Calorimetry Introduction
An important characteristic of heat must be noted from the very beginning. Unlike the quantities volume, pressure, temperature, etc., heat is not related to any equilibrium state of a body. Heat manifests itself only when a body undergoes a transition from one state to another.
Statements like ‘heat of a body’, ‘initial heat’, and ‘final heat’ have no physical meaning. Physically significant are the quantities like ‘heat gained’, or ‘heat lost’ by a body during any process. In this sense, heat is regarded as an energy in transit.
The rise or fall in temperature of a body implies the addition or extraction of heat from it. If two objects are in contact with each other then there will be heat flow between them till both attain an equilibrium temperature.
In such situations a need arises to know the quantity of heat transferred from one body to another, or, from an object to its surroundings. The study of problems that involve heat exchange between different bodies is called calorimetry.

Calorimetry Factors Affecting Heat Transfer
We know that it takes longer to boil some water than to warm it. Also, boiling water takes longer to cool down than lukewarm water does. So for rise and fall of different amount of temperature, different amounts of heat are absorbed or released.
- Again, increasing the amount of water to be boiled, increases the time taken to boil it too. From this we can conclude that the amounts cf heat absorbed (or released) by the bodies of same material but different mass for a fixed rise (or fall) in temperature depend on their masses.
- When a piece of iron and some water of the same mass are heated, the piece of iron heats up faster than the water.
- Clearly, the material of an object plays a role in determining the amount of heat absorbed (or released) by the object for a certain rise (or fall) in temperature.
- From these observations, it can be inferred that, the amount of heat absorbed or released by a body depends on the change of temperature of the body, mass of the body, and material of the body, provided that the material does not undergo any phase change.
Temperature: Amount of heat gained or lost by a body of fixed mass is directly proportional to the rise or fall in its temperature.
Mass: Amount of heat gained or lost by a body, for a fixed rise or fall in temperature, is directly proportional to its mass.
Material: Amount of heat gained or lost by a body depends on the material of the body.
When a body of mass m receives heat H, the rise in temperature is t,
H ∝ mt or, H = smt……(1)
where s is specific heat whose value depends on the material of the body.
Therefore, H (the heat absorbed or released by a body) = m (mass of the body) x s (specific heat of the material of the body) x t (rise or fall in temperature)
It is to be noted that t in equation (1) is the change in temperature of the body.
So only the heat gained or lost can be measured by this equation.
Calorimetry Units Of Heat
Some useful useful units for the measurement of heat are defined as follows:
Calorie: It is the unit of heat in CGS system. The amount of heat required to raise the temperature of 1 g of water from 14.5 °C to 15.5 °C is called 1 calorie (1 cal).
- Practically, the heat required to raise the temperature of 1 g of pure water from 0°C to PC is not the same as the heat required to raise Its temperature, for example, from 45T to 46°C though the rise in temperature is the same.
- Hence, quantity of heat differs at the different ranges in the scale of temperature.
- So, most acceptable definition of calorie is, 1 cal = 1/100 X heat required to raise the temperature of 1 g of pure water from 0°C to 100°C. It is often known as mean calorie.
- Its value is equal to the amount of heat required to raise the temperature of 1 g of pure water from 14.5°C to 15.5°C. So it is sometimes called 15°C calorie.
Kilocalorie or kilogram calorie: The quantity of heat required to raise the temperature of 1 kg of pure water from 14.5°C to 15.5°C, is called 1 kilocalorie.
As 1 kg = 1000 g, 1 kilocalorie (kcal) = 1000 calorie (cal).
Joule: This is the unit of heat in SI. 1 cal = 4.2 J.
Calorimetry Notes for Class 11 WBCHSE
Calorimetry Specific Heat Or Specific Heat Capacity
It has been stated that heat gained (or lost) by a body depends on its mass, the rise (or fall) in temperature and the material of the body.
- Let us take two bodies of same mass but made of different materials. If we try to change the temperature of both the bodies by the same amount, we will find that the heat required is different for them.
- Therefore it is obvious, the quantity of heat required also depends on a special property. This property is known as specific heat or specific heat capacity of the body. It depends on the material of the body.
- It is obvious that different materials possess different specific heat capacities.
Specific Heat Or Specific Heat Capacity Definition: Specific heat capacity is the quantity of heat required to raise the temperature of unit mass of a substance by unit amount.
Definition of specific heat capacity in CGS system: It is the quantity of heat required to raise the temperature of 1 g of a substance through 1 °C. In this system the unit of specific heat is cal · g-1 · °C-1.
For example, ‘the specific heat capacity of copper is 0.093 cal · g-1 · °C-1 means that to raise the temperature of 1 g of copper by 1 °C, the heat required is 0.093 cal.
Definition of specific heat capacity in SI: The quantity of heat required to raise the temperature of 1 kg of a substance through 1 K is known as specific heat capacity of the substance.
The unit of specific heat in this system is J · kg-1 · K-1
In SI, the specific heat capacity of water is 4186 J · kg-1 · K-1.
Relation between CGS and SI unit of specific heat capacity: \(1 \mathrm{cal} \cdot \mathrm{g}^{-1} \cdot{ }^{\circ} \mathrm{C}^{-1}=\frac{4.186 \mathrm{~J}}{10^{-3} \mathrm{~kg} \times 1 \mathrm{~K}}=4186 \mathrm{~J} \cdot \mathrm{kg}^{-1} \cdot \mathrm{K}^{-1}\)
Specific heat capacities of a few solids and liquids
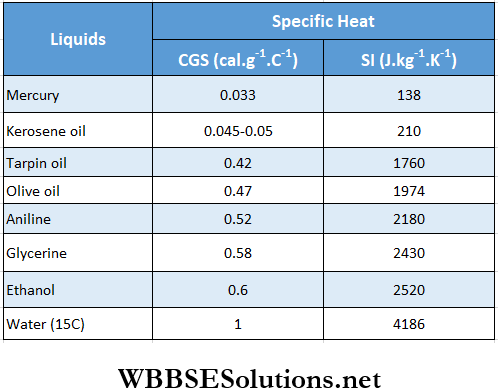
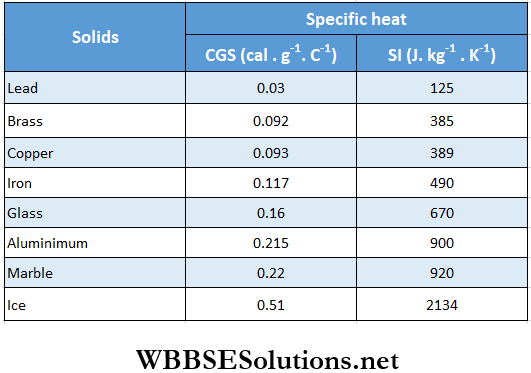
Calorimetry Fundamentals Principal Of Calorimetry
When two bodies at different temperatures are brought in contact, heat is transferred from the body at higher temperature to that at lower temperature. So, the former starts to cool down whereas the latter starts to warm up.
The flow of heat continues till both reach the same temperature. The state where both the bodies are at the same temperature is called thermal equilibrium.
Now we know that heat is a form of energy. According to the law of conservation of energy, energy can neither be created nor be destroyed.
So during such heat transfers, if heat does not transform into another form of energy (or, if no other form of energy transforms into heat energy) then, heat released by a body = heat absorbed by another body
This is the fundamental principle of calorimetry. This is also true for any number of bodies brought in contact with one another. In that case, we can write down the principle of calorimetry as: heat released by the hot bodies = heat absorbed by the cold bodies.
Calorimetry Calorimeter
A calorimeter is a cylindrical metallic container mostly used in calorimetric experiments which is generally made of copper. The container is partly filled with a proper liquid called a calorimetric substance.
- A stirrer (S) made of copper is used to stir the liquid and a thermometer (T) is used to measure the temperature of the liquid. Both are immersed in the liquid.
- In a calorimetric experiment when an object is dipped into the liquid of the calorimeter there must be a difference in temperature between the object and the liquid. After some time the object, the calorimeter, and the liquid inside the calorimeter reach thermal equilibrium.
Calorimetry Experiments for Class 11
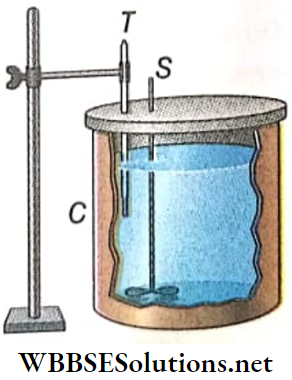
During this interval of time if
- No heat exchange occurs between the calorimeter and the surrounding,
- No exothermic or endothermic reaction (physical or chemical) happens inside the calorimeter and
- The object does not dissolve in the liquid, then according to the fundamental principle of calorimetry, heat released by the hot objects = heat absorbed by the cold objects.
There will be error in the experimental result if the above three conditions are not satisfied. Heat exchange may take place between the calorimeter and the environment by means of
- Conduction,
- Convection and
- Radiation.
A calorimeter is so constructed that heat exchange by any of the three processes can be minimised.
Calorimetric Substance
Water Advantages:
- For the same rise or fall in temperature, heat gained or lost by water is more than that gained or lost by the same mass of other solids or liquids of lower specific heat.
- Water is commonly used as a calorimetric substance because water is easily available.
Water Disadvantages:
- Again due to high specific heat of water, for absorption of same amount of heat, rise in the temperature of water is less than that of other liquids of lower specific heat. Now if the increase of temperature is low then there is high chance of error in reading.
- The boiling point of water is 100°C. If an object of temperature more than 100°C is mixed with water, a sudden vaporisation occurs. Due to these reasons water is not useful as a calorimetric substance.
Aniline: Recendy aniline has been recognized as the best calorimetric substance. Aniline is present in pure form. Its specific heat is low (0.62 cal · g-1 • °C-1) and boiling point is high (183.9°C).
Calorimetry Conclusion
If the state of a body remains unchanged, heat gained or lost by the body depends on
- Rise or fall in temperature of the body,
- Mass of the body and
- Nature of the material of the body.
Heat absorbed or released by a body only for a change in its temperature can be measured. Total heat contained in a body at a particular temperature cannot be measured.
- The amount of heat required by lg of pure water for the rise of its temperature from 14.5°C tol5.5°C is called 1 cal.
- The amount of heat required by lg of pure water for the rise in its temperature from 0° to 100°, divided by 100, gives the value of mean calorie.
- The SI unit of heat is joule. 1 cal = 4.2 J.
- Specific heat is the quantity of heat required to raise the temperature of unit mass of a substance through 1 degree.
According to this definition, units of specific heat: cal · g-1 · °C-1 (in CGS system) and J · kg-1 · K-1 (in SI).
Thermal capacity of a body is defined as the quantity of heat required to raise its temperature by unity.
Unit of thermal capacity: cal • °C-1 (in CGS system) and J · K-1 (in SI).
Water equivalent of a given body is the mass of water for which the rise in temperature is the same as that for the body, when the amount of heat supplied is the same for both.
Unit of water equivalent: g (in CGS system) and kg (in SI).
During heat transfer, if heat energy is not converted into any other form of energy (or no other form of energy is converted into heat energy), then
heat lost by one body = heat gained by another body.
This is the basic principle of calorimetry.
Calorimetry Useful Relations For Solving Numerical Problems
If no change of state occurs, then the heat gained or lost by a body, H = mst.
[where m = mass of the body, s = specific heat, t = change in temperature]
Thermal capacity of a body = ms = H/t
Thermal capacity per unit mass of a body = specific heat of the material of the body
Water equivalent of a body, W = \(\frac{m s}{s_w}\)
According to the basic principle of calorimetry, for more than two bodies at different temperatures in contact, heat lost by hot bodies = heat gained by cold bodies.
In CGS system, specific heat of water = 1 cal · g-1 · °C-1, and in SI, the specific heat of water = 4200 J · kg-1 · -1.
| Class 11 Physics | Class 12 Maths | Class 11 Chemistry |
| NEET Foundation | Class 12 Physics | NEET Physics |
Calorimetry Very Short Answer Type Questions
Examples of Calorimetry Problems
Question 1.Which substance, used in our everyday life, has the highest specific heat?
Answer: Water
Question 2. For a body of mass m and specific heat capacity s, how much heat is required to increase its temperature by t?
Answer: mst
Question 3. There are two spheres of the same radius. One is solid but the other is hollow. If both the spheres are heated to the same temperature and then allowed to cool down, which sphere will cool faster?
Answer: Hollow
Question 4. What is the CGS unit of thermal capacity?
Answer:
The CGS unit of thermal capacity
“cal • °C-1”
Question 5. What is the CGS unit of water equivalent?
Answer:
The CGS unit of water equivalent
“g”
Question 6. Which substance is treated as the best calorimetric substance nowadays?
Answer: Aniline
Calorimetry Assertion Reason Type Questions And Answers
Direction: These questions have statement 1 and statement 2. Of the four choices given below, choose the one that best describes the two statements.
- Statement 1 is true, statement 2 is true; statement 2 is a correct explanation for statement 1.
- Statement 1 is true, statement 2 is true; statement 2 is not a correct explanation for statement 1.
- Statement 1 is true, statement 2 is false.
- Statement 1 is false, statement 2 is true.
Question 1.
Statement 1: A gas have a unique value of specific heat.
Statement 2: Specific heat is defined as the amount of heat required to raise the temperature of unit mass of the substance through unit degree.
Answer: 4. Statement 1 is false, statement 2 is true.
Question 2.
Statement 1: When a body of mass M loses heat, the time rate of fall of temperature for given amount of loss of heat is inversely proportional to mass.
Statement 2: ΔQ = MsΔT where, ΔQ = amount of heat, s = specific heat, andΔT = decrease in temperature.
Answer: 1. Statement 1 is true, statement 2 is true; statement 2 is a correct explanation for statement 1.
Question 3.
Statement 1: Specific heat capacity is the cause of formation of land and sea breeze.
Statement 2: The specific heat of water is more than land.
Answer: 1. Statement 1 is true, statement 2 is true; statement 2 is a correct explanation for statement 1.
Question 4.
Statement 1: Specific heat of a body is always greater than its thermal capacity.
Statement 2: Thermal capacity is the heat required for raising temperature of a body by unity.
Answer: 4. Statement 1 is false, statement 2 is true.
Question 5.
Statement 1: Two bodies at different temperatures, if brought in thermal contact do not necessarily settle to the mean temperature.
Statement 2: The two bodies may have different thermal capacities.
Answer: 1. Statement 1: Two bodies at different temperatures, if brought in thermal contact do not necessarily settle to the mean temperature.
Calorimetry Match Column 1 And Column 2
Question 1. Three liquids A, B, and C having the same specific heat and masses m, 2 m, and 3 m have temperatures 20°C, 40°C, and 60°C respectively. Temperature of the mixture when
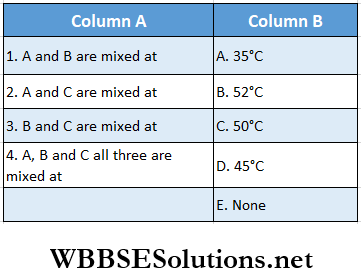
Answer: 1. E, 2. A, 3. D, 4. B
Calorimetry Comprehension Type Questions And Answers
Read the following passages carefully and answer the questions at the end of them.
Question 1. A vacuum flask contains 0.4 kg liquid paraffin whose temperature can be increased at the rate of 1°C per minute using an immersion heater of power 12.3 W. When a heater of 19.2 W is used to heat 0.5 kg of liquid paraffin, in the same flask, the rate of rise of temperature is 1.2°C per minute.
1. What is the specific heat of the paraffin?
- 0.529cal · g-1 · °C-1
- 0.429 cal · g-1 · °C-1
- 0.472cal · g-1 · °C-1
- 0.62.3 cal · g-1 °C-1
Answer: 1. 0.529 cal · g-1 · °C-1
2. What is the thermal capacity of the material of the flask?
- 17.67cal · °C-1
- 16.85 cal · °C-1
- 17.01cal · °C-1
- 16.63 cal · °C-1
Answer: 3. 17.01 cal · °C-1
Question 2. When a block of metal of specific heat 0.1 caI · g-1 · °C-1 and weighing 110 g is heated to 100°C and then quickly transferred to a calorimeter containing 200 g of a liquid at 10°C, the resulting temperature is 1°C. On repeating the experiment with 400 g of the same liquid in the same calorimeter at same initial temperature, the resulting temperature is 14.5°C.
1. Find the specific heat of the liquid.
- 0.42 cal · g-1 · °C-1
- 0.52 cal · g-1 · °C-1
- 0.48 cal · g-1 · °C-1
- 0.62 cal · g-1 · °C-1
Answer: 3. 0.48 cal · g-1 · °C-1
2. Find the water equivalent of calorimeter.
- 15.5 g
- 16.6 g
- 17.3 g
- 18.2 g
Answer: 2. 16.6 g
Calorimetry Integer Type Questions And Answers
In this type, the answer to each of the questions is a single-digit integer ranging from 0 to 9.
Question 1. 50 g of copper is heated to increase its temperature by 10°C. If the same quantity is given to 10 g of water, what will be the rise in its temperature (in °C)?
Answer: 5
Question 2. The water of volume 2 L in a container is heated with a coil of 1 kW at 27°C. The lid of the container is open and energy dissipates at rate of 160 J · s-1. In now much time (in minutes) temperature will rise from 27 °C to 75°C? [Given specific heat of water is 4.2 kJ • kg·-1 ]
Answer: 8
Question 3. If 0.5 kg of coal on burning raises the temperature of 50 L of water from 20 °C to 90 °C, then the heat of combustion of coal is n x 106 cal · kg-1. Find the value of n.
Answer: 7
Question 4. When x gram of steam is mixed with y grams of ice at 0°C, we obtain (x + y) grams of water at 100°C. Find the value of y/x
Answer: 3
Calorimetry Short Answer Type Questions
Question 1. A 10 Watt electric heater is used to heat a container filled with 0.5 kg of water. It is found that the tempera¬ture of water and the container rises by 3°K in 15 minutes. The container is then emptied, dried, and filled with 2 kg of oil. The same heater now raises the tem¬perature of the container-oil system by 2°K in 20 minutes. Assuming that there is no heat loss in the process and the specific heat of water as 4200 J • kg-1 • K-1, the specific heat of oil in the same unit is equal to
- 1.50 x 103
- 2.55 x 103
- 3.00 x 103
- 5.10 x 103
Answer:
Given
A 10 Watt electric heater is used to heat a container filled with 0.5 kg of water. It is found that the temperature of water and the container rises by 3°K in 15 minutes. The container is then emptied, dried, and filled with 2 kg of oil. The same heater now raises the tem¬perature of the container-oil system by 2°K in 20 minutes. Assuming that there is no heat loss in the process and the specific heat of water as 4200 J • kg-1 • K-1,
In first case the temperature of water and the container is increased by 3K in 15 min or (15 x 60)s by heating.
So, 10 x (15 x 60) = (0.5 x 4200 x 3) + (Wx 3)
[W = water equivalent]
or, \(W=\frac{9000-6300}{3}=900 \mathrm{~kg}\)
In second case, the heater increases the temperature of the oil and the container by 2K in 20 min or (20 x 60)s.
So, 10 x (20×60) = (2 x s x 2) + (W x 2)
[s = specific heat of oil]
or, s = \(\frac{12000-2 W}{4}=\frac{12000-1800}{4}=\frac{10200}{4}\)
= 2550 = 2.55 x 103 J • kg-1 · K-1
The option 2 is correct.
Thermal Properties of Matter and Calorimetry
Question 2. Three bodies of the same material and having masses m, m, and 3m are at temperatures 40°C, 50°C, and 60°C respectively. If the bodies are brought in the thermal contact, the final temperature will be
- 45°C
- 54°C
- 52°C
- 48°C
Answer:
Given
Three bodies of the same material and having masses m, m, and 3m are at temperatures 40°C, 50°C, and 60°C respectively. If the bodies are brought in the thermal contact,
If the final temperature is t°C,
ms(t-40) + ms(t-50) + 3ms(t-60) = 0
or, (t-40) + (t-50) + 3(t-60) = 0
or, 5t-270 = 0
∴ t = 54°C
The option 2 is correct.
Question 3. A copper ball of mass 100 g is at a temperature T. It is dropped in a copper calorimeter of mass 100 g, filled with 170 g of water at room temperature. Subsequently, the temperature of the system is found to be 75°C. T is given by: (given- room temperature = 30°C, specific heat of copper= 0.1 cal/g °C)
- 800°C
- 885°C
- 1250°C
- 825°C
Answer:
Given
A copper ball of mass 100 g is at a temperature T. It is dropped in a copper calorimeter of mass 100 g, filled with 170 g of water at room temperature. Subsequently, the temperature of the system is found to be 75°C.
From the fundamental principle of calorimetry, heat released by copper ball = heat absorbed by calorimeter and water
or, 100 x0.1(t-75) = 100 x 0.1 x (75-30) + 170 x 1 x (75-30)
or, t = 885°C
The option 2 is correct.
Question 4. Explain why the coolant in a chemical or a nuclear plant (i.e., the liquid used to prevent the different parts of a plant from getting too hot) should have high specific heat.
Answer:
This is due to the fact that heat absorbed by a substance is directly proportional to the specific heat of the substance.
Question 5. Explain why two bodies at different temperatures T1 and T2 if brought in thermal contact do not necessarily settle to the mean temperature (T1 + T2)/2.
Answer:
If two bodies, at different temperatures, come in thermal contact, heat flows from the body at higher temperature to the body at lower temperature till the temperature becomes same. The final temperature can be mean temperature, i.e., (T1 + T2)/2, only when both the bodies have equal thermal capacities.
