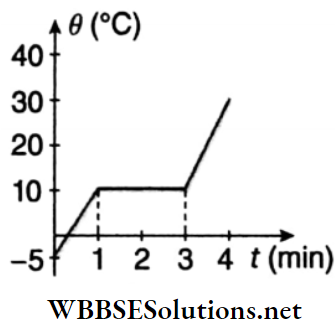
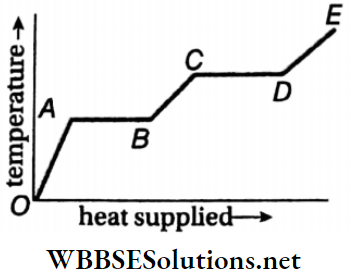
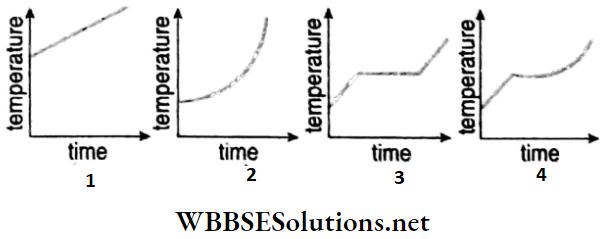
Unit 1 Physical World And Measurement Chapter 1 Measurement And Dimension Of Physical Quantity Short Answer Type Questions
WBBSE Class 11 Short Answer Questions on Measurement
Question 1. To determine an unknown resistance R in the laboratory, readings of potential difference V and electric current I are taken. From these readings, percentage error is calculated and V and l are obtained as V = (100±5) volt, l = (10±0.2) amp. The percentage error in the value of R determined from these V and l will be
- 3%
- 4.8%
- 5.2%
- 7%
Answer:
We know, R = \(\frac{V}{l}\)
∴ Percentage error in the value of R, \(\frac{\Delta R}{R} \times 100 \%=\frac{\Delta V}{V} \times 100 \%+\frac{\Delta I}{I} \times 100 \%\)
= 5%+ 2% = 7%
The option 4 is correct.
Question 2. How many significant digits are there in 0.06900?
Answer: Number of significant digits = 4
Question 3. Assuming velocity (V), time (T), and force (F) as the fundamental quantities, find the dimension of density.
Answer:
Assuming velocity (V), time (T), and force (F) as the fundamental quantities
Dimension of velocity, [V] = LT-1; dimension of time, [T] = T; dimension of force, [F] = MLT-2; dimension of density, [D] = ML-3
As the dimension of density does not involve T, we have
[D] = \(\mathrm{ML}^{-3}=\frac{M L T^{-2}}{L^4 T^{-4} \times T^2}\)
= \(\frac{[F]}{[V]^4 \cdot[T]^2}=[F][V]^{-4}[T]^{-2}\)
i.e., \(\mathrm{FV}^{-4} \mathrm{~T}^{-2}\) is the dimension of density, assuming y, T, and F as the fundamental quantities.
Question 4. The velocity of a particle in time t is v = \(a t+\frac{b}{t+c}\). The dimensions of a, b, and c are
- L2, T, LT
- LT-2,L,T
- LT2, LT, L
- L, LT, T2
Answer:
[v] = LT-1
∴ [at] = LT-1 or, \([a]=\frac{\mathrm{LT}^{-1}}{\mathrm{~T}}=\mathrm{LT}^{-2}\)
Again, [t + c] = T
∴ [c] = T
Now, \(\left[\frac{b}{t+c}\right]=\mathrm{LT}^{-1} \quad \text { or. }[b]=\mathrm{LT}^{-1} \cdot \mathrm{T}=\mathrm{L}\)
The option 2 is correct.

Question 5. If the error in measurement of the radius of a sphere is 2%. then the error in the determination of the volume of the sphere will be
- 4%
- 6%
- 8%
- 2%
Answer:
Let the actual and the incorrect radii be r and r’ respectively.
According to the question, \(\frac{r^{\prime}}{r}=\frac{102}{100}=1.02\)
(We could also take \(\frac{r^{\prime}}{r}=\frac{98}{100}=0.98\))
∴ \(\frac{\text { incorrect volume }}{\text { actual volume }}\)
= \(\frac{\frac{4}{3} \pi r^{\prime 3}}{\frac{4}{3} \pi r^3}=\left(\frac{r^{\prime}}{r}\right)^3\)
= \((1.02)^3 \approx 1.06\)
Therefore, percentage error =(1.06-1) x 100% = 6%
The option 2 is correct.
Alternate Method:
Volume, V = \(\frac{4}{3} \pi r^3 or, \ln V=\ln \left(\frac{4}{3} \pi\right)+3 \ln r\)
Differentiating, we get \(\frac{d V}{V}=0+3 \frac{d r}{r}\)
Here, error in measuring radius, \(\frac{d r}{r}=2 \%\)
Therefore, error in volume measurement, \(\frac{d V}{V}\) = 3×2% =6%
[This method is applicable only when percentage error << 100]
Dimensional Analysis Short Answer Questions for Class 11
Question 6. Given Z = \(\frac{A^4 B^{1 / 3}}{C D^{3 / 2}}\) where A, B, C, and D are physical quantities. What will be the maximum percentage error in Z.
Answer:
Given Z = \(\frac{A^4 B^{1 / 3}}{C D^{3 / 2}}\) where A, B, C, and D are physical quantities.
Maximum percentage error in Z
= \(\frac{d Z}{Z} \times 100\)
= \(4 \frac{d A}{A} \times 100+\frac{1}{3} \frac{d B}{B} \times 100+\frac{d C}{C} \times 100+\frac{3}{2} \frac{d D}{D} \times 100\)
Question 7. What will be the dimension of Young’s modulus if velocity (v), acceleration (A), and force (F) are taken as fundamental quantities?
Answer: The dimension of Young’s modulus, [Y] = V-4A2F.
Question 8. The number of significant figures in 6.0025 is
- 1
- 4
- 5
- 2
Answer: The option 3 is correct.
Question 9. If velocity (v), acceleration (A), and force (F) are three fundamental quantities in a system, then what will be the dimension of linear momentum in this system?
Answer:
If velocity (v), acceleration (A), and force (F) are three fundamental quantities in a system
Mass = \(\frac{\text { force }}{\text { acceleration }} ;\)
Dimension of mass = \(\frac{\text { dimension of force }}{\text { dimension of acceleration }}=\frac{F}{A}\)
∴ Dimension of linear momentum = \(\frac{F}{A}\)V= VA-1F
Question 10. For a moving particle, the relation between the distance (5) and time (t) is S = a+ bt+ ct2 + dt3; which one among a, b, c, and d will represent the dimension of acceleration?
Answer:
For a moving particle, the relation between the distance (5) and time (t) is S = a+ bt+ ct2 + dt3
[S] = L = dimension of each term on the right-hand side
Hence,[ct2] = L or, [c]T2 = L
or, [c] = LT-2= dimension of acceleration
∴ The constant c will represent the dimension of acceleration.
Question 11. If the error in the measurement of the radius of a circular disc is 2%, the error in determining the area of the disc will be
- 4%
- 2%
- 6%
- 8%
Answer:
The error in the measurement of the radius of the circular disc =2%
Area of the disc, A = πr²
∴ dA = 2πrdr
Now, \(\frac{d A}{A}=\frac{2 \pi r d r}{\pi r^2}\) or, \(\frac{d A}{A} \times 100=\frac{2 d r}{r} \times 100\)
= 2 x 2 =4
The option 1 is correct.
Question 12. What are the significant figures in the result of the addition of 9.8 and 15.298?
Answer:
9.8 And 15.298
9.8 + 15.298 = 25.098
The number of significant figures = 5.
Question 13. Which two of the following physical quantities are dimensionally alike?
- Surface tension,
- Pressure,
- Coefficient of viscosity,
- Coefficient of elasticity.
Answer:
The dimension of both pressure and coefficient of elasticity is ML-1T-2.
Key Concepts in Measurement and Dimensions: Short Answers
Question 14. If n denotes a positive integer, h the Planck’s constant, q the charge, and B the magnetic field, then the quantity \(\left(\frac{n h}{2 \pi q B}\right)\) has the dimension of
- Area
- Length
- Speed
- Acceleration
Answer:
⇒ \({\left[\frac{n h}{2 \pi q B}\right] }=\frac{[m \nu r]}{[q B]}=\frac{[m \nu r][v]}{[F]}\)
= \(\frac{\mathrm{ML}^2 \mathrm{~T}^{-2} \mathrm{~L}}{\mathrm{MLT}^{-2}}=\mathrm{L}^2\)
The option 1 is correct.
Question 15. In which of the following pairs, do the two physical quantities have different dimensions?
- Planck’s constant and angular momentum
- Impulse and linear momentum
- Moment of inertia and moment of force
- Energy and torque
Answer: The option 3 is correct.
Question 16. If x = at+bt² where x is in meter (m) and t is in an hour (h), then a unit of b will be
- m²/h
- m
- m/h
- m/h²
Answer:
The dimensional formula of x, |x| = \(\left[b t^2\right] \quad \text { or, }[b]=\frac{[x]}{\left[t^2\right]}\)
∴ Unit of b = m/h²
The option 4 is correct.
Question 17. The dimension of the universal constant of gravitation G is
- ML2T-1
- M-1L3T-2
- M-1L2T-2
- ML3T-2
Answer:
[G] = \(\left[\frac{F r^2}{m_1 m_2}\right]\) [from Newton’s law of gravitation]
= \(\frac{M L T^{-2} \times L^2}{M^2}=M^{-1} L^3 T^{-2}\)
Question 18. A spherical liquid drop is placed on a horizontal plane. A small disturbance causes the volume of the drop to oscillate. The time period of oscillation (T) of the liquid drop depends on the radius (r) of the drop, density (ρ), and surface tension (s) of the liquid. Which among the following will be a possible expression for T (where k is a dimensionless constant)?
- \(k \sqrt{\frac{\rho r}{s}}\)
- \(k \sqrt{\frac{\rho^2 r}{s}}\)
- \(k \sqrt{\frac{\rho r^3}{s}}\)
- \(k \sqrt{\frac{\rho r^3}{s^2}}\)
Answer:
Given
A spherical liquid drop is placed on a horizontal plane. A small disturbance causes the volume of the drop to oscillate. The time period of oscillation (T) of the liquid drop depends on the radius (r) of the drop, density (ρ), and surface tension (s) of the liquid.
Let, \(T \propto r^a \rho^b s^c\)
or, \(T=k r^a \rho^b s^c\)
From dimensional analysis, \(\mathrm{T}^1=\mathrm{L}^a\left(\mathrm{ML}^{-3}\right)^b\left(\mathrm{MT}^{-2}\right)^c\)
= \(\mathrm{L}^{(a-3 b)} \cdot \mathrm{M}^{(b+c)} \cdot \mathrm{T}^{-2 c}\)
Equating the power of both sides, c = \(-\frac{1}{2} ; b=\frac{1}{2} ; a=\frac{3}{2}\)
⇔ From equation (1), \(T=k r^{\frac{+3}{2}} \cdot \rho^{\frac{1}{2}} \cdot s^{-\frac{1}{2}}\)
or, \(T=k \sqrt{\frac{\rho r^3}{s}}\)
The option 3 is correct.
Applications of Dimensional Analysis: Short Answer Format
Question 19. The current-voltage relation of the diode is given by I = \(\left(e^{1000 \frac{V}{T}}-1\right) \mathrm{mA}\), where the applied voltage V is in volt and the temperature T is in kelvin. If a student makes an error of ±0.01 V in voltage while measuring the current of 5 mA at 300 K, what will be the error in the value of current in mA?
- 0.2 mA
- 0.02 mA
- 0.5 mA
- 0.05 mA
Answer:
Given
The current-voltage relation of the diode is given by I = \(\left(e^{1000 \frac{V}{T}}-1\right) \mathrm{mA}\), where the applied voltage V is in volt and the temperature T is in kelvin. If a student makes an error of ±0.01 V in voltage while measuring the current of 5 mA at 300 K,
5 = \(e^{1000 \frac{V}{T}}-1 \quad \text { or, } e^{1000 \frac{V}{T}}=6\)
Again, \(I=e^{1000 \frac{V}{T}}-1\)
∴ \(\frac{d I}{d V}=e^{\frac{1000 V}{T} \frac{1000}{T}} \text { or, } d I=\frac{1000}{T} e^{\frac{1000 V}{T}} d V\)
Using equation (1), \(\Delta I=\frac{1000}{T} \times 6 \times 0.01=\frac{60}{T}\)
ΔI = \(\frac{60}{300}=0.2 \mathrm{~mA}\)
Question 20. A student measured the length of a rod and wrote it as 3.50 cm. Which instrument did he use to measure it?
- A meter scale
- A vernier caliper where 10 divisions in the vernier scale match with 9 divisions in the main scale, and the main scale has 10 divisions in 1 cm (i.e. 1 smallest main scale division is 0.1 cm)
- A screw gauge having 100 divisions in the circular scale and a pitch as 1 mm
- A screw gauge having 50 divisions in the circular scale and a pitch as 1 mm
Answer:
Given
A student measured the length of a rod and wrote it as 3.50 cm.
The least count of vernier caliper is 1
1/10 mm = 0.1mm = 0.01 cm
The option 2 is correct
Question 21. The period of oscillation of a simple pendulum is T = \(2 \pi \sqrt{\frac{L}{g}}\) Measured value of L is 20.0 cm known to 1 mm accuracy and time for 100 oscillations of the pendulum is found to be 90 s using a wrist watch of 1 s resolution. The accuracy in the determination of g is
- 2%
- 3%
- 1%
- 5%
Answer:
Given
The period of oscillation of a simple pendulum is T = \(2 \pi \sqrt{\frac{L}{g}}\) Measured value of L is 20.0 cm known to 1 mm accuracy and time for 100 oscillations of the pendulum is found to be 90 s using a wrist watch of 1 s resolution.
g = \(4 \pi^2 \frac{L}{T^2}\)
or, lng = ln(4;r2) + InL-21nT
Differentiating, we get \(\frac{d g}{g}=\frac{d L}{L}+2\left(\frac{-d T}{T}\right) \)
= \(\frac{1 \mathrm{~mm}}{20.0 \mathrm{~cm}}+2 \times \frac{1 \mathrm{~s}}{90 \mathrm{~s}}\)
[To determine the maximum error, -dT has been taken as 1s]
= \(\frac{1}{200}\) + \(\frac{2}{90}\) = 0.0272 = 2.72% = 3%
The option 4 is correct.
Question 22. A student measures the time period of 100 oscillations of a simple pendulum four times. The data set is the 90s, 91s, 95s and 92s. If the minimum division in the measuring clock is ls, then the reported mean time should be
- 92±2 s
- 92±5.0 s
- 92±1.8s
- 92±3 s
Answer:
Given
A student measures the time period of 100 oscillations of a simple pendulum four times. The data set is the 90s, 91s, 95s and 92s. If the minimum division in the measuring clock is ls,
Average value of time period, \(\bar{x}=\frac{\sum_{i=1}^4 x_i}{N}=\frac{90+91+95+92}{4}=92\)
Average maximum error, \(\epsilon=\frac{\sum_{i=1}^4\left|\bar{x}-x_i\right|}{N}=\frac{2+1+3+0}{4}=1.5\)
As the clock is able to measure a minimum time of 1 s, the reported mean time = 92±2 s.
The option 1 is correct.
Question 23. A screw gauge with a pitch of 0.5 mm and a circular scale with 50 divisions is used to measure the thick¬ness of a thin sheet of Aluminum. Before starting the measurement, it is found that when the two jaws of the screw gauge are brought in contact, the 45th division coincides with the main scale line and that the zero of the main scale is barely visible. What is the thickness of the sheet if the main scale reading is 0.5 mm and the 25th division coincides with the main scale line?
- 0.75 mm
- 0.80 mm
- 0.70 mm
- 0.50 mm
Answer:
Given
A screw gauge with a pitch of 0.5 mm and a circular scale with 50 divisions is used to measure the thick¬ness of a thin sheet of Aluminum. Before starting the measurement, it is found that when the two jaws of the screw gauge are brought in contact, the 45th division coincides with the main scale line and that the zero of the main scale is barely visible.
Least count = \(\frac{\text { pitch }}{\text { total number of division in circular scale }}\)
= \(\frac{0.5}{50}\) = 0.01 mm
Error = -5 x least count = -0.05 mm
∴ Thickness = 0.5 mm + {(25 x 0.01) mm – (-0.05) mm}
= 0.5 mm + 0.3 mm = 0.80 mm
The option 2 is correct.
Short Answer Questions on Dimensional Formulas
Question 24. The following observations were taken for determining surface tension T of water by the capillary method: diameter of the capillary, D = 1.25 x 10-2 m rises of water, h = 1.45 x 10-2m. Using g = 9.80 m/s2 and the simplified relation T =\(\frac{ r h g}{2}\) x 103 N/m, the possible error in surface tension is closest to:
- 0.15%
- 1.5%
- 2.4%
- 10%
Answer:
Given
The following observations were taken for determining surface tension T of water by the capillary method: diameter of the capillary, D = 1.25 x 10-2 m rises of water, h = 1.45 x 10-2m. Using g = 9.80 m/s2 and the simplified relation T =\(\frac{ r h g}{2}\) x 103 N/m,
T = \(\frac{r h g}{2}x10^3\) = \(\frac{D h g}{4}x10^3\)
∴ \(\frac{\Delta T}{T}\) x 100 = \(\frac{\Delta D}{D}\) + \(\frac{\Delta h}{h}\) x 100
= \(\frac{0.01}{1.25}\) x 100 + \(\frac{0.01}{1.45}\) x 100
= 1.489 →1.5%
The option 2 is correct.
Question 25. The density of a material in the shape of a cube is determined by measuring three sides of the cube and its mass. If the relative errors in measuring the mass and length are respectively 1.5% and 1%, the maxi¬mum error in determining the density is
- 4.5%
- 6%
- 2.5%
- 3.5%
Answer:
Given
The density of a material in the shape of a cube is determined by measuring three sides of the cube and its mass. If the relative errors in measuring the mass and length are respectively 1.5% and 1%
ρ = \(\frac{M}{V}\) = \(\frac{M}{l^3}\)
∴ \(\left(\frac{d \rho}{\rho}\right)_{\max }\) = \(\frac{dM}{M}\) + 3\(\frac{dl}{l}\) = 1.5% + 3 x l% = 4.5%
Question 26. If energy (E), velocity (V), and time (T) are chosen as the fundamental quantities, the dimensional formula of surface tension will be
- EV-2T-1
- EV-1T-2
- EV-2T-2
- E-2V-1T-3
Answer:
Dimension of mass, [M] = ML2T-2 · L-2T2 = ML2T-2 ·(LT-1)-2 = EV-2
Therefore, the dimensional formula of surface tension, MT-2 = EV-2T-2
The option 3 is correct.
Question 27. A student performs an experiment of measuring the thickness of a slab with a vernier caliper whose 50 divisions of the vernier scale are equal to 49 divisions of the main scale. He noted that zero of the vernier scale is between the 7.00 cm and 7.05 cm mark of the main scale and 23rd division of the vernier scale exactly coincides with the main scale. The measured value of the thickness of the given slab using the caliper will be
- 7.73 cm
- 7.23 cm
- 7.023 cm
- 7.073 cm
Answer:
Given
A student performs an experiment of measuring the thickness of a slab with a vernier caliper whose 50 divisions of the vernier scale are equal to 49 divisions of the main scale. He noted that zero of the vernier scale is between the 7.00 cm and 7.05 cm mark of the main scale and 23rd division of the vernier scale exactly coincides with the main scale.
Smaller division of the main scale, a = 7.05 – 7.00 = 0.05 cm,
∴ Smallest division of vernier scale, b = 0.05 x 49/50 cm
∴ Vernier constant = a – b = 0.05( 1 – 49/50) = 0.05 x 1/50 = 0.001 cm
∴ Thickness of the slab = 7.00 + 23 x 0.001 = 7.023 cm
The option 3 is correct.
Measurement Techniques: Short Answer Questions
Question 28. A student measured the diameter of a small steel ball using a screw gauge of least count 0.001 cm. The main scale reading is 5 mm and zero of circular scale division coincides with 25 divisions above the reference level. If the screw gauge has a zero error of -0.004 cm, the correct diameter of the ball is
- 0.053 cm
- 0.525 cm
- 0.521 cm
- 0.529 cm
Answer:
Given
A student measured the diameter of a small steel ball using a screw gauge of least count 0.001 cm. The main scale reading is 5 mm and zero of circular scale division coincides with 25 divisions above the reference level. If the screw gauge has a zero error of -0.004 cm
Diameter of the ball,
D = main scale reading (m) + circular scale reading (n) x least count (c) – zero error
= 0.5 + 25 x 0.001 -(-0.004) = 0.529 cm
The option 4 is correct
Question 29. Percentage error in the measurement of the height and radius of the cylinder are x and y respectively. Find the percentage error in the measurement of volume. Which of the two measurements height or radius needs more attention?
Answer:
Given
Percentage error in the measurement of the height and radius of the cylinder are x and y respectively.
Height of cylinder = x
Radius of cylinder = y
Volume of cylinder = πy²x
Percentage error in volume, \(\frac{\Delta V}{V}\) x 100 = (2\(\frac{\Delta y}{y}\) + \(\frac{\Delta x}{x}\)) x 100
Hence, the radius needs more attention because any error in its measurement is multiplied 2 times.
Question 30. The length and breadth of a rectangle are measured as (a±Δa) and (b±Δb) respectively. Find:
- Relative error,
- Absolute error in the measurement of area.
Answer:
Relative error is \(\frac{ \pm \Delta A}{A}= \pm\left[\frac{\Delta a}{a}+\frac{\Delta b}{b}\right]\)
Absolute error is \(\pm \Delta A= \pm\left(\frac{\Delta a}{a}+\frac{\Delta b}{b}\right) A= \pm\left(\frac{\Delta a}{a}+\frac{\Delta b}{b}\right) a b\)
= \(\pm[(\Delta a) b+(\Delta b) a]\)
Question 31. A physical quantity P is related to four observables a,b,c, and d as follows: P = \(\frac{a^3 b^2}{\sqrt{c d}}\)
The percentage errors of measurement in a, b, c, and d are 1%, 3%, 4%, and 2% respectively. What is the percentage error in the quantity P? If the value of P calculated using the given relation turns out to be 3.763, to what value should the result be rounded off?
Answer:
Given
A physical quantity P is related to four observables a,b,c, and d as follows: P = \(\frac{a^3 b^2}{\sqrt{c d}}\)
The percentage errors of measurement in a, b, c, and d are 1%, 3%, 4%, and 2% respectively.
P = \(\frac{a^3 b^2}{\sqrt{c} d}=\frac{a^3 b^2}{c^{1 / 2} d}\)
So the errors are related as, \(\frac{\Delta P}{P}=3 \frac{\Delta a}{a}+2 \frac{\Delta b}{b}+\frac{1}{2} \frac{\Delta c}{c}+\frac{\Delta d}{d}\)
Putting the given values, \(\frac{\Delta P}{P}=3 \times \frac{1}{100}+2 \times \frac{3}{100}+\frac{1}{2} \times \frac{4}{100}+\frac{2}{100}\) = 13/100
Therefore, the percentage error in P = 13%
As the relative error 0.13 has 2 significant digits, it is useless to keep the subsequent digits.
So the calculated value of P should reasonably be rounded off as, P = 3.763 ≈ 3.8
Question 32. Define relative error and percentage error in a measurement.
Answer:
Relative error and percentage error in a measurement
Let the accurate value of a physical quantity = x and a measured value = x+Δx (Δx may be positive or negative).
Then in this measurement, relative error = \(\frac{\Delta x}{x}\) ; percentage error = \(\frac{\Delta x}{x}\) x 100%
Question 33. Write the dimensions of ‘light year’.
Answer:
The dimensions of ‘light year’
Dimension = L (as the light year is a unit of length).
Question 33. If A = (12.01±0.1) cm and B = (8.5±0.5) cm, find the value of (A – B).
Answer:
If A = (12.01±0.1) cm and B = (8.5±0.5) cm,
A = (12.0±0.1) cm
B = (8.5±0.5) cm
A-B =[(12.0±0.1)-(8.5±0.5)] cm
= [12.0-8.5]±1(0.1+0.5)
= (3.5±0.6) cm
Question 34. Name two physical quantities having dimension MLT-1.
Answer:
- Momentum,
- Impulse of force.
Question 35. A potential difference of V = (20±1) volt is applied across a resistance of (8±2) ohm. Calculate the current with error limits.
Answer:
Given
A potential difference of V = (20±1) volt is applied across a resistance of (8±2) ohm.
We know from Ohm’s law, V = IR or, l = \(\frac{V}{R}\)
Current without error limit is, l = \(\frac{20}{8}\) = 2.5 A
Next, the maximum fractional error in l is \(\frac{\Delta I}{I}\) = \(\frac{\Delta V}{V}\) + \(\frac{\Delta R}{R}\) = \(\frac{1}{20}\) + \(\frac{2}{8}\)
or, \(\frac{\Delta I}{I}\) = 0.05 10.25 = 0.30
∴ ΔI = 0.30 x 2.5 = 0.75 A
∴ The current with error limits is (2.5±0.75) A or, (2.5 ±0.8) A
Question 36. If x = a+ bt+ ct², where x is in meters and t is in seconds, what is the dimensional formula of c?
Answer:
If x = a+ bt+ ct², where x is in meters and t is in seconds,
Dimension of x = dimension of c x dimension of t² or, L = [c] x T²;
∴ [c] = LT-2
Question 37. A physical quantity X is connected from X = ab²/c. Calculate the percentage error in X, when the % error in a, b, and c are 4,2 and 3 respectively.
Answer:
A physical quantity X is connected from X = ab²/c.
n = ab²
The percentage error in n is, \(\frac{\Delta n \times 100}{n}=\frac{\Delta a \times 100}{a}+\frac{2 \Delta b \times 100}{b}+\frac{\Delta c \times 100}{c}\)
∴ \(\frac{\Delta n \times 100}{n}=4 \%+4 \%+3 \%=11 \%\)
Question 38. Write the dimensional formula of
- Pressure and
- Impulse.
Answer:
- The dimensional formula of pressure is ML-1T-2.
- The dimensional formula of impulse is MLT-1.
Question 39. Write the dimensional formula for
- Planck’s constant and
- Surface Tension.
Answer:
Planck’s Constant: ML2T-1
Surface Tension: ML0T-2
Question 40. A physical quantity, P = \(\) the percentage errors in measurement in a, b,c, and d are 1%, 3%, 4%, and 2% respectively. What is the percentage error in the measurement of quantity P?
Answer:
Given
A physical quantity, P = \(\) the percentage errors in measurement in a, b,c, and d are 1%, 3%, 4%, and 2% respectively.
P = \(\frac{a^3 b^2}{\sqrt{c d}}\)
Percentage error in P
= \(\frac{\Delta P}{P}=\left(3 \frac{\Delta a}{a}+2 \frac{\Delta b}{b}+\frac{1}{2} \frac{\Delta c}{c}+\frac{\Delta d}{d}\right) \times 100\)
So, \(\frac{\Delta P}{P} \times 100=3 \times \frac{\Delta a}{a} \times 100+2 \times \frac{\Delta b}{b} \times 100+\frac{1}{2}\)
x \(\frac{\Delta c}{c} \times 100+\frac{1}{2} \times \frac{\Delta d}{d} \times 100\)
∴ Percentage error in P
= 3(% error in a) + 2(% error in b) + 1/2 (% error in c) + 1/2(% error in d)
= 3 x 1% + 2 x 3% + 1/2 x 4% + 1/2 x 2%
= 3% + 6% + 2% + 1% = 12%
Question 41. Name the forces having the longest and shortest range of operation.
Answer:
The gravitational force has the longest range of operation, and weak nuclear force has the shortest range of operation.
Question 42. What is the difference between mN and N • m?
Answer:
mN is millinewton which is the smallest unit of force.
N • m is newton • meter which is unit of work.
Question 43. If heat dissipated in a resistance can be determined from the relation: H = l²Rt joule If the maximum error in the measurement of current, resistance, and time are 2%, 1%, and 1% respectively, what would be the maximum error in the dissipated heat?
Answer:
Given
If heat dissipated in a resistance can be determined from the relation: H = l²Rt joule If the maximum error in the measurement of current, resistance, and time are 2%, 1%, and 1% respectively
H = l²RT
⇒ \(\frac{\Delta H}{H}\) = 2\(\frac{\Delta l}{l}\) + \(\frac{\Delta R}{R}\) + \(\frac{\Delta T}{T}\)
In terms of percentage error,( \(\frac{\Delta H}{H}\)x 100)
= 2(\(\frac{\Delta l}{l}\) x100) + (\(\frac{\Delta R}{R}\) x100) + \(\frac{\Delta T}{T}\) x 100)
= [2(2)+ 1 + 1]% = 6%
Question 44. The frequency (v) of a transverse wave on a string may depend upon
- Length L of string
- Tension T in the string and
- Mass per unit length m of the string. Derive the formula for frequency with the help of dimensions.
Answer:
Let the frequency of vibration of the string depend upon L, m, and T in the following way:
v ∝La …(1)
v ∝ mt ….(2)
v ∝ Tc …. (3)
Combining them, v ∝ LamtTc
or, v = kLamtTc…(4)
Putting dimensions on both sides,
(T-1) = (L)al(ML-1)t(MLT-2)c
or, (M0L0T-1) = (Mt+cLa-t+cT-2c)
Equating powers of M, L, T we get, t+ c = 0, a- t+ c = 0, -2c = -1
On solving we get a = -1, t = ^ , c = |
Putting the value of a, b, c in equation (4), v = \(\frac{k}{L} \sqrt{\frac{T}{m}}\)