NEET Foundation Notes For Chemistry Chapter 2 Is Matter Around Us Pure
Matter Around Us
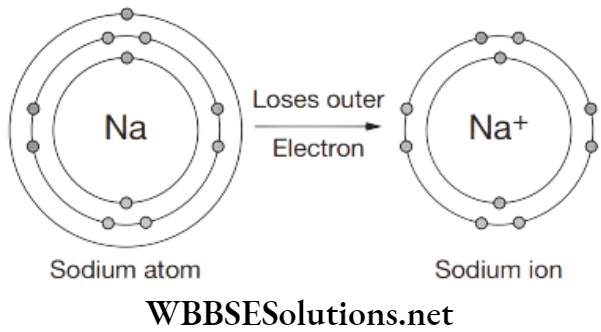
How to be sure whether the products brought from the market are pure? Is the word ‘pure’ written on the packs enough to tell us that the substance is pure? What does this ‘pure’ means? For a layman, pure means that the product is without any adulteration.
But according to scientists, these are actually mixtures which aren’t pure. For example, when we buy milk, we often find ‘pure’ written on it but for a scientist, it is a mixture of fat, protein, water etc. For a scientist, A pure substance consists of a single type of particles. It is a pure single form of matter.
Anything that has mass and takes up space is considered as matter. So, matter is everything including your desk, clothes, food, and buildings, etc. All matters are not of same kind. Matter can be classified into two categories—mixtures or substances.

NEET Foundation Notes For Chemistry Chapter 2 Is Matter Around Us Pure Substances
Substance is simply a pure form of matter, i.e., it contains only one type of atom or molecule. While mixture contains a combination of different atoms or molecules and is therefore an impure ‘substance’. Substances refer to either an element or a compound—but not a mixture, as ‘substance’ always has a definite composition.
Read and Learn More: NEET Foundation Notes
Examples of substance:
- Iron is an element; therefore, it is also a substance.
- Methane is a compound; therefore, it is also a substance.
Examples of non-substances:
- Salt water is not a substance, because it is a mixture of two substances, i.e., sodium chloride and water. Its composition and properties are not fixed.
- Gasoline is not a substance; as it is mixture of hydrocarbons and depending on the composition of the gasoline in a mixture, the properties of gasoline vary.
A pure substance has definite and constant composition with distinct chemical properties. Pure substance is any single type of material that was not contaminated by any another substance.
Examples of pure substances include elements and compounds, such as:
- Water
- Diamond
- Gold
- Sodium chloride
- Ethanol
- Brass
- Bronze
Examples of substance, which are not pure are:
- Rocks
- An orange
- Wheat
- Light bulb
- A shoe
- A sandwich

As described in the above, Matter is anything, which occupies space and has mass. Matter is broadly classified as pure substance and mixture.
- A pure substance is made of only one type of particle or matter. Example: Sugar, distilled water.
- Mixture is made up of two or more than two different types of particle or mater. Example: Apple juice, as it is made of water, sugar and fruit juice.
Difference between pure substance and mixtures

NEET Foundation Notes For Chemistry Chapter 2 Is Matter Around Us Pure Mixture
Is Matter Around Us Pure
Mixture is made up of two or more than two different substances that are mixed and are not combined chemically. Substances in a mixture combine physically; their identities are retained and are mixed in the form of solutions, suspensions, and colloids.
Is Matter Around Us Pure Example: Air, is a mixture of different gases.
Types of Mixture: There exist different types of mixture depending on the nature of its components, and the appearance of the mixture. Based on their appearance, mixtures are classified.
Difference between pure substance and mixtures

Based on the physical state of components, mixtures are classified as (Table 2.3).
Main properties of the three families of mixture

NEET Foundation Notes For Chemistry Chapter 2 Is Matter Around Us Pure Solution
Solution is a mixture of two or more components in which the minor component (the solute) is uniformly distributed within the major component (the solvent). Scientists say that solutions are homogenous systems as all its constituents are evenly spread out and are thoroughly mixed.
Components of a Solution
Component which is dissolved is solute, and is present in small amount. Solution may or may not be in the same state of matter as the solute. Medium in which solute is dissolved is solvent and is present in large amount; it is in the same state of matter as the solvent.
Can Anything Become a Solution
Solutions can be solids dissolved in liquids. When you work with chemistry or even cook in your kitchen, you will usually be dissolving solids into liquids. Solutions can also be gases dissolved in liquids, such as carbonated water. There can also be gases in other gases and liquids in liquids.
If you mix things up and they stay at an even distribution, it is a solution. Solution can be solid–solid, as they usually start as solid/gas/liquid–liquid solutions and then harden at room temperature. Example of Solid–Solid is alloys with all types of metals at room temperature.
Solutions can be:
- Solids dissolved in liquids: Sugar in water
- Gases dissolved in liquids: Carbon dioxide in soda gas in other gas: Air
- Liquids in liquids: Gasoline
- Gas dissolved in solid: Hydrogen in palladium metal
- Liquid dissolved in solid: Dental filling
- Solid dissolved in Solid: Metal alloys such as brass.
Depending on the nature of the solvent, Solutions can be classified as:
- Solid solutions: Solvent is solid
- Liquid Solutions: Solvent is liquid
- Gaseous Solution: Solvent is gas.
How to make a Solution?
Solution is made by dissolving the solute in the solvent. Simple solution consists of two substances, which are evenly mixed together, also called binary solution. One of them is called solute and the other is the solvent.
Binary Solution
Mixture of two liquids are completely miscible with one another. The boiling point of binary solution depends upon the composition of the solution so formed and the range of boiling point is:
- Binary Solution may lie between the boiling points of clean liquids.
- Binary Solution may lie above the boiling points of clean liquids.
- Binary Solution may lie below the boiling points of clean liquids.

Binary Solution Example: Alcohol and water
Types of binary solution:
- Solids dissolved in solid: Alloys
- Liquid dissolved in solid: Dental filling
- Gas dissolved in solid: Hydrogen in palladium metal
- Solids dissolved in liquids: Sugar in water
- Liquids dissolved in liquids: Gasoline
- Gases dissolved in liquids: Carbon dioxide in soda
- Solid dissolved in Gas: Camphor in air
- Gas in other gas: Air
- Solid dissolved in Solid: Metal alloys such as brass.
- Liquid dissolved in gas: Air
Properties of a Solution
- The particles of solute are the size of individual small molecules, 1 nanometre is the maximum diameter for a solute particle.
- In a gravity environment, the solution will not be separated due to any difference in the density of the materials in the solution.
- It does not separate by common fibre filter; in fact an entire solution will pass through the filter.
- Once it is completely mixed, it became homogeneous.
- The mixture appears clear rather than cloudy.
- The solute is completely dissolved into the solvent up to a point characteristic of the solvent, solute, and temperature. At a saturation point, the solvent can no longer dissolve any more of the solute. If there is a saturation point, the point is distinct and characteristic of the type of materials and temperature of the solution.
- The solution shows an increase in boiling point as the amount of solute is increased.
- The solution shows a decrease in melting point as the amount of solute is increased.
Difference between Solutions and Mixtures: Mixture is a combination of two or more than two substances which are not chemically united and do not exist in fixed proportions to each other. Most natural substances are mixtures.
Different Types of Solution
- Aqueous and Non-Aqueous Solution: Solution in which water acts as a solvent is aqueous solution (Solution of common salt or sugar in water) and the solution in which any other liquid acts as a solvent is non-aqueous solution (Solution of sulphur in carbon disulphide).
- Saturated, Unsaturated and Supersaturated Solution:
- Solution in which no more solute can be dissolved at a particular temperature as it contains maximum amount of solute that can be dissolved into it and if more solutes are added then they will get settle at the bottom.
- Solution in which more solute can be dissolved at a given temperature is Unsaturated solution.
- Solution may temporary contain more solute than a saturation level at a particular temperature, then a solution is called as supersaturated solution.
- Concentrated and dilute solution: Solution having larger proportion of solute is concentrated and the one having lesser proportion of solute is dilute solution.
- True solution: In this, particles of the solute are thoroughly mixed with the solvent so that they cannot be separated from each other.
Solubility: Maximum amount of solute in grams which can be dissolved in 100 grams of the solvent at a given temperature to form a saturated solution.
⇒ \(\text { Solubility }=\frac{\text { weight of the solute in saturated solution }}{\text { weight of solvent in saturated solution }}\)
Alloy: Alloys are homogeneous mixtures of two or more than two metals or it may be a mixture of metal and a non-metal, which cannot be separated into their components by any physical methods.
Substance In Common Use
Alloy can be described as a ‘mixture of metals’. The best way to think of an alloy is that it is a material, which is made up of at least two different kinds of chemical elements, one of which is a metal.
The most important metallic component of an alloy is called main metal, the parent metal, or the base metal and the other components of an alloy can be either metals or non-metals and it is present in much smaller quantity. Alloy can sometimes be a compound. Atoms from inside are arranged in a regular structure known as crystalline lattice.
Different types of alloys and their composition

Concentration of Solution: It is the amount of solute, which is present in a given amount either by mass or by volume of solution, or it is the amount of solute that dissolved in a given mass or volume of solvent.
⇒ \(\text { Concentration of solution }=\frac{\text { Amount of solute }}{\text { Amount of solution }}\)
Ways of expressing the concentration of a solution are:
- Mass by mass percentage of a solution = \(\frac{\text { mass of solute }}{\text { mass of solution }} \times 100\)
- Mass by volume percentage of a solution = \(\frac{\text { mass of solute }}{\text { volume of solution }} \times 100\)
- Parts per Million Or Parts per Billion (ppm or ppb)
Parts per Million is used for expressing concentration of trace amount of substance present in the total amount of solution.
It can be calculated as:
ppm = \(\left(\frac{\text { mass of solute }}{\text { mass of solution }}\right) \times 10^6\)
ppb = \(\left(\frac{\text { mass of solute }}{\text { mass of solution }}\right) \times 10^9\)
There are many ways to represent the relative amounts of solute and solvent in a solution and those ways are:
- Molarity
- Molality
- Mole Fraction
Molarity
Molarity tells the number of moles of solute present in exactly one litre of a solution.
To calculate the molarity of a solute in a solution, one should know:
- The moles of solute present in the solution.
- The volume of solution containing the solute.
To calculate molarity, use the equation:
\(\text { Molarity }=\frac{\text { Moles of solute }}{\text { Volume of solution in liters }}\)
Molality
Molality tells us the number of moles of solute, which are dissolved in exactly one kilogram of solvent.
To calculate the molality of a solute in a solution, one should know:
- The moles of solute present in the solution.
- The mass of solvent (in kilograms) in the solution.
To calculate molality, use the equation:
⇒ \(\text { Molarity }=\frac{\text { Moles of solute }}{\text { Mass of solvent in kilograms }}\)
Mole Fraction
The mole fraction, X, of a component in a solution is the ratio of the number of moles of that component to the total number of moles of all components in the solution.
- To calculate mole fraction, one should know:
- The number of moles of each component present in the solution.
The mole fraction of A, XA, in a solution consisting of A, B, C … is calculated using the equation:
⇒ \(\mathrm{X}_{\mathrm{A}}=\frac{\text { Moles of } \mathrm{A}}{\text { mass of } \mathrm{A}+\text { moles of } \mathrm{B}+\text { moles of } \mathrm{C}+\cdots}\)
To calculate the mole fraction of B, XB, use:
⇒ \(X_B=\frac{\text { Moles of } B}{\text { mass of } A+\text { moles of } B+\text { moles of } C+\cdots}\)
Question 4. Which among the following is a heterogeneous mixture?
- Rainwater
- BrassMuddy
- Water
- Vinegar
Answer. 3. Muddy Water
Fill In The Blanks
Question 1. A ____________ is a material made up of two or more substances.
Answer. Mixture
Question 3. A _____________ has sharp melting and boiling point.
Answer.Pure Substance
Question 1. Alloys are mixtures of two or more than two __________________.
Answer. Metals
Question 2. Molarity tells the number of moles of solute present in exactly one litre of a solution. (True/False)
Answer. True
Question 3. To calculate mole fraction, one should know _________ of each component in the solution.
Answer. No. of moles
Question 4. Concentration of solution = Amount of solute/_________.
Answer. Amount of Solution
NEET Foundation Notes For Chemistry Chapter 2 Is Matter Around Us Pure Suspension
Non-homogeneous systems like mixture of sodium chloride and iron fillings in which solids are dispersed in liquids are called suspensions. The solute particles do not dissolve but remain suspended throughout the bulk of the medium in a heterogeneous mixture. The particles of a suspension are visible to the naked eye.
It contains solid particles, which are sufficiently large for sedimentation; size of a particle must be larger than one micrometre. The internal phase, which is solid, is dispersed throughout the external phase, which is fluid through mechanical mixing.
Example: Sand in water, in this the suspended particles are visible under a microscope and are settled over time if left undisturbed.
Properties of Suspension
- It is a heterogeneous mixture.
- The particles of a suspension can be seen through naked eye.
- The particles scatter a beam of light passing through it which make its path visible.
- The solute particles settle down when a suspension is left undisturbed which shows that it is unstable. They can be separated from the mixture by filtration.
Suspensions are classified based on the dispersed phase and the dispersion medium, where the former is essentially solid while the latter may be a solid, a liquid, or a gas.
NEET Foundation Notes For Chemistry Chapter 2 Is Matter Around Us Pure Colloidal Solution
In colloid, particles are uniformly spread throughout the solution, because of the relatively smaller size of particles. However, in reality, a colloidal solution is a heterogeneous mixture, example, milk and water.
Because of the small size of colloidal particles, we cannot see them with naked eyes, but these particles can scatter a beam of visible light. This scattering of a beam of light is called Tyndall effect.
Properties of Colloid
- It is heterogeneous in nature. The particles can be seen only through powerful microscope.
- The size of particles in a colloid lies between 10-9 m to 10-7 m.
- Colloidal particles can easily pass through the pores of a filter paper. Therefore, colloidal particles cannot be separated by filtration.
- Colloids are unstable; particles tend to come together and settle down.
- When viewed under a microscope, the colloidal particles are seen to be moving in a random (zigzag) fashion called Brownian motion.
- Particles scatter the beam of light and make its path visible.
Tyndall Effect
Tyndall effect can be observed when a fine beam of light enters a room through a small hole and then the light get scattered in the room. The particles of dust and smoke present in the room get visible due to this scattering of light.
So, Tyndall effect is the scattering of light, as a light beam passes through a colloid, all suspension particles present in the colloid get scatter and reflects under the light, thus makes the beam of light visible.
Where one can observe the Tyndall effect?
When sunlight passes through the canopy of a dense forest, the mist contains tiny droplets of water that acts as particles of colloid and is dispersed in air.

The Tyndall effect is caused by the reflection of light through small particles in suspension in a transparent medium. Tyndall Effect can be seen when headlight beams are visible on foggy nights.
Tyndall effect can be easily seen by using a laser pointer, which is aimed at the mist from the ultrasonic humidifier’s mist. In liquids, the Tyndall effect can be easily seen by using a laser pointer. If you dilute milk and then pass the beam of the laser, it will be easily seen as it travels through the liquid.

Tyndall effect, which is shown above, uses a laser pointer. The glass on the left contains 5 ppm of HVAC colloidal silver and the one, which is present on the right side, is from the tap after the bubbles have settled out.
For any particular particle size, Tyndall effect will increase linearly with the concentration (ppm). Since Tyndall will increase to the third power of particle size for any given concentration, it is very difficult to use Tyndall effect to determine the concentration of a sol. Tyndall can be used as a go/no go test to determine if a colloid is present, not its concentration. common examples of colloid.
Common examples of colloid

The solute-like component or the dispersed particles in a colloid, form the dispersed phase.
Component in which the dispersed phase is suspended is known as the dispersing medium. Colloids are classified according to the state, which are solid, liquid, or gas of the dispersing medium and the dispersed phase. A few common examples are given.
Emulsion
It is a colloidal solution in which dispersing medium and dispersed phase both are liquid. An emulsion is a mixture of two immiscible substances. One substance (the dispersed phase) is dispersed in the other (the continuous phase). Example – milk, butter, face cream, etc.
An emulsion is termed an oil/water (o/w) emulsion if the dispersed phase is an organic material and the continuous phase is water or an aqueous solution and is termed water/oil (w/o) if the dispersed phase is water or an aqueous solution and the continuous phase is an organic liquid (an “oil”).
A w/o emulsion is sometimes called an inverse emulsion. Its properties that are the opposite of those of an emulsion. Its use is, therefore, not recommended.
Electrophoresis and Coagulation
Electrophoresis It is the motion of dispersed particles relative to a fluid under the influence of a spatially uniform electric field. It is caused by the presence of a charged interface between the particle surface and the surrounding fluid.
Coagulation Process by which the colloidal particles are separated by addition of small amount of electrolyte is coagulation. It is carried by addition of electrolyte like Sodium chloride, barium chloride.
Difference between true solution, suspension and colloidal solution

NEET Foundation Notes For Chemistry Chapter 2 Is Matter Around Us Pure Separating the Components of a Mixture
Most of the natural substances are not pure. There are different methods of separation used to get an individual component from a mixture. Heterogeneous mixtures can be separated into its constituents by simple physical methods like handpicking, sieving.
To get coloured component (dye) from blue/black ink, heat the beaker containing water and place a watch glass containing ink over it, water from ink get separated by the process of evaporation and only dye is left behind in the watch glass.
- Sometimes the solid particles in a liquid are very small and pass through a filter paper. For such particles, the filtration technique is used. Such mixtures are separated by centrifugation. The principle is that the denser particles are forced to the bottom and the lighter particles stay at the top when spun rapidly.
- To separate a mixture of two immiscible liquids a separating funnel is used, which works on the principle that immiscible liquids separate out in layers depending on their densities.
- To separate mixtures, which contain a sublimable volatile component from a non-sublimable impurity, the sublimation process is used.
- To separate a mixture of two miscible liquids the method is used called distillation and is used for the separation of components of a mixture, which contains two miscible liquids which boil without decomposition and have sufficient difference in their boiling points.
Evaporation To get coloured component (dye) from blue/ black ink
Blue or black dye, can be separated from ink by the process of evaporation. As we know that ink is a colloidal solution, as it is a heterogeneous mixture of dye and water, heating it leads to the evaporation of water and leaves behind the dye in the watch glass.
Procedure to separate coloured component from blue/black ink:
- Heat the beaker containing water.
- Place a watch glass over it ink
- After some time, water from ink get separated by the process of evaporation and only ink is left behind in the watch glass.

Centrifugation
Sometimes the solid particles in a liquid are very small that they get pass through a filter paper. For such particles the filtration technique cannot be employed, such mixtures are separated by centrifugation. The principle is that the denser particles are forced to the bottom and the lighter particles stay at the top when spun rapidly.
In the method of centrifugation, the centripetal and centrifugal forces are used to separate lighter and heavier components of mixture of two immiscible liquids. This process is used to separate very small solids particles from a liquid mixture.
Consider an example of milk, which is a mixture of fat, water, and other constituents. By using centrifugation, most of the fat can be separated from milk as fat is suspended throughout the milk which is separated out using the method of centrifugation.
Centrifugation Procedure:
When milk is churned rapidly, water which is heavier than fat, moves away from the centre of centrifuge while fat is forced towards the bottom, which is drained out.
Centrifugation Applications:
- Used in dairies and home to separate butter from cream.
- Used in washing machines to squeeze out water from wet clothes.
- Used in diagnostic laboratories for blood and urine tests.
Separating Funnel
To separate a mixture of two immiscible liquids. Separating funnel is used to separate two immiscible liquids.
Decantation is used to separate the components from a mixture of two immiscible liquids (mixture of oil and water). In a mixture of two immiscible liquids, lighter one and heavier one form separate layer. The lighter one can be separated after settling of mixture, carefully in another container.
In the process of decantation some of the heavier liquid also poured out with lighter one. Therefore, components from a mixture of two immiscible liquids; can be separated more easily and accurately using a separating funnel.
Separating Funnel Applications
- In the extraction of iron from its ore, the lighter slag is removed from the top by this method to leave the molten iron at the bottom in the furnace.
- To separate mixture of oil and water.
Sublimation
To separate mixtures of sublimable volatile component from a non-sublimable impurity, the sublimation process is used.
There are many substances which get converted into gas from solid when heated, and converted from gas to solid when cooled without converting into liquid. Such substances are known as sublime. Example of such substance are: ammonium chloride, naphthalene balls, camphor, etc.
Sublimation Procedure
- Take the mixture of ammonium chloride and common salt.
- Heat the mixture in a China dish (note: cover the china dish with an inverted funnel).
- Plug the cotton in the opening of the funnel.
- After heating, ammonium chloride is converted into vapour and gets deposited over the inner surface of funnel; due to cooling.
- This leaves the common salt in China dish.
- Ammonium chloride can be taken out by scratching from the inner wall of funnel.
Distillation
Distillation is used to separate a mixture of two miscible liquids, and is used for the separation of components of a mixture which contains two miscible liquids which boil without decomposition and have sufficient difference in their boiling points.
If you want to separate a mixture of two or more miscible liquids whose difference in boiling points is less than 25 K, then fractional distillation process is used.
Fractional distillation is used to separate different gases from air, different fractions from petroleum products etc. Apparatus of fractional distillation is similar to that of simple distillation and in fractional distillation; fractionating column is fitted in between the distillation flask and the condenser.
A simple fractionating column is a tube, which is packed with glass beads, which provide surface for the vapours to cool and condense repeatedly. Most of the natural substances are not pure. There are different methods of separation used to get an individual component from a mixture.
Heterogeneous mixtures can be separated into its constituents by simple physical methods like handpicking, sieving.
To Obtain Different Gases from Air
Air is a homogeneous mixture, which can be separated into its components by fractional distillation.
If you want oxygen gas from air, separate out all the other gases present in the air. The air is compressed by increasing the pressure and is then cooled by decreasing the temperature to get liquid air. This
liquid air is then allowed to warm-up slowly in a fractional distillation column, where gases are separated at different heights depending upon their boiling points.
Fractional distillation of liquid air is a process, which converts air into a liquid form and then allows it to be portioned out into layers and separated from one another. Because pure oxygen and nitrogen have a number of applications, this is a useful technique.

To perform this separation on air, it must be first cooled down to a very low temperature so that it liquefies. Once this is done, air is then passed into the bottom of a fractionating column, in which the temperature until the oxygen and nitrogen in the air separates from one another. From there, two tubes separately pipe off the gases.
This process can be repeated on the oxygen, as there are still trace amounts of argon found in the oxygen. Pure nitrogen, oxygen and argon can be removed from the air by the end of the final fractional distillation. The nitrogen is used in a number of different settings, especially the food and grocery industries.

Steps involved in separation of the components of air:
- First the air is filtered and the dust particles are removed.
- The air is then compressed under high pressure in a chamber.
- It is then passed through a water condenser to lower its temperature.
- The compressed air then moves into a separator where carbon dioxide separates out as dry ice.
- The air now becomes cold and turns into a liquid because of repeated compression.
- The liquid air then moves into the distillation column through expansion jet where it is warmed slowly.
- The boiling point of liquid nitrogen is –196°C so it boils out first to form liquid nitrogen gas.
- Argon is collected next having a boiling point of –186°C and finally oxygen having a boiling point of –183°C is collected last.
- The process is called fractional distillation
Chromatography
Chromatography is a technique used for separating the components, or solutes, of a mixture based on the relative amounts of each solute, which is distributed between a moving fluid stream called mobile phase and a contiguous stationary phase. The mobile phase may be either a liquid or a gas, while the stationary phase is either a solid or a liquid.
Chromatography is a differential migration from a narrow initial zone. Electrophoresis is another member of this group, but it is used mostly in biological labs and forensic labs. In electrophoresis, driving force is an electric field, which exerts different forces on solutes of different ionic charge.
The resistive force is the viscosity of the non-flowing solvent. The combination of these forces yields ion mobilities peculiar to each solute.
It is used to separate
- Colours in a dye;
- Pigments from natural colours; and
- Drugs from blood.
Crystallization
Crystallization is one of the very important purification techniques. Crystalline compounds are generally purified via crystallization process.
In crystallization, the impure substance is dissolved in a suitable solvent till it become a supersaturated solution by heating the solute in its solution form. Filtration of the hot solution is carried out so that if the hot solution contains any impurities, they can be filtered out.
Filtrate is then cooled and crystals of pure substance is obtained. The liquid left behind is called mother liquor. The crystals formed are separated by either decanting the mother liquor or by the process of filtration.
Crystallization is a process that separates a pure solid in the form of crystals from its solution. It is better than evaporation due to following reasons:
- Some solids get charred or they decompose on heating to dryness.
- Some impurities may remain dissolved in the solution which on evaporation contaminates the solid.
Crystallization Applications
- Purification of salt that we get from sea-water.
- In the pharmaceutical industry, crystallization is used as a separation and purification process.
- Separation of crystals of alum from impure samples.
NEET Foundation Notes For Chemistry Chapter 2 Is Matter Around Us Pure Water Purification System in Cities
In cities, drinking water is supplied from water works. A flow diagram of typical water system.
Water purification plays an important role in ensuring access to safe drinking water. Systems are in place to ensure the on-going quality of water, including water quality testing. The testing helps ensure that the water treatment process results in a product that meets federal water quality guidelines.
Water analysis involves looking for several kinds of contaminants, including unsafe levels of organic, inorganic, microbial and/or radioactive contaminants.

Screening
Water from lakes, rivers or the ground passes through a screen as it enters the water treatment plant. When the water source is a lake or river, the screen serves an important function, keeping out large natural contaminants such as plants and wood, or fish. If ground water is used, screening may not be necessary since the water has passed through layers of the earth in what is essentially a natural screening function.
Coagulation
Treatment plant workers add alum and other chemicals to the water, which cause tiny sticky particles, or floc to form. This floc attracts dirt particles, making them eventually heavy enough to sink to the bottom of the water storage tank.
Sedimentation
The water and floc flow into a sedimentation basin. As the water sits there, the heavy floc settle to the bottom, where they remain until removal.
Filtration
Water passes through layers of gravel, sand and perhaps charcoal, which serve to filter out any remaining particles. The gravel layer is often about one foot deep and the sand layer is about 2½ feet deep.
Disinfection
Water goes into a closed tank or reservoir. Chlorine or other disinfecting chemicals kill any remaining microorganisms or bacteria in the water and help keep the water clean until distribution. If a water treatment facility uses ground water as its only water source, disinfection may be the only step required to sufficiently treat the water. After it is disinfected, the purified water sits in the closed tank or reservoir until it flows through pipes to homes and businesses.
Let us summarize the separation of mixtures

NEET Foundation Notes For Chemistry Chapter 2 Is Matter Around Us Pure Physical and Chemical Changes
Properties which can be observed and can be specified like colour, hardness, rigidity, fluidity, density, melting point, boiling point, etc., are the physical properties. The inter conversion of states is a physical change as it occurs without a change in composition of the substance.
Ice, water and water vapour all of these look different and also display different physical properties, but are chemically same.
Water and cooking oil are liquid but their chemical characteristics are different. They differ in odour and in flammability as oil burns in air whereas water extinguishes fire. So, it is chemical property of oil which makes it different from water.
Burning is also a chemical change, as in this one substance reacts with another and undergo a change in chemical composition. Chemical change always brings change in the chemical properties of matter and as a result new substances. A chemical change is also called chemical reaction.
Difference between physical and chemical change

Features of Physical and Chemical Changes
- When physical change occurs in a substance, no new substance is created. The substance will remain in its original state.
- When chemical change occurs in the substance, you will be able to produce a different kind of substance. This means you will lose the original substance and a new one will form.
- A physical change is superficial and can possibly be reversed; a chemical change is complete and permanent.
- Physical change occurs faster and sometimes instantaneously. Most chemical changes, on the other hand, take longer time to become discernible.
- With physical change, you are not transforming the original molecular composition of the substance. But with chemical change, the molecular structure is being transformed thus you will get a new substance.
- A chemical change may also cause a physical change; a physical change alone cannot lead to a chemical change.
- Physical reactions can or cannot be initiated but chemical reactions start only after they are initiated.
- Energy changes are small in a physical reaction when compared to a chemical reaction.
- Chemical changes take place on the molecular level but physical changes are concerned with energy and states of matter.
NEET Foundation Notes For Chemistry Chapter 2 Is Matter Around Us Pure Types of Pure Substances
Substances can be classified as elements or compounds on the basis of their chemical composition.
Elements
In 1661, Boyle was the first scientist who used the term element. Antoine Laurent Lavoisier (1743–94), a French chemist, was the first to establish an useful definition for element and defines an element as a basic form of matter which cannot be broken down into simpler substances by chemical reactions.
Elements can be normally divided into:
- Metal
- Non-metals
- Metalloids.
Metals
Most elements are metals. It includes alkali metals, alkaline earth metals, transition metals, lanthanides and actinides. On the periodic table, metals are separated from non-metals by a zig-zag line stepping through carbon, phosphorus, selenium, iodine and radon. These elements and those to the right of them are non-metals.
Elements just to the left of the line may be termed metalloids or semi-metals and have properties intermediate between those of the metals and non-metals. The physical and chemical properties of the metals and nonmetals may be used to tell them apart.
Properties of metal:
- They have a lustre.
- They conduct heat and electricity.
- High melting point.
- High density.
- They are ductile.
- They are malleable.
- They are sonorous.
Examples: gold, silver, copper, iron, sodium, potassium, etc.
Non-metals
Non-metals, with the exception of hydrogen, are located on the right side of the periodic table. Some elements that are non-metals are hydrogen, carbon, nitrogen, phosphorus, oxygen, sulphur, selenium etc.
Properties of non-metal:
- They do not have lustre.
- They are poor conductors of heat and electricity.
- They are not ductile.
- They are not malleable.
- They are not sonorous.
Examples: hydrogen, oxygen, iodine, carbon (coal, coke),
Metalloids
Elements having an intermediate property of both metals and non-metals.
Example: boron, silicon, germanium etc.
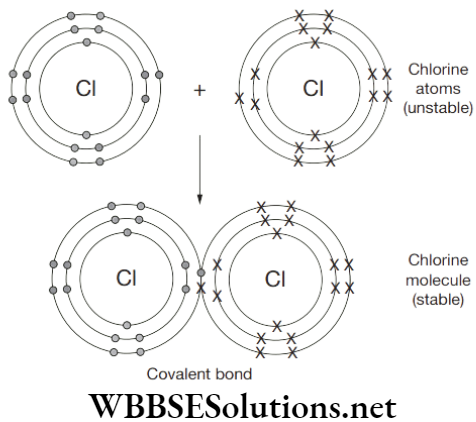
Compounds
Substance that is composed of two or more than two elements and is chemically combined with one another in a fixed proportion is called compounds.
Properties of compound are entirely different from its constituents which can be separated by physical means. Each compound has a fixed and sharp melting point. It is homogenous in nature. Energy in form of heat and light is either evolved or absorbed during the formation of a compound.
Example: Water, alcohol.
Compounds can also be classified as:
- 1. Inorganic and organic compounds of the basis of their structures.
- 2. Acids, bases and salts on the basis of their properties.
Difference between elements and compound

Important Terms to remember

NEET Foundation Notes For Chemistry Chapter 2 Is Matter Around Us Pure Classroom Corner Fill In the Blanks
Question 1. The phenomenon of crystallization is based on _________ of solution.
Answer. Heating
Question 2. The major components of air can be separated by ____________.
Answer. Fractional distillation
Question 3. ____________ is the solvent for Sulphur.
Answer. Carbon disulphide
Question 4. Blood is a type of a __________.
Answer. Mixture
Question 5. Brass is a/an _______.
Answer. Alloy
Question 6. Carbon is an example of _________.
Answer. Non-metal
Question 7. Ammonium chloride is an example of ____________.
Answer. Sublime
Question 8. ___________ do not scatter the light.
Answer. True solutions
Question 9. Particles of __________ are unstable.
Answer. Suspension
Question 10. A solution in which the solvent is not water is called ________ solution.
Answer. Non-aqueous
Question 11. In ___________ elements are chemically joined.
Answer. Compounds
Question 12. ____________ are not ductile.
Answer. Non-Metals
Question 13. The constituents of a __________ can be separated by physical methods.
Answer. Mixture
Question 14. Cooking is an example of __________ change.
Answer. Chemical
Question 15. A chemical change is usually irreversible. (True/False)
Answer. True
Question 16. No new product is formed in chemical change. (True/ False)
Answer. False
Question 17. __________ change is a process in which the substance experiences a change in its physical properties.
Answer. Physical
Question 18. Melting of wax is a __________ process.
Answer. Physical
NEET Foundation Notes For Chemistry Chapter 2 Is Matter Around Us Pure Match the Column
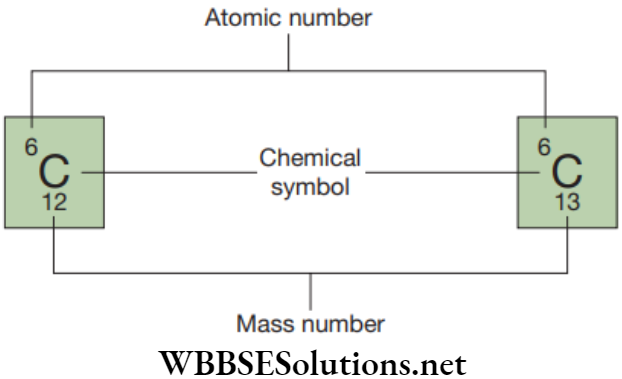
Question 1. Match the following separation techniques with the respective mixtures

Select the correct option:
- A-1, B-3, C-2, D-4
- A-3, B-2, C-4, D-1
- A-3, B-1, C-2, D-4
- A-4, B-2, C-1, D-3
Answer. 2. A-3, B-2, C-4, D-1
Question 2. Match the following separation techniques with the respective mixtures

Select the correct option:
- A-2, B-3, C-1, D-4
- A-1, B-2, C-4, D-3
- A-2, B-4, C-1, D-3
- A-3, B-1, C-4, D-2
Answer. 3. A-2, B-4, C-1, D-3
Question 3. Match the following separation techniques with the respective mixtures

Select the correct option:
- A-3, B-4, C-1, D-2
- A-3, B-2, C-4, D-1
- A-1, B-4, C-2, D-3
- A-4, B-2, C-1, D-3
Answer. 1. A-3, B-4, C-1, D-2
NEET Foundation Notes For Chemistry Chapter 2 Is Matter Around Us Pure Assertion Reason Type
For the following questions, the options will remain as follows:
- Both A and R are correct and R is the explanation of A
- Both A and R are correct, but R is not the logical explanation of A
- A is correct but R is incorrect
- A is incorrect but R is correct
Question 1. Assertion: The mixture of ammonium chloride and sand is separated by sublimation
Reason: Sand does not sublime, ammonium chloride sublimes
Answer. 1. Both A and R are correct and R is the explanation of A
Question 2. Assertion: Cream is separated from milk by centrifugation
Reason: Milk is a heterogeneous mixture
Answer. 2. Both A and R are correct, but R is not the logical explanation of A
Question 3. Assertion: Alum is used in the purification of water
Reason: Alum decreases the rate of sedimentation
Answer. 3. A is correct but R is incorrect
NEET Foundation Notes For Chemistry Chapter 2 Is Matter Around Us Pure Comprehension Passage
Read the passage and answer the questions:
Element is the simplest form of a pure substance which cannot be divided further into another simple substance. Thus, we can say that it is made up of only one kind of atoms. Out of 114 elements known today, 92 are natural and rest are made by man. Elements can be classified as:
Metals, non-metals, metalloids, inert gases.
To identify the elements, usually a symbol is assigned, e.g., C is the symbol for carbon. Elements are different from compounds, as they cannot be broken down further, whereas a compound can be broken down .both elements and compounds are pure substances.
Question 1. Which of the following elements is not solid at room temperature?
- Bi
- As
- Rn
- Br
Answer. 4. Br
Question 2. What can you say about sugar?
- It is an element
- It is a compound
- It is a mixture
- It is an alloy
Answer. 2. It is a compound
Question 3. Rn, Ar, Xe are all symbols of _________?
- Metals
- Non metals
- Metalloids
- Noble gases
Answer. 4. Noble gases
Question 4. Which of the following is a compound?
- Chlorine
- Gold
- Calcium Chloride
- Iron
Answer. 2. Gold
Question 5. The best method to separate the components of ink:
- Evaporation
- Vaporization
- Distillation
- Sublimation
Answer. 1. Evaporation