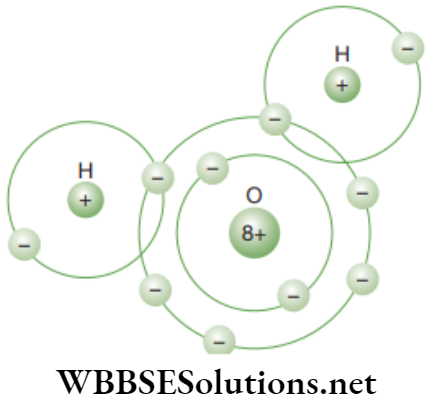
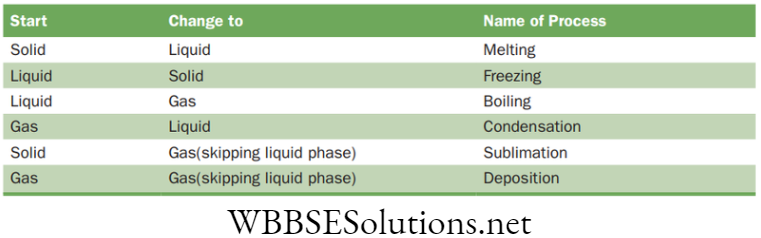
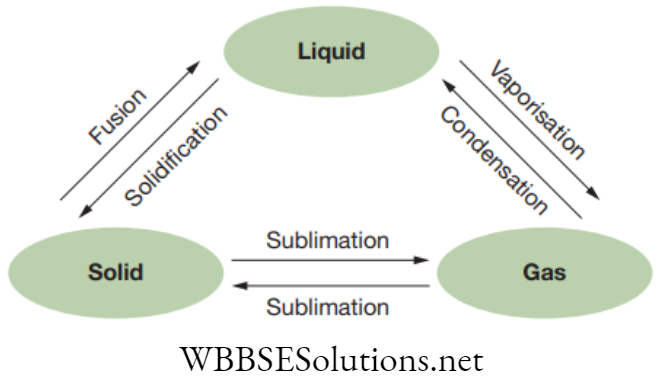
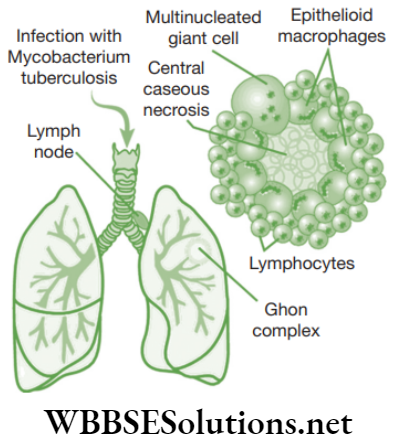
Chapter 2 Tissues
Tissue
It is evidently known that multicellular organisms are made up of trillions of cells, but each and every cell performs specific functions. A group of cells that are similar in structure and which performs a specific function are described as tissues. Owing to this, a specific function is performed by a group of cells at a particular site in the organism’s body.
Hence, different functions are performed by different groups of cells in an organism, and this is called division of labour in organisms.
Division of labour in organisms enables a smooth and efficient functioning in the body for better survival and also different functions can be performed at the same time. For example, in human body, several processes occur such as digestion. A tissue is a group of cells having similar structure to perform a specific function.
In a particular tissue, all the cells have a common origin. For example, in human beings, nervous tissue that is present in brain, spinal cord and nerves has nerve cells or neurons having the same basic structure and function. Both plants and animals possess tissues, but these are different from each other. The study of tissues is known as Histology.
Chapter 2 Tissues Are Plants and Animals Made of the Same Types of Tissues
The world we live is diverse and complex. Each form is different from other to a lesser or greater extent. When we compare the structure and functions of plant and animal tissues there are noticeable differences between them. Plants are fixed and they do not move. Plant tissues provide structural strength.
Read and Learn More: NEET Foundation Notes
Animals move around and consume more energy as compared to plants. The growth in plants is limited to certain regions also some plant tissues divide throughout their life. Based on dividing capacity of the tissues, plant tissues are classified into growing or permanent tissue.
Types Of Tissue
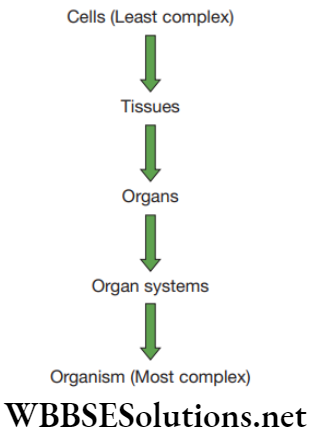
The cell differentiation and growth in animal tissue is more uniform and there is no clear distinction of dividing and non-dividing cells. Tissues get organized to form organs and organs into organ systems. The science of cells is called cytology, and science of organs is called organology. The embryonic derivation of tissues is called histogenesis.
Tissue Structure
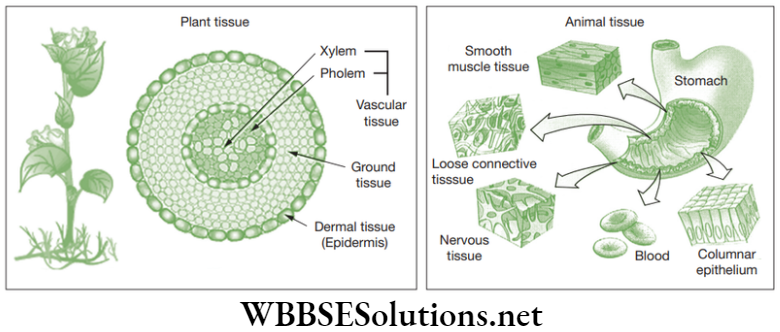
Chapter 2 Tissues Plant Tissues
Plants are multicellular organisms made up of trillions of cells which cluster together to perform a specific function. Plant tissues provide mechanical strength to internal as well as external organs. They provide elasticity and flexibility to the plant organs; for example, the leaves, stems and branches of trees can bend without causing any damage to the plant body.
They also help in transportation of materials across the plant body and prevents loss of water. They undergo division to help the plants to grow both in length and girth. The plant tissues are involved in many metabolic processes, such as photosynthesis, respiration, etc.
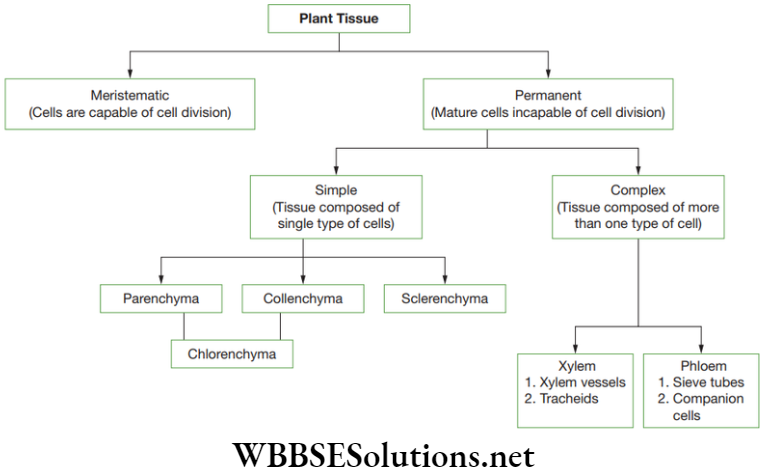
Plant tissues can be categorized into two types – (1) Meristematic, (2) Permanent
Meristematic Tissues (Meristems)
Meristematic tissue or simple meristems is a group of dividing cells that has the capacity to divide and re-divide and help in the growth of plants. Nageli (1858) gave the term ‘meristem’. Meristems are found in the apices of stem and root, leaf primordia, vascular cambium, cork cambium, etc.
Characteristics of Meristematic Tissue
- The cells are oval, rounded or polygonal in shape.
- The cells are living with thin cellulose walls and without any intercellular spaces.
- The cells are rich in cytoplasm due to cell division which constantly produce a lot of biomolecules.
- The cells are diploid and undergo mitosis.
- The cells do not contain reserve food materials, endoplasmic reticulum and plastids. Hence, the cells lack vacuoles.
Types of Meristems
Meristems or meristematic tissues are categorized into many types based on the following criteria.
- Classification based on origin and development
- Classification based on position
- Classification based on function
- Classification based on plane of division
- Classification based on origin and development: Meristems can be categorized into three types.
-
- Promeristem or primordial meristem: It is a group of young meristematic cells present in the growing organ, such as the tips of stem and root. It is the early embryonic meristem that gives rise to other advanced meristems. It further divides to produce primary meristem.
- Primary meristem: It is derived from promeristem and is found below the promeristem at shoot and root apices. These cells divide to produce permanent tissues, such as apical meristem, intercalary meristem and intrafascicular cambium.
- Secondary meristem: It is derived from primary permanent tissues that have the capacity to divide. For example, cork cambium, root cambium and interfascicular cambium of stem.
- Classification based on position: Meristems can be categorized to three types:
-
- Apical meristem: It is found at the apices or tips (i.e., growing points) of root and stem (shoot) and brings about an increase in length. It includes promeristem and as well as primary meristem. Many theories have been proposed to explain the activity of apical meristem.
- Apical cell theory proposes that a single apical cell is the structural and functional unit of apical meristem that controls the whole process of apical growth. This type of organization is found only in cryptogams.
- Intercalary meristem: This meristematic tissue is found between the regions of permanent tissues. It is considered as a part of primary meristem that has detached owing to the formation of intermediate permanent tissues. It is located either at the base of leaf, for example, Pinus or at the base of internodes, for example, grasses.
- Lateral meristem: This meristem is arranged parallel to the sides of origin and normally divides periclinally or radially and produces secondary permanent tissues. This meristem helps in increasing the thickness of the plant and its parts.

- Classification based on function: Meristems are classified into three types.
-
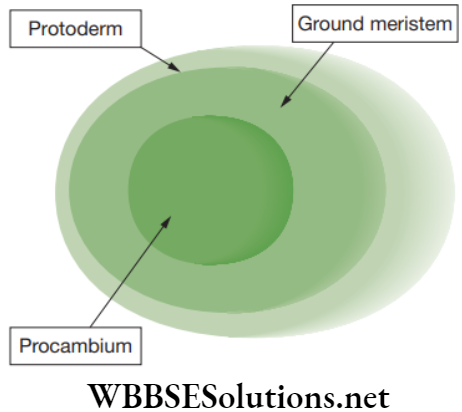
- Protoderm meristem: This is the outermost layer of the young growing region that develops to give rise to epidermal tissue system.
- Procambium meristem: This consists of narrow, elongated, prosenchymatous, meristematic cells producing the vascular tissues system.
-
- Ground meristem: This consists of large, thick-walled cells that gives rise to ground tissue system, which includes hypodermis, cortex and pith.
- Classification based on plane of divisions: Meristems are categorized into three types.
- Mass meristem: The cell divisions occur in all planes resulting in the increase of volume. It can be found in meristems of cortex and pith.
- Rib or file meristem: The cells divide only on one plane, for example, formation of filaments in algae.
- Plate meristem: These cells divide in two planes resulting to an increase in the area of an organ, for example, leaf formation.
Chapter 2 Tissues Permanent Tissues
Permanent tissues are those tissues whose cells have lost the power of division or ability to multiply. The process by which the cells derived from meristematic tissue attain a permanent shape, size and function is known as differentiation. Hence, the cells of meristematic tissue differentiate to form cells of permanent tissues.
They are of various sizes, shape and other characteristic to make them suitable for different functions. Permanent tissues are classified into two main types, such as simple and complex permanent tissues.
Simple Permanent Tissues
A simple tissue is composed of one type of cells forming a uniform mass. Based on their functions, they are classified into three types—(i) Parenchyma; (2) Collenchyma and (3) Sclerenchyma
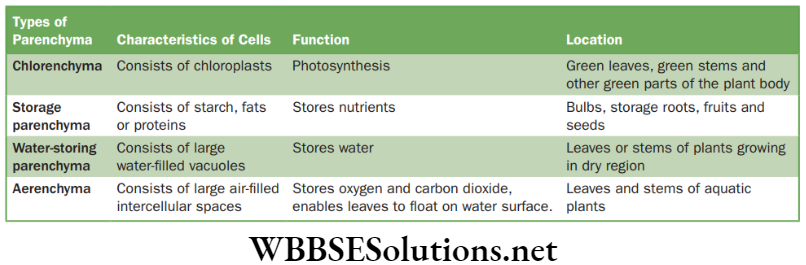
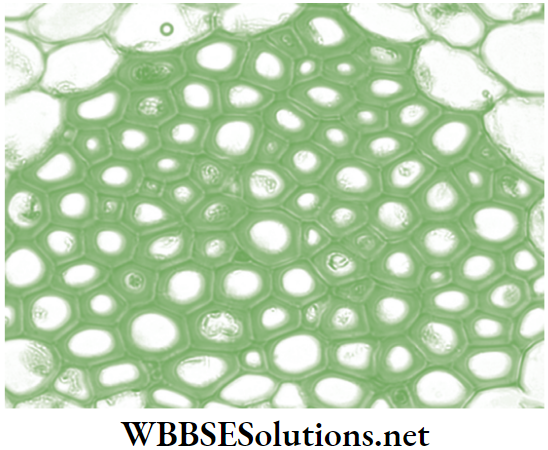
- Parenchyma: Parenchyma forms the bulk of the plant body and is found in the cortex of root, ground tissue in stems and mesophyll of leaves. The cells are isodiametric, i.e., equally expanded on all sides. The shape of the cells may be oval, round, polygonal or elongated.
The cells consist of nucleus, and so are living in nature. The thin cell walls are made up of cellulose. The cell has dense cytoplasm with a single large vacuole. Intercellular air spaces are present.
-
- Location of parenchymatous tissue: Parenchyma tissue is found in soft parts of stems and roots of the plant and forms the bulk tissue of the plant body.
- Functions of parenchymatous tissue
- The main function of parenchyma is to store and assimilate food.
- Provides mechanical strength by maintaining turgidity.
- Manufactures food if chlorophyll is present.
- Stores waste material such as tannins, gum, crystals and resins.
- Types of parenchymatous tissue: These are mainly of four types.
Functions and locations of parenchyma cells
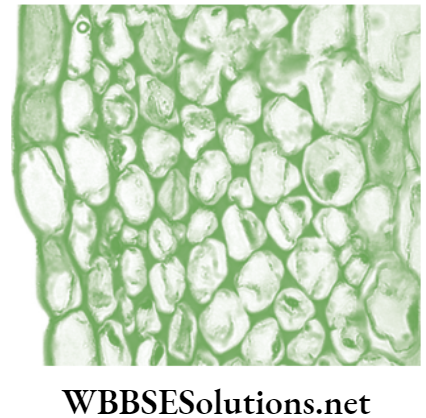
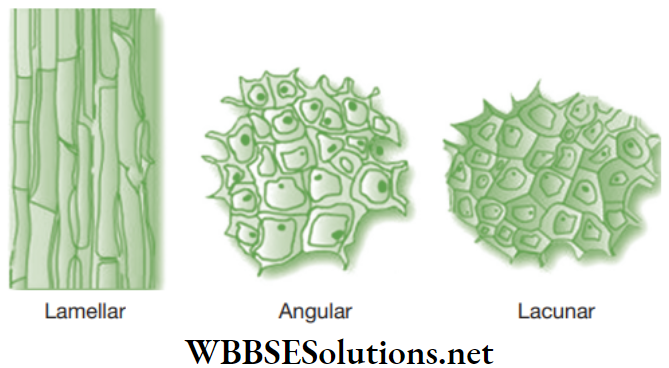
- Collenchyma: Collenchyma is composed of elongated cells with irregularly thickened walls, having deposition of extra cellulose at the corners of the cells. The intercellular spaces are usually absent . The cells are usually living and have only a thick primary cell made up of cellulose and pectin. The thickness of cell wall is strongly influenced by mechanical stress upon the plant. For example, the cell walls of collenchyma in wind-exposed plants are 40–100% thicker as compared to those plants inhabiting in the less windy area.
-
- Location of collenchyma: Found in leaf and below the outer protective layer of stems and leaves.
- Functions of collenchyma
- Provides structural support, specifically in growing stems and leaves.
- Sometimes collenchyma consists of chloroplasts, which then manufactures sugar and starch.
- Provides flexibility and tensile strength to the branches and facilitates easy bending of the plant.
- Types of collenchyma: There are three main types of collenchyma.
Structure and location of collenchyma cells
Human Tissue
- Sclerenchyma: Sclerenchyma cells are dead cells that are devoid of cytoplasm. The cell walls are very thick made up of lignin. Due to the presence of excessing thickening of a sclerenchyma cell wall, its cell cavity becomes almost absent. A visible middle lamella is present between two sclerenchymatous cells.
-
- Location: Sclerenchyma is found in stems (around the vascular bundles), roots, veins of leaves, hard coverings of seeds and nuts.
- Function: Sclerenchyma tissue provides strength and mechanical support to various parts of the plant.
- Types of sclerenchyma: The sclerenchyma is of two types and they are fibres and sclereids.
- Fibres: They are pointed, needle-like structures. The cell walls are mainly composed of cellulose. Fibres generally originate from meristems. These are produced mainly in cambium and procambium. The fibres are generally associated with the xylem and phloem of the vascular bundles. The fibres of the xylem are lignified, whereas those of the phloem are cellulosic. Fibres that do not belong to the xylem are called bast (outside the ring of cambium). For example such as jute and coir (husk of coconut).
- Sclereids: Sclereids are a reduced form of sclerenchyma cells with highly thickened and lignified walls. They are small bundles of sclerenchyma tissue in plants forming durable layers. The cells may be isodiametric, prosenchymatous, forked or elaborately branched. These can be grouped into bundles and it can form complete tubes located at the periphery or can occur as single cells or small groups of cells within parenchyma, for example such as hard grit of pear fruit. These structures are used to protect other cells.
Complex Permanent Tissues
The complex permanent tissues are composed of more than one type of cells that have a common origin and coordinate to perform a similar function. Complex tissue is responsible for the conduction of substances through the plants. Therefore, this tissue is called vascular tissue or conducting tissue.
These tissues are involved in the transport of water, mineral salts (nutrients) and food material. The complex permanent tissues are of two types, namely xylem or wood and phloem or bast. Usually, in higher plants, xylem and phloem occurs together in the roots, stems and leaves and they are called vascular bundles.
For the growth of tree, it is essential that new vessels within the stem should be formed, which is done by the vascular cambium. The vascular cambium is highly active during summer and spring seasons, generating a lighter band made of large diameter vessels.
During winter season, small diameter vessels and a darker band appears around the previous lighter band. Therefore, two rings are made per year, one of which is lighter and the other of which is darker. By counting these pairs directly, the age of the tree can be estimated.
Xylem
Xylem cells are thick-walled. They consists of cells (or ‘elements’) of four different kinds.
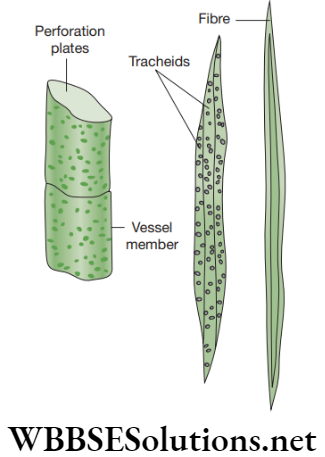
- Tracheids: These are non-living elongated cells with tapering ends. In these, the water passes from cell to cell through the pits.
- Vessels: These are shorter and wider than tracheids. They are non-living cylindrical tube-like cells. Vessels are made up of lignin. These are very long tubular structures formed by a row of cells placed end to end. The transverse walls between the vessel elements are partially or wholly dissolved to form continuous channels or water-pipes.
- Xylem parenchyma: Contains living cells. It stores food and helps in lateral conduction of water.
- Xylem sclerenchyma: They are non-living, thick-walled cells. It provides mechanical strength to the plant body.
Functions of Xylem
- Its main function is to carry water and mineral salts upward from the root to different parts of stem.
- It also provides mechanical strength to the plant body.
Phloem
Phloem consists of tubes but do not provide mechanical support. Phloem consists of four types of cells or elements as discussed below.
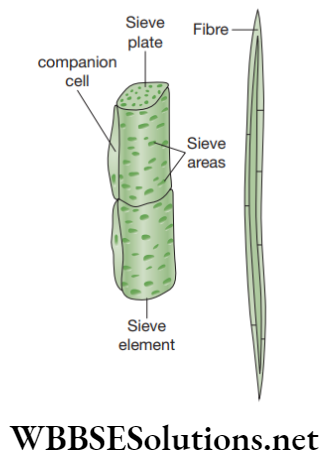
- Sieve tubes: These are slender, tube-like structures that consist of elongated thin-walled cells, placed end to end. The end walls of these cells are perforated by several pores and are known as sieve plates. The nucleus of the sieve cell degenerates at maturity, but cytoplasm still remains in the mature cell.
- Companion cells: These are smallthin-walled cells consisting of dense and very active cytoplasm with large elongated nucleus. These are connected to the sieve tube with several plasmodesmata. Companion cells consists of additional mitochondria and chloroplasts.
- Phloem parenchyma: These are thin-walled, living cells of parenchyma of phloem. They help in storage and slow lateral conduction of food.
- Phloem fibres or bast fibres: These are thickwalled, elongated spindle-shaped dead cells that possess narrow lumen. They provide mechanical strength to the tissue.
Functions of Phloem
Phloem helps in transporting photosynthetically prepared food materials from the leaves to the storage organs, from where these are supplied to the growing regions of the plant body.
Characteristics of Plant Tissue Systems
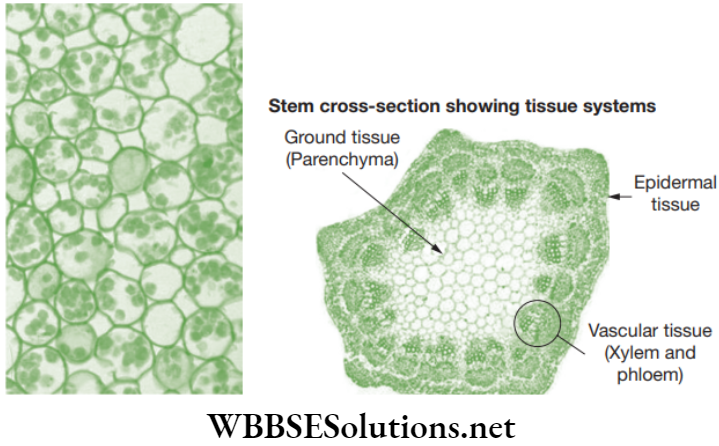
The structural and functional tissue systems of plants. The tissues of a plant are organized into three systems: the dermal tissue system, the ground tissue system, and the vascular tissue system and discussed below in Table 2.3.
- Dermal tissue covers the outer surface of the stem. It provides protection to the plant and it also helps in gaseous exchange.
- Ground tissue usually consists mainly of parenchyma cells and it surrounds the vascular tissue. It gets involved in photosynthesis sometimes.
- Vascular tissue provides long distance transport and mechanical support.
| Class 11 Physics | Class 12 Maths | Class 11 Chemistry |
| NEET Foundation | Class 12 Physics | NEET Physics |
Characteristics of plant tissue system
Chapter 2 Tissues Animal Tissues
Depending on the location and functions of the tissues in the human body, animal tissues are classified into four main types and they are listed below.
- Epithelial tissue
- Muscle or muscular tissue
- Connective tissue
- Nervous tissue
Epithelial Tissue (Epithelium)
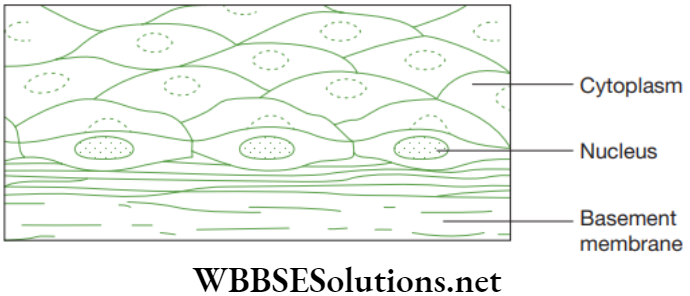
Epithelial tissue is one of the simplest tissues, whose main function is to protect the animal body. In this tissue, the cells are tightly packed and form a continuous sheet. Cells of epithelium consist of no intercellular matrix. The epithelial tissue covers most of the organs and cavities within the animal body.
It also acts a barrier by keeping different organ systems separated. For instance, the skin and lining of buccal cavity, blood vessels, alveoli of lungs, kidney tubules and many others are made up of epithelial tissue. Beneath the epithelial cells is a non-cellular basement membrane that contains a special form of matrix protein known as collagen.
Functions of Epithelial Tissue
- The epithelial cells form the outer layer of the skin and protect the underlying cells from drying, injury and other chemical and physical effects.
- The epithelial cells form the lining of mouth and alimentary canal to protect these organs.
- The epithelial tissues help in the absorption of water and nutrients.
- The epithelial tissues help in the elimination of waste material.
- Some epithelial tissues secrete numerous substances like sweat, saliva, enzymes, etc.
Types of Epithelial Tissue
Epithelial tissue is a protective, excretory and sensory in function. Based on the shape and function of the cells, the epithelial tissues are categorized into five types as listed below.
- Squamous epithelium
- Cuboidal epithelium
- Columnar epithelium
- Glandular epithelium
- Ciliated epithelium
- Squamous epithelium: It is composed of thin, flat, irregular-shaped cells that fit together to form a compact tissue. Squamous epithelium is also called tessellated and pavement epithelium as it appears like floor tiles.
-
- Stratified keratinized squamous epithelium: This tissue is found in skin and covers the external dry surface of the skin. Deeper layers of the tissue are composed of cuboidal cells that become polygonal and finally flattened (‘squamous’) towards the free surface. The flattened cells of superficial layer consist of a fibrous protein, the keratin, and become dead cells. Horny, scaly remnants of dead squamous cells, finally flakes away. This tissue is water-proof and highly resistant to mechanical injury.
-
- Location: Squamous epithelium forms the delicate lining of cavities (mouth, oesophagus, nose, pericardium, alveoli, etc.) and of blood vessels and covering of the tongue and skin.
- Functions
- This tissue protects the underlying parts of body from mechanical injury, invasion of germs and chemicals and drying.
- It also forms a selectively permeable lining through which filtration takes place.
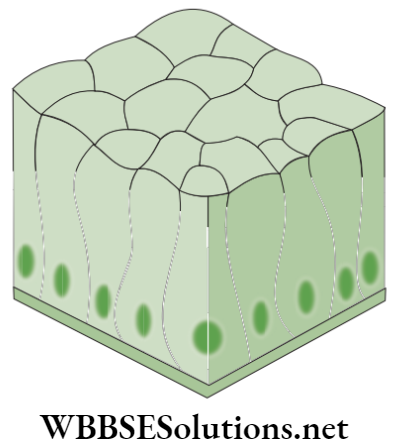
- Cuboidal epithelium: This epithelium consists of cube-like (‘cuboidal’) cells that appear as square shaped in cross section but the free surface looks hexagonal.
- Location: The cuboidal epithelium is found in kidney tubules, thyroid vesicles and in glands, such as salivary glands, sweat glands and exocrine glands. This epithelium forms germinal epithelium of gonads (testes and ovaries).
- Functions: Cuboidal epithelium helps in absorption, excretion and secretion, and also provides mechanical support.
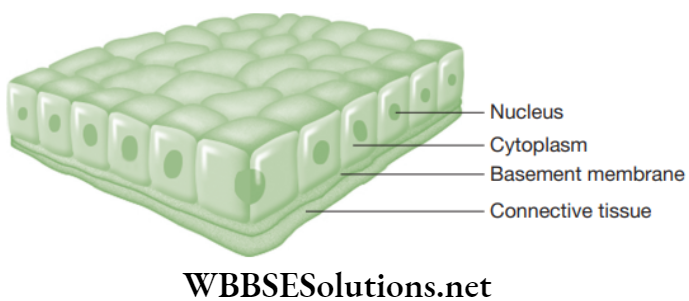
- Columnar epithelium: This epithelium is composed of the cells that are taller than broader and looks like pillars. The nuclei are present at the base and sometimes the free ends of cells have a brush border consisting of microvilli.
-
- Location: The columnar epithelium forms the lining of stomach, small intestine and colon forming mucous membrane. It also lines the gall bladder, oviducts and facilitates movement across the cells.
- Functions: It helps in the absorption (for example, stomach, intestine) and secretion (for example, mucous by goblet cells).
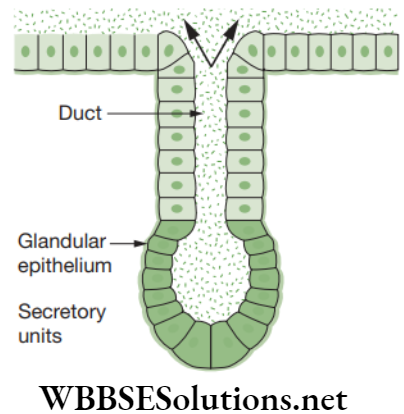
- Glandular epithelium: This epithelium consists of specialized cells that secrete bodily products and are called glands. Glands are of two types, such as endocrine and exocrine.
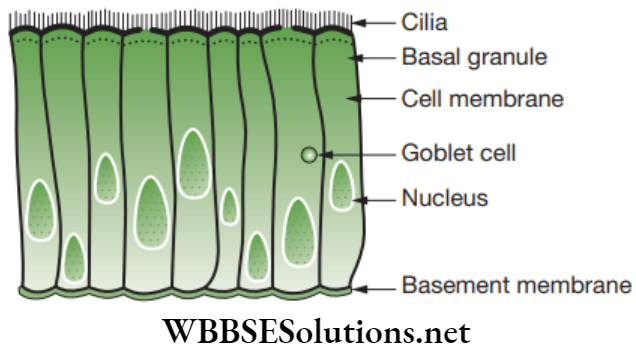
Exocrine glands are generally the glands associated with the term ‘glandular epithelium’. These glands are of two types based on the morphology and they are unicellular glands and multicellular glands. - Ciliated epithelium: This epithelium is composed of cuboidal or columnar cells having a free border that bear thread-like cytoplasmic outgrowths known as cilia.
-
- Location: It occurs in the sperm ducts and lines the trachea (wind pipe), bronchi (lungs), kidney tubules and oviducts (Fallopian tubes).
- Functions: The rhythmic, concerted beating of the cilia helps in the movement of solid particles in one direction through the ducts.
Chapter 2 Tissues Muscle or Muscular Tissue
The muscle tissues of the body form the contractile tissue and consist of muscle cells. Muscle cells are elongated and large-sized, and hence, they are also called muscle fibres. In muscle cells, the contractile proteins are present that help in the movements of the body or limbs through contraction and relaxation.
Typically, the muscle cells are arranged in parallel arrangement permitting them to work together effectively. Muscles help in body movements (local and gross). Major movements of the body rely on the action of skeletal muscles which are connected to bones. Based on their location, structure and function, muscles are of three types, such as striated muscles, smooth muscles and cardiac muscles.
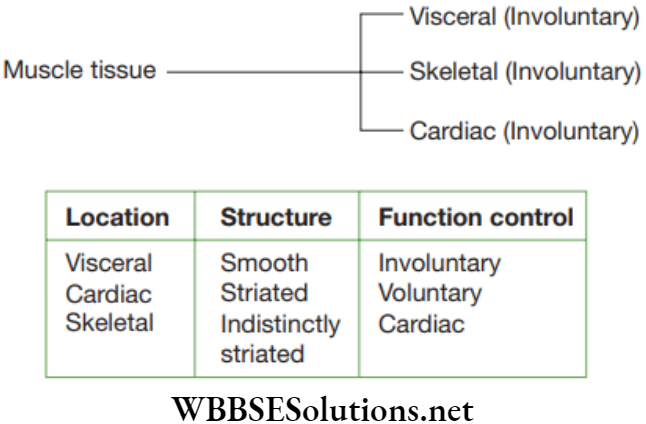
Striated muscles: The entire muscle fibres show alternate dark and light stripes or bands or striations, they are also known as striped muscles. As they are attached to the bones and are responsible for body movements, they are also called skeletal muscles. Since these muscles work according to our will, they are also called voluntary muscles.
The striated muscle cells are elongated, non-tapering, cylindrical, unbranched and multinucleate cells. Each muscle cell is enclosed in a thin and distinct plasma membrane known as sarcolemma. In the sarcoplasm (cytoplasm) of the muscle cell are embedded numerous contractile elements known as sarcostyles or myofibrils.
Striated muscles are found in the muscles of limbs, body wall, face, neck, etc. Striated muscles found in tongue, pharynx, diaphragm and upper part of oesophagus are known as visceral striated muscles.
Functions of Striated Muscles
- These muscles undergo rapid contraction and can get tired and may need rest.
- These muscles provide the force for locomotion and all other voluntary movements of the body.
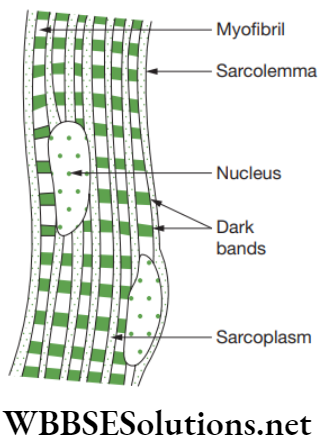
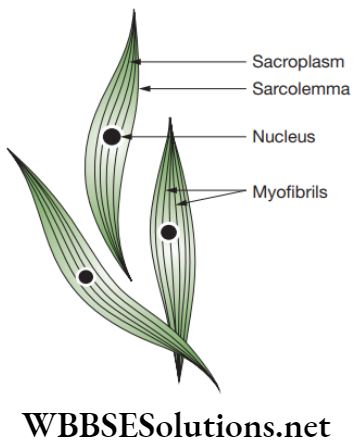
Smooth muscles: Smooth muscles are present as bundles or sheets of elongated fusiform or spindle-shaped cells or fibres. These are held together by loose connective tissue. Each muscle cell is covered by a plasma membrane. A single centrally located nucleus is present in the cytoplasm or sarcoplasm. Myofibrils run longitudinally throughout the cell. As these myofibrils are devoid of any bands or stripes or striations, these are known as smooth or unstriated muscles.
Smooth muscles are located in the walls of the hollow visceral organs except that of the heart, and hence, they are called visceral muscles. These muscles are found in the wall of alimentary canal and internal organs, ducts of glands, urogenital ducts and blood vessels. Smooth muscles are present in stomach, intestine, ureter, bronchi, iris of eye, etc.
Functions of Smooth Muscles
- Smooth muscles are also called involuntary muscles, as they do not work according to our will. For example, movement of food in the alimentary canal, opening and closing of tubes are involuntary movements.
- Smooth muscle contracts slowly but can remain contracted for long duration. Due to this characteristic, smooth muscles cause peristaltic movements in the tubes. Peristaltic movements are the rhythmic progressive waves of muscular contraction and relaxation. These movements happen in the gastrointestinal tract and male genital tract.
- In few organs, the smooth muscles contract throughout the organ to produce extrusive movements as in the urinary bladder, the gall bladder and the uterus.
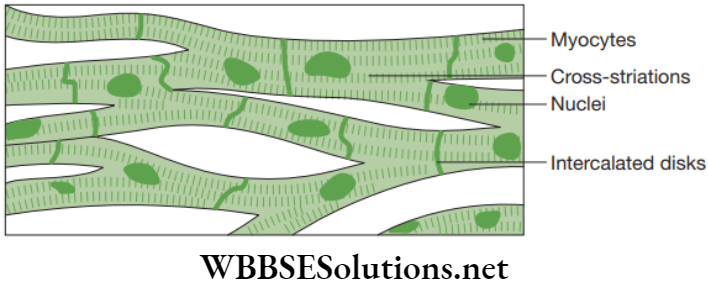
Cardiac muscles: Cardiac muscles consist of branched fibres and these branches join to form a network. Each fibre or cell is enclosed in sarcolemma, sarcoplasm with longitudinal myofibrils and a centrally located nucleus. The intercellular spaces of cardiac muscles are filled with plenty of loose connective tissue provided by blood capillaries.
Cardiac muscles show stripes of light and dark bands. Also, these muscle fibres show densely stained crossbands known as intercalated impulse. These are zones of interdigitations of plasma membranes consisting of adjacent muscle cells or fibres. Cardiac muscles are found in the walls of the heart.
Cardiac muscles have excellent blood supply and are specialized to avoid becoming fatigued. Since asphyxiation can result in death within few minutes, cardiac muscles must be able to fulfil their duty transporting oxygen in blood haemoglobin. Cardiac muscles possess mitochondria that help with energy production in rhythmic capacity, even at high force when required.
Functions of Cardiac muscles
- Cardiac muscles contract and relax speedily, rhythmically and without getting tired throughout the life of a human.
- The contraction and relaxation of the heart muscles facilitate the pumping and distribution of blood to all parts of the body.
Chapter 2 Tissues Connective Tissue
The connective tissue is the most abundant tissue found in human body and it helps to connect and anchor various body organs. These tissues can connect bones to each other, muscles to bones, bind tissues and can also provide support to various other body parts by building a packing around the organs.
The packing would prevent the organs from getting displaced by body movements. Hence, the main functions of connective tissue are binding, supporting and packing together various organs of the body.
The tissue consists of three components, such as (a) non-living intercellular substance called ground substance, (b) fibres made up of proteins and (c) living cells. Ground substance and fibres together form the extracellular matrix. Fibres are of three types such as and they are listed below.
- White fibres are inelastic, unbranched, made up of collagen fibres and most abundant in nature.
- Yellow fibres are elastic, branched and made up of protein elastin.
- Reticular fibres are inelastic, straight and made up of protein reticulin.
The cells of connective tissue are living, loosely spaced and few in number. Homogeneous, gel-like intercellular substance known as matrix forms the main bulk of the connective tissue. Therefore, the space between the cells is filled with a non-living matrix that may be solid as in bone and cartilage and may be fluid as in the blood.
Connective tissues are classified into four types and they are as follows.
- Loose connective tissue
- Dense connective tissue
- Specialized connective tissue
- Fluid connective tissue
Loose Connective Tissue
Loose and cellular connective tissue, whose matrix consists of two types of fibres are – (1) Areolar connective tissue, (2) Adipose connective tissue.
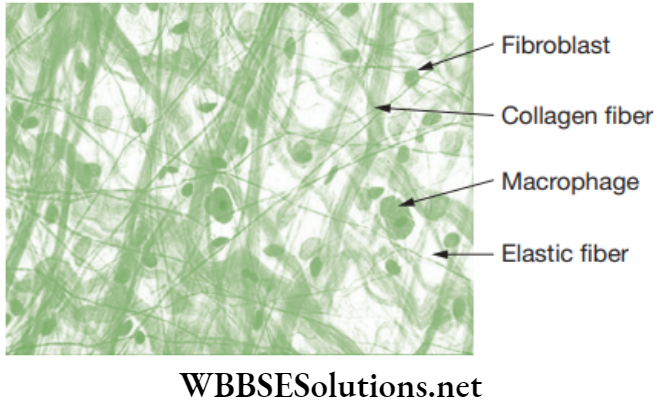
Areolar connective tissue: Areolar tissue is the simplest and most widely distributed connective tissue. It joins skin to muscles, fills spaces inside organs and is found around muscles, blood vessels and nerves.
Functions of Areolar Tissue
- Behaves as a supporting and packing tissue between organs lying in the body cavity. In this tissue, the matrix is important in diffusion of oxygen and nutrients from small blood vessels.
- Helps in the repairment of injured tissues after an injury.
- Helps in combating foreign toxins.
- It cements skin to underlying muscles.
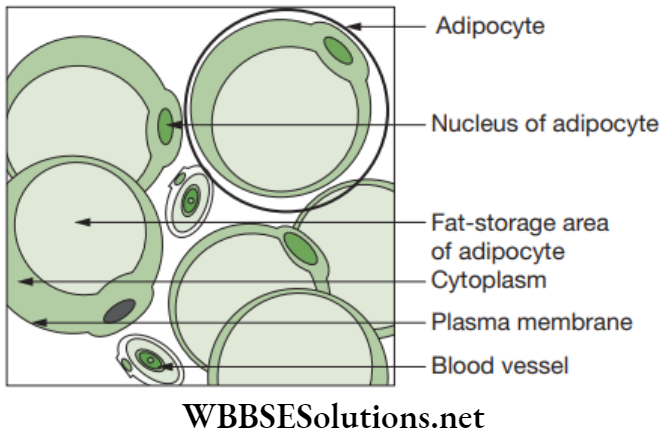
Adipose connective tissue: Adipose tissue is an aggregation of fat cells called adipocytes.
Each fat cell is rounded or oval in shape and consists of a large droplet of fat that fills it.
The fat cells are arranged into lobules separated by partitions of collagen and elastin fibres, which carry blood vessels of lobules. Adipose tissue is found below the skin between the internal organs and in yellow bone marrow.
Functions of Adipose Tissue
- Adipose tissue is a reservoir for fats.
- Provides shape to the limbs and the body.
- Keeps visceral organs in position, and forms shock-absorbing cushions around kidneys and eye balls.
Dense Regular Connective Tissue
It consists of ordered and densely packed collection of fibres and cells. This tissue is mainly present in the tendons and ligaments and aponeurosis.
- Tendons: A tendon is a white fibrous tissue that has great strength but restricted flexibility. It is composed of parallel bundles of collagen fibres and between these fibres, the rows of fibroblasts called tendinocytes are present. Collagen fibres are bounded by areolar connective tissue. This tissue helps in the movement of bones.
- Ligaments: These are elastic structures that connect bones to bones. In ligament, some elastic and many collagen fibres are bound together by areolar connective tissue. Fibroblasts are compressed in between regular rows of fibres. Ligament strengthens the joint and allows normal movement but prevents over-flexing. By excessive pulling of ligaments, sprain is caused.
- Apneuroses: These are broad sheets of dense, fibrous, collagenous connective tissues that cover, invest and form the terminations and attachments of several muscles.
Special Connective Tissues
This tissue forms the endoskeleton of the organisms. They form the rigid framework which supports the body and provides protection of all vital organisms. Depending on the composition and characteristic, this tissue is classified into two types—cartilage and bone.
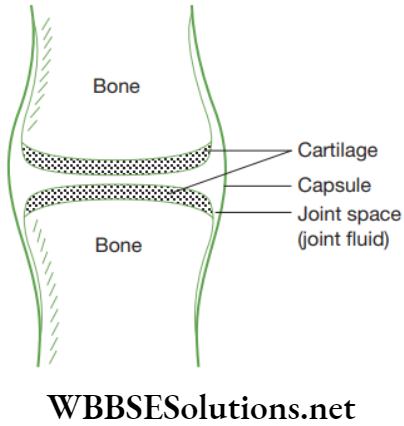
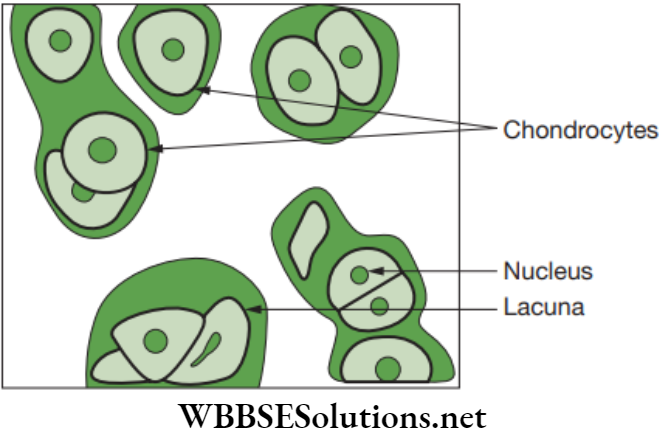
Cartilage: The cartilage is a connective tissue that is compact and less vascular. Its extensive matrix consists of proteins and is somewhat hardened by calcium salts. Its matrix is produced and maintained by the chondrocytes. The matrix is solid and firm but slightly elastic, which gives it a flexible nature.
The matrix of cartilage has a delicate network of collagen fibres and living cells called chondrocytes. There are three cartilages in human body, such as hyaline, fibrous and elastic. Cartilage is found in ear pinna, nose tip, epiglottis, intervertebral discs, end of long bones, lower ends of ribs and rings of trachea (wind pipe). The comparative study of three types of cartilage are discussed below in.
Characteristics of three types of cartilage
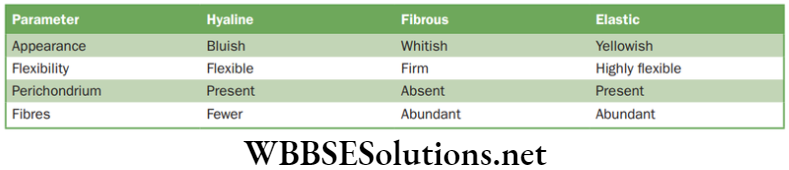
Functions of Cartilage
- It gives support and flexibility to the body organs.
- It makes the surface at joints smooth.
Bone: Bone is a very strong and non-flexible connective tissue. It is porous, highly vascular, mineralized, hard and rigid. Its matrix consists of proteins (for example, osteonectin, osteocalcin, proteoglycan and collagen). The matrix of bone consists of salts of calcium and magnesium like phosphates and carbonates of calcium and magnesium (for example, hydroxyapatite).
These minerals impart hardness to the bones. The matrix of the bone is in the form of thin concentric rings known as lamellae. Bone cells or osteoblasts or osteocytes are present between the lamellae in fluid-filled spaces called lacunae.
All lacunae of the bone communicate with each other by a network of fine canals called canaliculi. Each canaliculus is filled with delicate cytoplasmic processes of the bone cell. The canaliculi helps bone cell to receive food and oxygen and eliminate wastes.
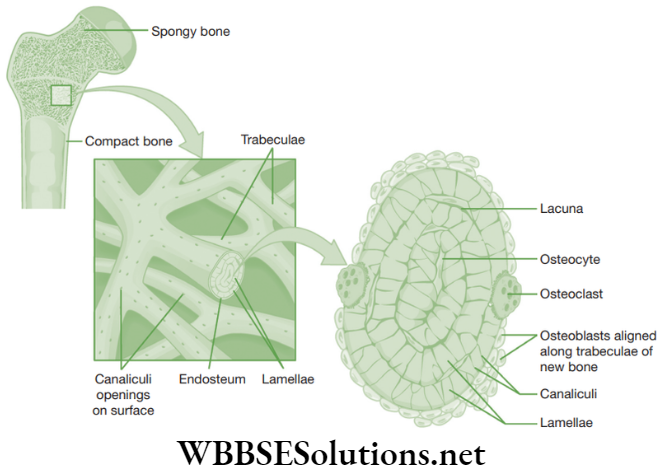
Functions of Bone
- Gives shape to the body.
- Provides skeletal support to the body.
- Protects important body organs, like brain, lungs, etc.
- Acts as a storage site of calcium and phosphate.
- Anchor the muscles.
Fluid Connective Tissue
This tissue links the various parts of the body and maintains continuity in the body. Blood and lymph are two kinds of fluid connective tissue.
Blood: Blood is a fluid connective tissue and is the main circulating medium that helps in the transport of various substances. In this tissue, the cells or corpuscles move in a fluid or liquid matrix or a medium known as blood plasma. This plasma forms 55 per cent of the total volume of blood (Fig. 2.29). It is a complex fluid and it consists of inorganic salts and organic compounds.
Other organic compounds present include glucose, amino acids, lipids, vitamins, enzymes, hormones and waste substances, such as urea, uric acid, etc.
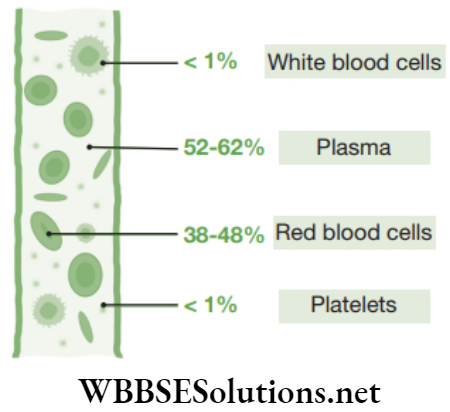

The blood plasma does not consist of protein fibres but possess cells known as blood corpuscles or blood cells. These blood corpuscles include (a) Red blood corpuscles (RBCs) or erythrocytes (b) White blood cells (WBCs) or leucocytes (c) Platelets.
-
- Red blood corpuscles (RBCs): They are present in large number and possess iron-containing red respiratory pigment, the haemoglobin. In mammals, erythrocytes are circular, biconcave, disc-like and devoid of nuclei. Hence, mammalian erythrocytes have an increased surface area for gaseous exchange and they bear much more haemoglobin in them as compared to RBCs of other animals. RBCs play a very crucial role in the transport of oxygen.
- White blood cells (WBCs): The WBCs are present in much smaller number and nucleated. They play an important role in the body’s defense mechanism.
- Phagocytes: These are capable of phagocytosis and helps in the defence of body by engulfing bacteria and other foreign substances. Phagocytes are of two types, namely granulocytes and agranular leukocytes.
- Granulocytes have irregular-shaped nuclei and cytoplasmic granules with specific staining properties. They include neutrophils, basophils and eosinophils.
- Agranular leukocytes have no cytoplasmic granules and include the monocytes. Monocytes consist of a large nucleus on one side and large amount of cytoplasm. They ultimately migrate to body tissues and transform into macrophages and histiocytes.
- Immunocytes: These produce antibodies and are involved in immune response. They include lymphocytes that have approximately spherical nucleus and meagre cytoplasm devoid of granules. Few of the lymphocytes later on transform into plasma cells.
Blood platelets: They are very small, anucleated, fragile fragments of giant bone marrow cells termed as megakaryocytes. Blood is found in blood vessels known as arteries, veins and capillaries that are connected together to form the circulatory system. The elaborate branching network of vessels enables blood to reach every part of the body.
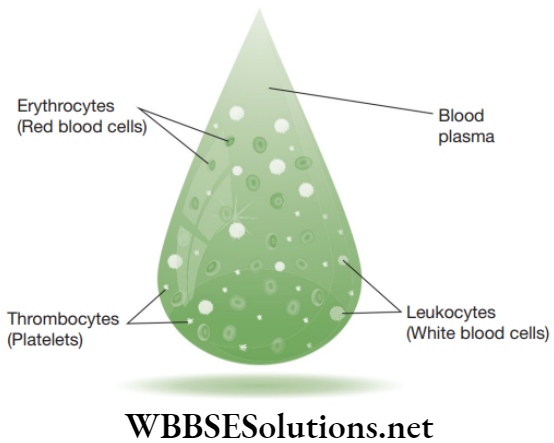
Lymph: Lymph is a colourless fluid that is filtered out of the blood capillaries. In lymph, WBCs are found abundantly. Lymph is devoid of RBCs and some blood proteins.
Functions of Lymph
- Lymph transports nutrients filtered by the blood capillaries and it comes back into the heart to be recirculated in the body.
- Lymph brings carbon dioxide and nitrogenous wastes from tissue fluid to blood.
- Lymph protects the body against infection as it contains WBCs in abundance. It makes up the immune system of the body.
Functions of Fluid Connective Tissue
- Blood transports nutrients, hormones and vitamins to the tissues.
- Blood transports excretory substances from the tissues to the liver and kidney.
- RBCs carry oxygen to the tissues.
- WBCs helps in producing antibodies to destroy pathogens and it also produces antitoxins that neutralize the toxins released by pathogens.
- Blood platelets disintegrate at the site of injury and helps in the clotting of blood, which prevents unnecessary loss of blood from the body.
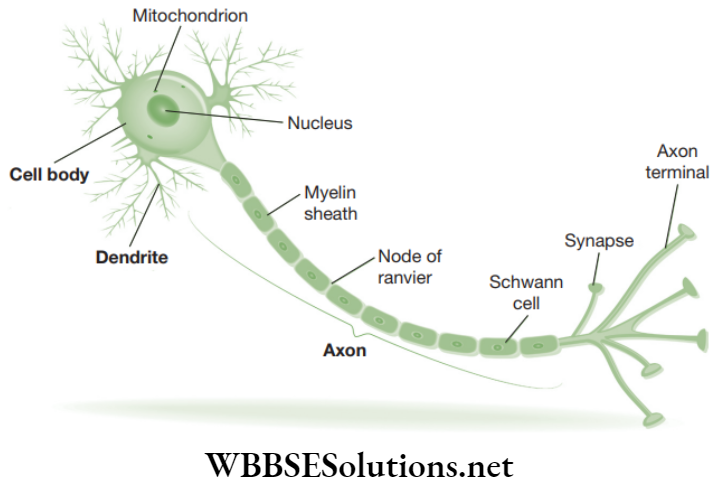
Chapter 2 Tissues Nervous Tissue
Nervous tissue is a specialized tissue to transmit messages within the body. Brain, spinal cord and nerves are all made up of nervous tissue. Nervous tissue consists of highly specialized unit cells known as nerve cells or neurons.
Neurons are capable of receiving stimuli from within or outside the body and of conducting impulses or signals to various parts of the body. The impulse travels from one neuron to another neuron.
A neuron consists of the following three parts.
- Cyton or cell body: It consists of a central nucleus and cytoplasm with deeply stained particles called Nissl’s granules (i.e., clumps of ribosomes).
- Dendrons: These are short processes originating from the cyton and further branching into dendrites.
- Axon: It is a single, long, cylindrical process of uniform diameter. It forms fine branches at its end. Each such twig-like branch of axon ends in a swollen structure termed as synaptic knob or bouton. Bouton consists of acetylcholine-filled vesicles. Acetylcholine (Ach) is a vital neurotransmitter (i.e., a substance playing a crucial role in the transmission of nerve impulses inside the nervous system). Axon is also known as nerve fibre.
Functions: The dendrites receive impulses and the axon takes away impulses from the cell body.
Chapter 2 Tissues Fill In The Blanks
Question 1. ______ group of young meristematic cells of a growing organ, such as the tips of stem and root.
Answer. primordial meristem
Question 2. Parenchyma serves as ______ storage tissues.
Answer. Food
Question 3. Collenchyma is ______ in monocot stems, roots and leaves.
Answer. Absent
Question 4. Collenchyma is a ______.
Answer. Mechanical Tissue
Question 5. ______ provides mechanical support and elasticity.
Answer. Collenchyma
Question 6. ______ gives strength, rigidity, flexibility and elasticity to the plant body.
Answer. Sclerenchyma
Question 7. ______ enables plant body to withstand various strains.
Answer. Sclerenchyma
Question 8. Xylem and ______ is a vascular and mechanical tissues.
Answer. Phloem
Question 9. ______ of xylem are elongated cells with tapering ends.
Answer. Tracheids
Question 10. ______ forms the delicate lining of cavities (mouth, oesophagus, nose, pericardium, alveoli, etc.) and of blood vessels and covering of the tongue and skin.
Answer. Squamous epithelium
Question 11. ______ are found in the muscles of limbs, body wall, face, neck, etc.
Answer. Striated epithelium
Question 12. Smooth muscles are located in the walls of the hollow visceral organs except that of the heart, and hence, they are called ______.
Answer. visceral muscles
Question 13. Cardiac muscles show stripes of light and dark bands. Also, these muscle fibres show densely stained cross bands known as ______.
Answer. intercalated impulse
Question 14. ______ protects the body against infection, as it contains WBCs in abundance. It makes up the immune system of the body.
Answer. Lymph
Question 15. A group of cells that are similar in structure and which perform a specific function are called ______.
Answer. Tissues
Question 16. Founder of Histology ______.
Answer. Marcello Malphigi
Question 17. Arrange the order: Cells ______ Organ ______ Organism.
Answer. 1. Tissue 2. Organ System
Question 18. Cell growth is distributed uniformly in ______.
Answer. Animal tissues
Question 19. Full form of ATP ______.
Answer. Adenosine triphosphate
Question 20. ______ divides in two planes resulting to an increase in an organ, for example, leaf formation.
Answer. Plate meristem
Question 21. Cell divisions occur in all planes resulting in the increase of volume is ______.
Answer. Mass meristem
Question 22. ______ outermost layer of the young growing region that develops to give rise to epidermal tissue system.
Answer. Protoderm meristem
Question 23. ______ is found between the regions of permanent tissues.
Answer. Intercalary meristem
Question 24. ______ was first proposed by Hanstein (1868).
Answer. Histogen Cell Theory
Chapter 2 Tissues True or False
Question 1. Epithelial tissue is a protective tissue in animal body.
Answer. True
Question 2. The lining of blood vessels, lung alveoli and kidney tubules are all made up of epithelial tissue.
Answer. True
Question 3. Epithelial cells have a lot of intercellular spaces.
Answer. False
Question 4. Epithelial layer is a permeable layer.
Answer. True
Question 5. Epithelial layer does not allow the regulation of materials between body and external environment.
Answer. False
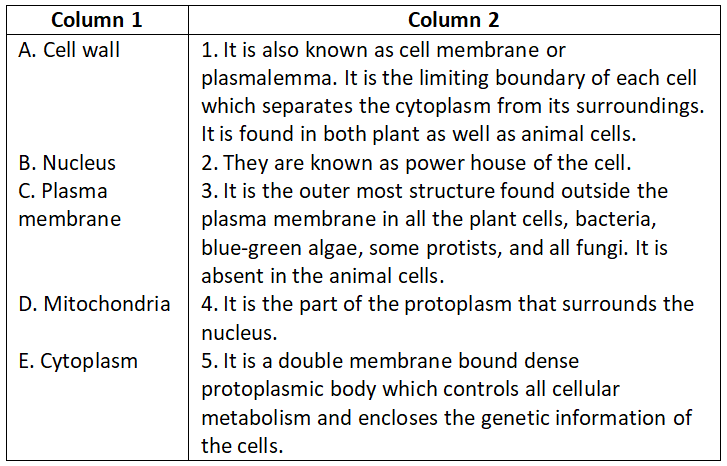
Chapter 2 Tissues Match The Columns
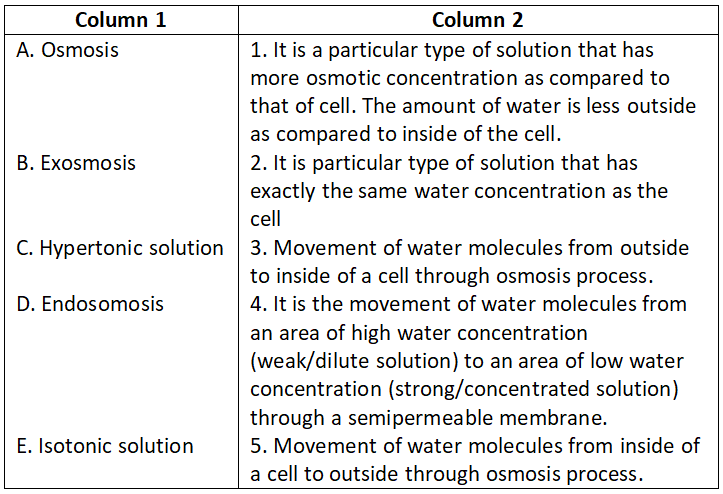
Question 1.
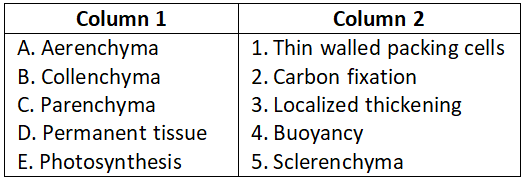
Select the correct option.
- A-4, B-3, C-1, D-5, E-2
- A-2, B-4, C-5, D-1, E-3
- A-5, B-3, C-2, D-4, E-1
- A-4, B-2, C-5, D-3, E-1
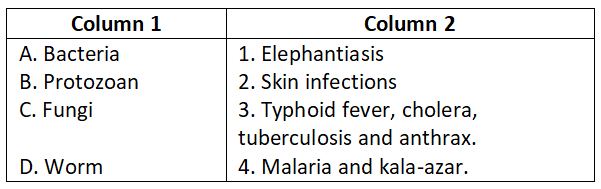
Answer. 1. A-4, B-3, C-1, D-5, E-2
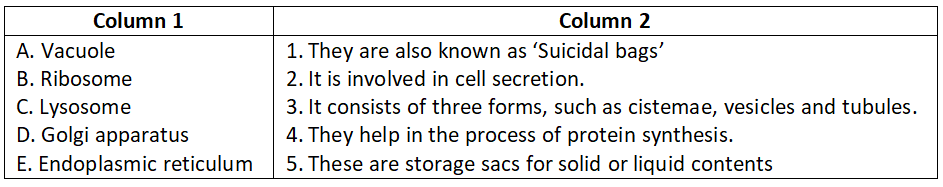
Question 2.
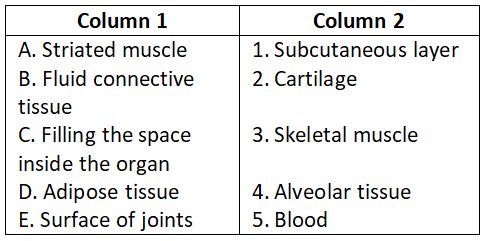
Select the correct option.
- A-4, B-3, C-1, D-5, E-2
- A-3, B-5, C-4, D-1, E-2
- A-5, B-1, C-3, D-2, E-4
- A-2, B-3, C-4, D-1, E-5
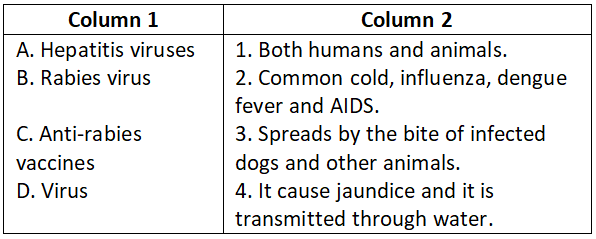
Answer. 2. A-3, B-5, C-4, D-1, E-2
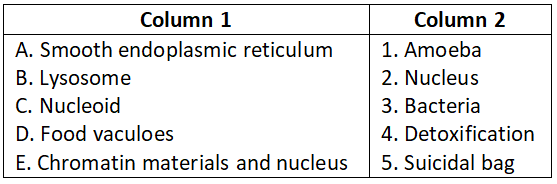
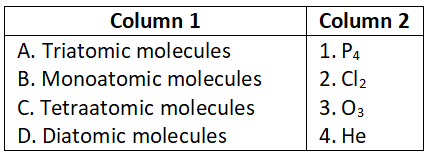
Question 3.
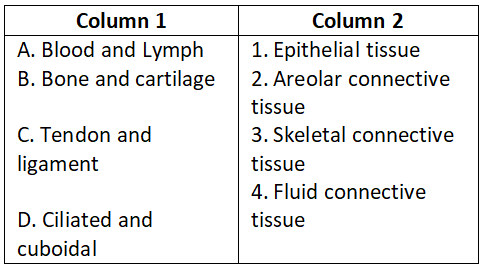
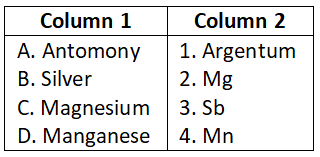
Select the correct option.
- A-1, B-2, C-3, D-4
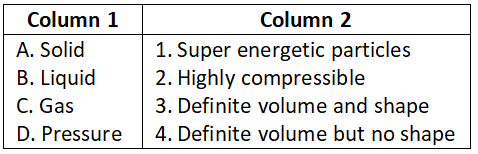
- A-2, B-3, C-4, D-1
- A-4, B-3, C-2, D-1
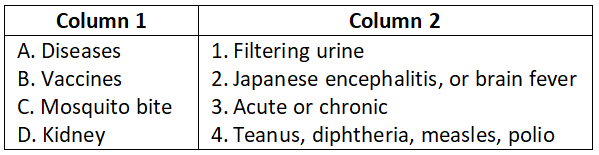
- A-1, B-3, C-4, D-2
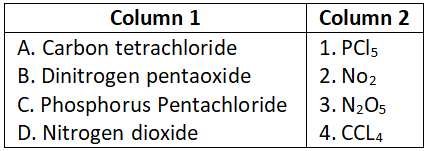
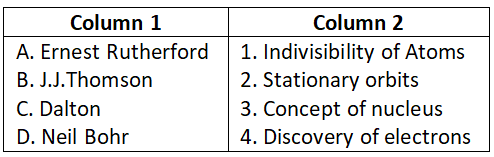
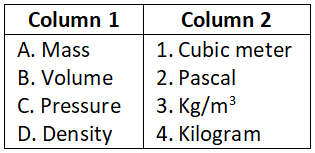
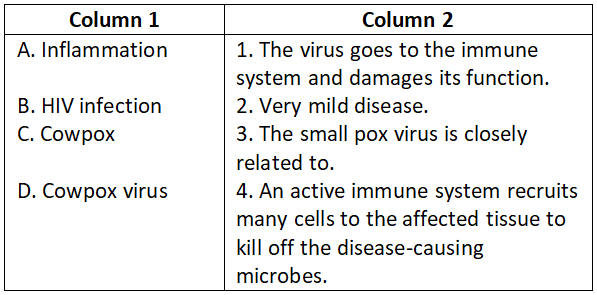
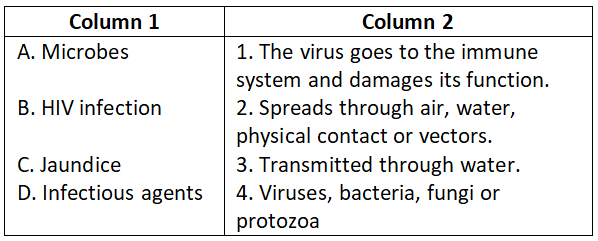
Answer. 3. A-4, B-3, C-2, D-1
Question 4.
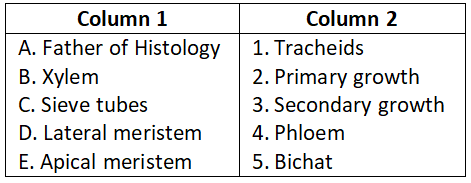
Select the correct option.
- A-3, B-4, C-1, D-2, E-5
- A-5, B-1, C-4, D-3, E-2
- A-3, B-2, C-4, D-1, E-5
- A-4, B-5, C-3, D-2, E-1
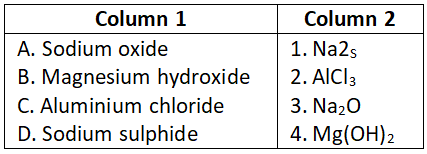
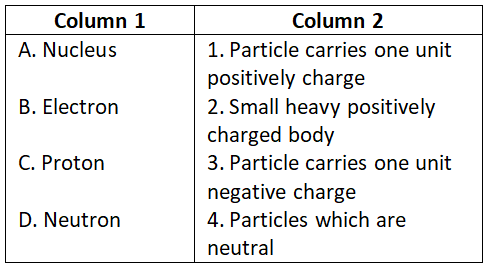
Answer. 2. A-5, B-1, C-4, D-3, E-2
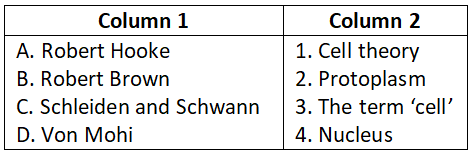
Question 5.
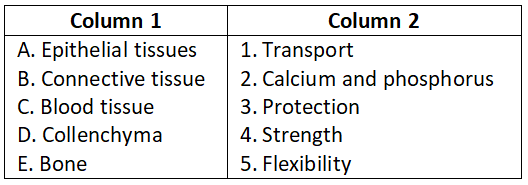
Select the correct option.
- A-2, B-1, C-5, D-3, E-4
- A-1, B-2, C-3, D-4, E-5
- A-5, B-4, C-3, D-2, E-1
- A-3, B-4, C-1, D-5, E-2
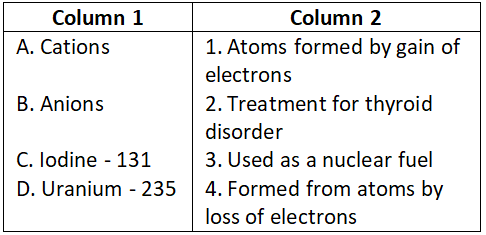
Answer. 4. A-3, B-4, C-1, D-5, E-2