NEET Biology Chapter 3 Diversity In Living Organisms MCQs
Chapter 3 Diversity In Living Organisms Multiple Choice Question And Answers
Direction: Choose the correct option for each question. There is only one correct response for each question.
Question 1. Binomial nomenclature was given by
- R. H. Whittaker
- Linnaeus
- Aristotle
- Theophrastus
Answer. 2. Linnaeus
Question 2. The science of naming organism is called
- identification
- nomenclature
- classification
- taxonomy
Answer. 2. nomenclature
Question 3. The term biodiversity was coined by
- Rosen
- Linnnaeus
- Darwin
- Aristotle
Answer. 1. Rosen
Question 4. Which of the following categories contain least common features as compared to genus?
- Genus
- Division
- Class
- Family
Answer. 4. Family
NEET Biology Chapter 3 Diversity in Living Organisms MCQs
Read and Learn More NEET Foundation Multiple Choice Questions
Question 5. Taxon is the unit of
- order
- species
- genus
- taxonomy
Answer. 4. taxonomy
Question 6. Binomial nomenclature consists of two words
- genus and species
- order and family
- family and genus
- species and variety
Answer. 1. genus and species
Question 7. A plant body not differentiated into root, stem and leaves is termed as
- thallus
- mycelium
- hyphae
- herb
Answer. 1. thallus

Question 8. The phylum of sedentary animals is
- Mollusca
- Echinodermata
- Porifera
- Both (a) and (b)
Answer. 3. Porifera
Question 9. Tapeworms is a members of the phylum
- Annelida
- Porifera
- Nematoda
- Platyhelminthes
Answer. 4. Platyhelminthes
Question 10. True coelom appeared for the first time in
- Mollusca
- Nematoda
- Annelida
- Arthropoda
Answer. 3. Annelida
Question 11. Phylum Chordata is named after the occurrence of
- notochord
- dorsal hollow nerve cord.
- occurrence of postnatal tail.
- presence of gill slits.
Answer. 1. notochord
Question 12. Naked seeds are present in
- Mango
- Lemon
- Pinus
- Mustard
Answer. 3. Pinus
Question 13. The book System of Nature was written by
- Linnaeus
- Haeckel
- Whittaker
- Robert Brown
Answer. 1. Linnaeus
Question 14. The book Origin of Species was written by
- Haeckel
- Robert Brown
- Darwin
- Linnaeus
Answer. 3. Darwin
Question 15. Real organs are absent in
- Mollusca
- Cnidaria
- Arthropoda
- Echinodermata
Answer. 2. Cnidaria
Question 16. Two-chambered heart occurs in
- crocodiles
- amphibians
- aves
- fish
Answer. 4. fish
Question 17. Which of the following feature is not a characteristic of Protochordata?
- Presence of notochord
- Bilateral symmetry and coelom
- Jointed leg
- Presence of circulatory system
Answer. 3. Jointed leg
Question 18. The locomotory organs of Echinodermata are
- parapodia
- tube feet
- muscular feet
- jointed legs
Answer. 2. tube feet
NEET Biology Chapter 3 Diversity in Living Organisms MCQs
Question 19. Which one is a true fish?
- Jelly fish
- Star fish
- Dogfish
- Silverfish
Answer. 3. Dogfish
Question 20. Comb jellies belong to
- Scyphozoa
- Hydrozoa
- Ctenophora
- Rhyzopus
Answer. 3. Ctenophora
Question 21. Which of the following is triploid in nature in angiosperm?
- Embryo
- Endosperm
- Cotyledons
- Anther
Answer. 2. Endosperm
Question 22. Pick the odd one out.
- Maize
- Grass
- Wheat
- Mango
Answer. 4. Mango
Question 23. Which of the following is not an aquatic animal?
- Filaria
- Hydra
- Jelly fish
- Corals
Answer. 1. Filaria
Question 24. Differentiation in segmental fashion occurs in which of the following?
- Starfish
- Leech
- Snails
- Ascaris
Answer. 2. Leech
Question 25. Five-kingdom classification was given by
- Morgan
- Linnaeus
- R.H.Whittaker
- Haeckel
Answer. 3. R.H.Whittaker
NEET Foundation Biology Diversity in Living Organisms MCQs
Question 26. Carl von Linnaeus was involved with which branch of science?
- Morphology
- Taxonomy
- Physiology
- Medicine
Answer. 2. Taxonomy
Question 27. Amphibians do not have which of the following?
- Three chambered heart
- Gills or lungs
- Scales
- Mucous gland
Answer. 3. Scales
Question 28. Skeleton is made entirely of cartilage in
- Shark
- Tuna
- Rohu
- None of these
Answer. 1. Shark
Question 29. Well-defined nucleus is absent in which of the following?
- Diatoms
- Algae
- Yeast
- Blue-green algae
Answer. 4. Blue-green algae
Question 30. Tissue level organization is found in which of the following?
- Porifera
- Coelenterata
- Protozoa
- Round worms
Answer. 2. Coelenterata
Question 31. In higher chordates, notochordis transformedinto
(1) Cranium
(2) Limbs
(3) Vertebral column
(4) Canal system
Select the correct option.
- Only (1) and (2)
- Only (2) and (3)
- Only (3) and (4)
- Only (1) and (4)
Answer. 2. Only (2) and (3)
Question 32. Cnidaria is characterized by
(1) Nematoblasts
(2) Coelenteron
(3) Jointed legs
(4) Cellular level of organization
Select the correct option.
- Only (1) and (2)
- Only (2) and (3)
- Only (3) and (4)
- Only (1) and (4)
Answer. 1. Only (1) and (2)
Question 33. Bilateral symmetry means_____________
(1) Limbs and organs are paired.
(2) Limbs and organs occur all around the central axis.
(3) Cephalization is present.
(4) Cephalization is absent.
Select the correct option:
- Only (1) and (2)
- Only (2) and (3)
- Only (1) and (3)
- Only (3) and (4)
Answer. 3. Only (1) and (3)
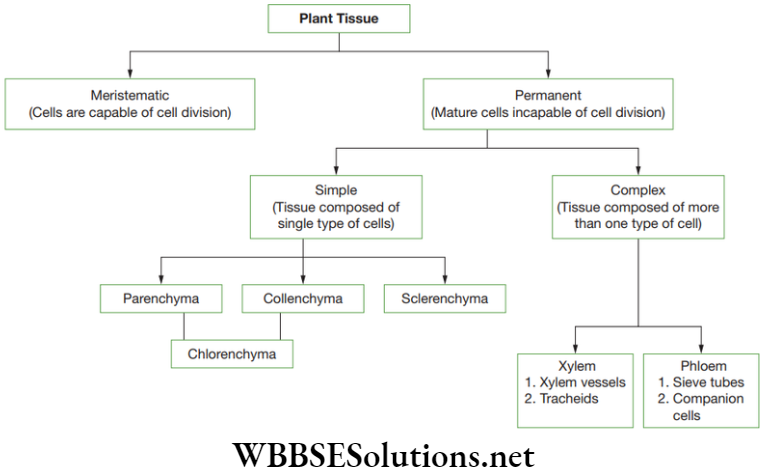
Question 34. The characteristic features of Bryophyta is_____
(1) Plant body is sporophytic.
(2) They are vascular plants.
(3) Plant body is gametophytic.
(4) They are non-vascular in nature.
Select the correct option.
- Only (1) and (2)
- Only (2) and (3)
- Only (3) and (4)
- Only (1) and (4)
Answer. 3. Only (3) and (4)
NEET Foundation Biology Diversity in Living Organisms MCQs
Question 35. Binomial nomenclature consists of two words
(1) Order
(2) Family
(3) Genus
(4) Species
Select the correct option.
- Only (1) and (2)
- Only (2) and (3)
- Only (1) and (4)
- Only (3) and (4)
Answer. 4. Only (3) and (4)
Question 36. Characteristic features of Fungi are
(1) Presence of photosynthetic pigments.
(2) The cell wall is made of chitin.
(3) The cell wall is made up of cellulose.
(4) Absence of photosynthetic pigments.
Select the correct option.
- Only (1) and (2)
- Only (2) and (4)
- Only (1) and (4)
- Only (3) and (4)
Answer. 2. Only (2) and (4)
| Class 11 Physics | Class 12 Maths | Class 11 Chemistry |
| NEET Foundation | Class 12 Physics | NEET Physics |
Question 37. Which among the following have open circulatory system?
(1) Arthropoda
(2) Mollusca
(3) Annelida
(4) Cnidaria
Select the correct option.
- Only (1) and (2)
- Only (2) and (3)
- Only (3) and (4)
- Only (1) and (4)
Answer. 1. Only (1) and (2)
NEET Foundation Biology Diversity in Living Organisms MCQs
Question 38. Organisms without nucleus and cell organelles belong to
(1) Fungi
(2) Protista
(3) Archaebacteria
(4) Cyanobacteria
Select the correct option.
- Only (1) and (2)
- Only (2) and (3)
- Only (3) and (4)
- Only (1) and (4)
Answer. 3. Only (3) and (4)
Question 39. Which among the following has specialized tissue for conduction of water?
(1) Gymnospermae
(2) Bryophyta
(3) Thallophyta
(4) Pteridophyta
Select the correct option.
- Only (1) and (2)
- Only (2) and (3)
- Only (3) and (4)
- Only (1) and (4)
Answer. 4. Only (1) and (4)
Question 40. Which among the following have scales?
(1) Amphibians
(2) Pisces
(3) Reptiles
(4) Mammals
Select the correct option.
- Only (1) and (3)
- Only (3) and (4)
- Only (2) and (3)
- Only (1) and (2)
Answer. 3. Only (2) and (3)
Question 41. The characteristic features of Aves are
(1) Mammary glands are absent.
(2) Mammary glands are present.
(3) Forelimbs are modified into wings.
(4) Wings are absent.
Select the correct option.
- Only (1) and (2)
- Only (1) and (3)
- Only (2) and (3)
- Only (2) and (4)
Answer. 2. Only (1) and (3)
NEET Chapter 3 Biology MCQs Diversity in Living Organisms
Question 42. Bony fishes are characterized by _______
(1) Cartilaginous endoskeleton
(2) 5–7 pairs of gill slits
(3) Terminal positioned mouth
(4) 4 pairs of gill slits
Select the correct option.
- Only (1) and (2)
- Only (2) and (3)
- Only (3) and (4)
- Only (1) and (4)
Answer. 3. Only (3) and (4)
Question 43. Annelids have ______________
(1) Unjointed appendages
(2) Closed circulatory system
(3) Jointed appendages
(4) Open circulatory system
Select the correct option.
- Only (1) and (2)
- Only (2) and (3)
- Only (3) and (4)
- Only (1) and (4)
Answer. 1. Only (1) and (2)
Question 44. Reptilia are characterized by ____________
(1) Three-chambered heart
(2) Incompletely four-chambered heart
(3) Fertilization is external
(4) Fertilization is internal
Select the correct option.
- Only (1) and (2)
- Only (2) and (4)
- Only (3) and (4)
- Only (1) and (4)
Answer. 2. Only (2) and (4)
NEET Chapter 3 Biology MCQs Diversity in Living Organisms
Question 45. Male ascaris can be distinguished from female one by the______________
(1) Curved posterior
(2) Round shape end
(3) Presence of penial setae
(4) Straight posterior
Select the correct option.
- Only (1) and (2)
- Only (2) and (4)
- Only (3) and (4)
- Only (1) and (4)
Answer. 1. Only (1) and (2)
Question 46. The lowest category of classification is
- Phylum
- Genus
- Species
- Family
Answer. 3. Species
Question 47. The mode of nutrition in most fungi is
- Autotrophic
- Saprophytic
- Holozoic
- Symbiotic
Answer. 2. Saprophytic
Question 48. In Whittaker’s system of classification, unicellular organisms are kept under
- Monera
- Fungi
- Protista
- Protozoa
Answer. 3. Protista
Question 49. Pinus is a ___________ plant.
- Bryophytic
- Gymnospermous
- Pteridophytic
- Angiospermous
Answer. 2. Gymnospermous
Question 50. Find out incorrect sentence
- Protista includes unicelluar eukaryotic organisms.
- Whittaker considered cell sturcture, mode and source of nutrition for classifying the organisms in five kingdoms.
- Both Monera and Protista may be autotrophic and heterotrophic.
- Monerans have well defined nucleus.
Answer. 4. Monerans have well defined nucleus.
Question 51. Which among the following has specialized tissue for conduction of water?
(1) Thallophyta
(2) Bryophyta
(3) Pteridophyta
(4) Gymnosperms
- (1) and (2)
- (2) and (3)
- (3) and (4)
- (1) and (4)
Answer. 3. (3) and (4)
Question 52. Which among the following produce seeds?
- Thallophyta
- Bryophyta
- Pteridophyta
- Gymnosperms
Answer. 4. Gymnosperms
NEET Chapter 3 Biology MCQs Diversity in Living Organisms
Question 53. Organisms without nucleus and cell organelles belong to
(1) fungi
(2) protista
(3) cyanobacteria
(4) archaebacteria
- (1) and (2)
- (3) and (4)
- (1) and (4)
- (2) and (3)
Answer. 2. (3) and (4)
Question 54. Which of the following is not a criterion for classification of living organisms?
- Body design of the organism
- Ability to produce one’s own food
- Membrane bound nucleus and cell organelles
- Height to the plant
Answer. 4. Height to the plant
Question 55. The book System a Nature was written by
- Linnaeus
- Haeckel
- Whittaker
- Robert Brown
Answer. 1. Linnaeus
Question 56. Karl von Linne was involved with which branch of science?
- Morphology
- Taxonomy
- Physiology
- Medicine
Answer. 2. Taxonomy
Question 57. 5-kingdom classification was given by
- Morgan
- R. Whittaker
- Linnaeus
- Haeckel
Answer. 2. R. Whittaker
Question 58. Well defined nucleus is absent in
- blue green algae
- diatoms
- algae
- yeast
Answer. 1. blue green algae
NEET Chapter 3 Biology MCQs Diversity in Living Organisms
Question 59. The ‘Origin of Species’ is written by
- Linnaeus
- Darwin
- Haeckel
- Whittaker
Answer. 2. Darwin
Question 60. The most simple and primitive plants are
- bacteria
- algae
- protista
- fungi
Answer. 2. algae
Question 61. Which of the following organism lacks chlorophyll?
- Rhizopus
- Cycas
- Spirogyra
- Wheat
Answer. 1. Rhizopus
Question 62. Prokaryotic organisms are found in kingdom
- Protista
- Fungi
- Monera
- Plantae
Answer. 3. Monera
Question 63. To which group of animals, the nematocysts are unique:
- Cnidaria
- Porifera
- Platyhelminthes
- Annelida
Answer. 1. Cnidaria
Diversity in Living Organisms MCQs for NEET Preparation
Question 64. Tube-within-tube plain is shown by:
- Coelenterates
- Flatworms
- Round worms
- Sponges
Answer. 3. Round worms
Question 65. Excretory organs of annelids are:
- Protonephridia
- Nephridia
- Green glands
- Kidneys
Answer. 2. Nephridia
Question 66. Study of molluscs is called:
- Malacology
- Conchology
- Mycology
- Phycology
Answer. 1. Malacology
Question 67. Aristotle’s lantern is found in:
- Star fish
- Brittle star
- Sea urchin
- Sea cucumber
Answer. 3. Sea urchin
Question 68. Link between animal kingdom and plant kingdom is:
- Euglena
- Amoeba
- Trypansoma
- Paramecium
Answer. 1. Euglena
Question 69. Metameric segmentation first appeared in:
- Platyhelminthes
- Annelida
- Cockroach
- Arthropoda
Answer. 2. Annelida
Question 70. The following vertebrate respires by skin:
- Fish
- Frog
- Crocodile
- Whale
Answer. 2. Frog
Diversity in Living Organisms MCQs for NEET Preparation
Question 71. True coelom is lined with:
- Ectoderm
- Endoderm
- Mesoderm
- Ectoderm and endoderm
Answer. 3. Mesoderm
Question 72. Excretory cells of platyhelminthes are:
- Flame cells
- Nephridia
- Solenocytes
- Both (a) and (c)
Answer. 4. Both (a) and (c)
Question 73. Green glands present in some arthropod help in:
- Respiration
- Digestion
- Excretion
- None of these
Answer. 3. Excretion
Diversity in Living Organisms MCQs for NEET Preparation
Question 74. Which of the following classes has largest number of animals?
- Pisces
- Reptilia
- Mammalia
- Insecta
Answer. 4. Insecta
Question 75. An open circulatory system occurs in the:
- Reptiles
- Birds
- Insects
- Annelids
Answer. 3. Insects
Question 76. In Panthera pardus, the genus name is
- Pardus
- Panthera
- None of these
- Both Panthera and pardus
Answer. 2. Panthera
Question 77. Name the field of biology that deals with the identification, nomenclature and classification of organisms.
- Systematics
- Biogeography
- Taxonomy
- Vernacular science
Answer. 3. Taxonomy
Question 78. The naming system where every organism’s scientific names consists of two names is called
- binomial nomenclature
- binary nomenclature
- bilateral nomenclature
- coded nomenclature
Answer. 1. binomial nomenclature
Question 79. ______ is the study of evidences of the past life in the form of fossils.
- Taxonomy
- Classification
- Palaeontology
- Systematics
Answer. 3. Palaeontology
NEET Biology Diversity in Living Organisms Practice Questions
Question 80. Which theory states that organisms with different variant forms leave different numbers of offspring to future generations?
- Perpetual change
- Common descent
- Graduation
- Natural selection
Answer. 4. Natural selection
Question 81. Which theory states that the large differences in anatomical characteristics that make different species originate through the accumulation of many small incremental changes over long duration of time?
- Perpetual change
- Common descent
- Graduation
- Natural selection
Answer. 3. Graduation
Question 82. _______ includes all organisms who share a set of distinguishing common characters.
- Order
- Phylum
- Kingdom
- Family
Answer. 3. Kingdom
Question 83. Which of the following archaea favour extremely hot and acidic environments like hot springs?
- Methanogens
- Thermoacidophiles
- Halophiles
- Eubacteria
Answer. 1. Methanogens
Question 84. Which of the following archaea grow in very salty environments?
- Methanogens
- Thermoacidophiles
- Halophiles
- Eubacteria
Answer. 3. Halophiles
Question 85. Blue-green algae is also known as
- Actinomycetes
- Enterobacteria
- Spirochaetes
- Cyanobacteria
Answer. 2. Enterobacteria
Question 86. In which of the following, blue-green algae and fungi live in symbiotic relationship?
- Rhizopoda
- Lichens
- Protista
- Protozoa
Answer. 3. Protista
Question 87. Which of the following lack locomotory organs?
- Sporozoa
- Ciliata
- Mastigophora
- Sarcodina
Answer. 4. Sarcodina
NEET Biology Diversity in Living Organisms Practice Questions
Question 88. The mode of nutrition in fungi is
- only saprotrophic.
- saprotrophic or parasitic.
- only parasitic.
- none of above
Answer. 2. saprotrophic or parasitic.
Question 89. Which of the following is not possessed by bacteria?
- Membrane bound organelles
- Nucleus
- Nucleolus
- All of these
Answer. 4. All of these
Question 90. Which sub-group in plant kingdom produces flowers?
- Angiosperms
- Fungi
- Mosses
- Ferns
Answer. 1. Angiosperms
Question 91. What does a bacteria lack?
- Endoplasmic reticulum
- DNA.
- Cell wall
- Cytoplasm
Answer. 1. DNA.
Question 92. What is the mode of nutrition in bacteria?
- Autotrophic
- Heterotrophic
- Autotrophic and heterotrophic
- None of these
Answer. 3. Autotrophic and heterotrophic
Question 93. In algae, asexual reproduction usually takes place by
- spores
- gametes
- single-celled sex organs
- embryo
Answer. 2. gametes
Question 94. Pteridophytes have sex organs that are
- unicellular only.
- multicellular without any jacket.
- multicellular and jacketed by sterile cells.
- bicellular
Answer. 3. multicellular and jacketed by sterile cells.
Question 95. Which of the following are known as the ‘Amphibians of Plant Kingdom’?
- Pteridophytes
- Bryophytes
- Gymnosperms
- Green algae
Answer. 4. Green algae
Question 96. Name the most primitive and simple seed plants.
- Pteridophytes
- Bryophytes
- Gymnosperms
- Angiosperms
Answer. 2. Bryophytes
NEET Biology Diversity in Living Organisms Practice Questions
Question 97. In which of the following the reproductive organs are hidden?
- Cryptogamae
- Phanerogamae
- Gymnosperms
- Angiosperms
Answer. 1. Cryptogamae
Question 98. Choose the vascular plants out of these.
- Mosses
- Liverworts
- Hornworts
- Ferns
Answer. 4. Ferns
Question 99. Which of the these are found filamentous?
- Spirogyra
- Euglena
- Chlamydomonas
- Amoeba
Answer. 1. Spirogyra
Question 100. Which of the following does a bacteria lack?
- Endoplasmic reticulum
- DNA
- Cell wall
- Cytoplasm
Answer. 1. Endoplasmic reticulum
Question 101. What is the phylum of octopus?
- Arthropoda
- Mollusca
- Annelida
- cnidarian
Answer. 2. Mollusca
NEET Biology Diversity in Living Organisms Practice Questions
Question 102. Gametophytic and sporophytic generations are seen in the life history of which of the following?
- Fungi
- Algae
- Ferns
- Diatoms
Answer. 3. Ferns