{1}{2}\) × (sum of parallel sides) × height
= \(\frac{1}{2}\) × (30 + 5) × 60
= \(\frac{1}{2}\) × 35 × 60
= 1050 m
Area of triangle = \(\frac{1}{2}\) × base × height
= \(\frac{1}{2}\) × 25 × 40 = 500 m
Displacement of object = area of trapezium – area of triangle
= 1050 m – 500 m
= 550 m
Distance travelled by object = area of trapezium + Area of triangle
= 1050 m + 500 m
= 1550 m
Determination of Acceleration from Velocity–time Graph
\(\text { Acceleration }=\frac{\text { change in velocity }}{\text { time taken }}\)
= Slope of velocity – time

- Case 1: If the object is moving with uniform velocity, the velocity-time graph is a straight line parallel to the time axis.
- Case 2: If the object is moving with uniform acceleration, the velocity-time graph is a straight line inclined to the time axis. The slope of the line gives the acceleration.
NEET Foundation Notes For Physics Chapter 1 Motion Acceleration–Time Graph

In the above graph, time is on x axis and acceleration is on y axis (Fig. 1.14). Through this graph, we can calculate the change in speed in a certain time interval.
The area enclosed between the acceleration-time sketch and the time axis determines the change in speed of the object. There are various cases discussed below:

Case 1:
- If the object is stationary or if it is moving with uniform velocity, the acceleration is zero.
- The acceleration-time graph in this case is a straight line coinciding with the time axis.
Case 2:
- If the velocity of body in motion increases uniformly with time, the acceleration is constant.
- The acceleration-time graph in this case is a straight line parallel to time axis.
Case 3:

- If the velocity of the object decreases at a constant rate, the retardation is constant.
- The acceleration-time graph is a straight line parallel to the time axis on the negative acceleration axis.
Case 4:
- If the velocity of the object changes in an irregular manner, the acceleration is variable.
- The acceleration-time graph will be a curve of any shape in this case.
NEET Foundation Notes For Physics Chapter 1 Motion Equations of Motion by Graphical Method
For an object moving with a uniform acceleration, the following three equations give the relationship between initial velocity (u), final velocity (v), acceleration (a), time of journey (t) and distance travelled (S).
1. v = u + at
2. S = \(\frac{1}{2}(u+v) t\)
= ut + \(\frac{1}{2} a t^2\)
3. v2 = u2 + 2aS
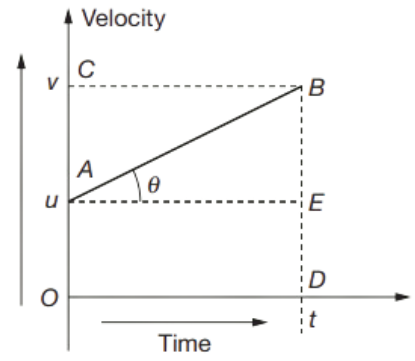
Equation for Velocity–Time Relation

The initial velocity of an object is u which gradually increases to v in time t. The graph shows change in velocity.
Initial velocity (at t = 0) = OA = u
Final velocity ( at t = t) = OC = v
Acceleration a = Slope of the line AB
a = EB/AE
= AC/OD
= (OC – OA)/OD
= (v – u)/t
at = v – u
v = u + at
Equation for Position–Time Relation
Distance S travelled in time t = area of trapezium OABD
S = area of rectangle OAED + area of triangle ABE
S = OA × OD + ½ x BE x AE
S = u × t + ½ × (v – u) × t
As we know v – u = at
Therefore, S = ut + \(\frac{1}{2} a t^2\)
Equation for Position–Velocity Relation
Distance S travelled in time t = area of trapezium OABD
= \(\frac{1}{2}(O A+D B) \times O D\)
= \(\frac{1}{2}(u+v) \times t\)
As we know t = (v-u)/a
Therefore, S = \(\frac{1}{2}(u+v)\left(\frac{v-u}{a}\right)\)
= \(\frac{1}{2}\left(\frac{v^2-u^2}{a}\right)\)
2 a S = v2 – u2
v2 = u2 + 2aS
NEET Foundation Notes For Physics Chapter 1 Motion Uniform Circular Motion
An object is said to be in uniform circular motion when it moves in a circular path at a constant speed. The moving object is accelerating in a circle. Accelerating objects are the one who keeps changing their velocity, either the speed or direction. The object moving in a circular motion has a constant speed.
There can be different closed tracks where an object moves. It can be rectangular, hexagonal, octagonal or circular.
Velocity changes either due to the change in magnitude or direction of the motion or due to both. If an object runs with a uniform speed in a rectangular track, he has to run fast at the corners in order to keep himself in track. Same goes for hexagonal and octagonal tracks.
If the object is moving with a velocity of constant magnitude in a circular path, his velocity will change only if he changes his direction.

NEET Foundation Notes For Physics Chapter 1 Motion Fill in the Blanks
Question 1. Negative acceleration is called ______.
Answer. Retardation
Question 2. The shortest distance between the initial and final position is ______.
Answer. Displacement
Question 3. The speed of 10 m/s in km/h would be ______.
Answer. 36 Km/h
Question 4. Velocity -time graph of a body at rest would be a ______.
Answer. Coincides with time axis
Question 5. Speedometer of bikes show ______ speed.
Answer. Instantaneous
Question 6. Uniform motion suggests that the ______ of the body is constant.
Answer. Speed
Question 7. The equation of motion v = u + at can be applied for a body having ______ acceleration.
Answer. Constant
Question 8. In uniform circular motion, ______ is uniform.
Answer. Speed
Question 9. The SI unit of the rate of change of velocity is ______.
Answer. Acceleration
Question 10. In a rectilinear motion, the path of the moving object is ______.
Answer. Straight line
Question 11. An object is said to be in uniform circular motion when it moves in a circular path at a ______.
Answer. Constant Speed
Question 12. Accelerating objects are the one who keeps changing their __________.
Answer. Velocity
Question 13. Velocity changes due to the change in ____________ of the motion.
Answer. magnitude or direction
Question 14. _______________ is the rate of change of velocity with time.
Answer. Acceleration
Question 15. Acceleration can only be positive. (True/False)
Answer. False
Question 16. The acceleration produced by the earth is called ________________.
Answer. Acceleration due to gravity
NEET Foundation Notes For Physics Chapter 1 Motion Match the Columns
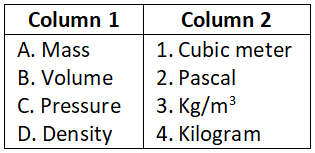
Question 1. Match the following units and choose the correct code.

Select the correct option:
- A-1, B-2, C-3, D-4
- A-4, B-3, C-2, D-1
- A-2, B-1, C-4, D-3
- A-3, B-4, C-1, D-2
Answer. 3. A-2, B-1, C-4, D-3
Question 2. Match the following equations where u = initial velocity v = final velocity, a = acceleration, t = time of journey and S = distance travelled.

Select the correct option:
- A-1, B-2, C-3, D-4
- A-4, B-3, C-2, D-1
- A-2, B-3, C-1, D-4
- A-1, B-3, C-2, D-4
Answer. 3. A-2, B-3, C-1, D-4
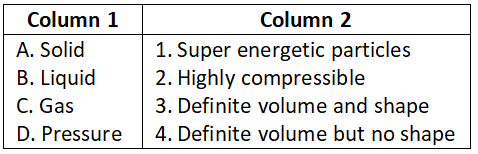
Question 3. Match the following.

Select the correct option:
- A-1, B-2, C-3, D-4
- A-4, B-3, C-2, D-1
- A-1, B-2, C-4, D-3
- A-3, B-4, C-1, D-2
Answer. 3. A-1, B-2, C-4, D-3
Question 4. Choose the correct code.

Select the correct option:
- A-1, B-2, C-3, D-4
- A-4, B-3, C-2, D-1
- A-1, B-2, C-4, D-3
- A-3, B-4, C-1, D-2
Answer. 2. A-4, B-3, C-2, D-1
Question 5. Choose the correct option.

Select the correct option:
- A-2, B-1, C-4, D-3
- A-4, B-3, C-2, D-1
- A-1, B-2, C-4, D-3
- A-3, B-4, C-1, D-2
Answer. 1. A-2, B-1, C-4, D-3
NEET Foundation Notes For Physics Chapter 1 Motion Assertion Reasoning
Direction: For the following questions the options will remain the following:
- Both A and R are correct and R is correct explanation of A.
- Both A and R are correct but R is not a logical explanation of A.
- A is correct but R is incorrect.
- R is correct but A is incorrect.
- Assertion: When object moves with uniform velocity, displacement – time graph is a straight line inclined to the time axis.
Reason: Displacement increases by the same amount in each second.
- Assertion: When an object is moving with linear velocity, the slope of the graph is always a straight line.
Reason: The diagonal line shows that the distance is increasing at a non-uniform rate.
- Assertion: If object is moving with a uniform retardation, v = u – at where u = initial velocity, v = final velocity, a = acceleration, t = time of journey.
Reason: a will be negative.