Preparation Of Dihydrogen, H2
WBBSE Class 11 Dihydrogen Preparation Methods
Preparation Of Dihydrogen In The Laboratory
Dihydrogen
Principle: In the laboratory, dihydrogen is prepared by the action of dilute sulphuric acid on granulated zinc (commercial variety).
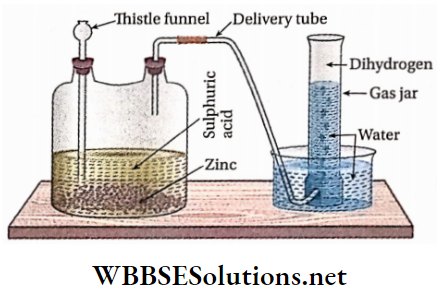
Laboratory Preparation of Dihydrogen Notes
Process: Dilute sulphuric acid is added through a thistle funnel to some pieces of granulated zinc taken in a Wolff’s bottle. The liberated hydrogen gas is collected by the downward displacement of water.
Pure zinc cannot be used in this process because it reacts sluggishly with dilute H2SO4. Impurities like Cu, Cd, etc. present in commercial zinc help to speed up tire reactions by constituting electrochemical couples (local Galvanic cells). If pure zinc is used, a few drops of CuSO4 solution should be added to the reaction mixture to increase the reaction rate.
Concentrated H2SO4 cannot be used in this preparative method because being oxidizing in nature it oxidizes dihydrogen into the water and itself gets reduced to SO2 For the same basic reason, concentrated HNO3 is not used.
Read and Learn More WBCHSE Class 11 Chemistry
⇒ \(\mathrm{Zn}+2 \mathrm{H}_2 \mathrm{SO}_4 \text { (conc.) } \rightarrow \mathrm{ZnSO}_4+\mathrm{SO}_2+2 \mathrm{H}_2 \mathrm{O}\)
⇒ \(\left.\mathrm{Zn}+4 \mathrm{HNO}_3 \text { (conc. }\right) \rightarrow \mathrm{Zn}\left(\mathrm{NO}_3\right)_2+2 \mathrm{NO}_2+2 \mathrm{H}_2 \mathrm{O}\)
Concentrated HCl cannot be used in the preparation of dihydrogen because the produced dihydrogen will contain times of volatile HCl. Moreover, ZnCl2 formed in the reaction is insoluble in HCl and hence the reaction stops after some time
⇒ \(\mathrm{Zn}+2 \mathrm{HCl} \rightarrow \mathrm{ZnCl}_2+\mathrm{H}_2 \uparrow\)
Various Methods Of Preparation Of Dihydrogen
By the reaction of dilute mineral acids with metals:
⇒ \(\mathrm{Zn}+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{ZnSO}_4+\mathrm{H}_2 \uparrow ; \mathrm{Mg}+2 \mathrm{HCl} \rightarrow \mathrm{MgCl}_2+\mathrm{H}_2 \uparrow\)
By the reaction of water with metals:

Chemical Properties of Dihydrogen for Class 11
1. By the reaction of certain alkali and alkaline earth metals with water at room temperature:
⇒ \(2 \mathrm{M}+2 \mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{MOH}+\mathrm{H}_2 \uparrow[\mathrm{M}=\mathrm{Na}, \mathrm{K}]\)
⇒ \(\mathrm{M}^{\prime}+2 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{M}^{\prime}(\mathrm{OH})_2+\mathrm{H}_2 \uparrow\left[\mathrm{M}^{\prime}=\mathrm{Ca}, \mathrm{Sr}, \mathrm{Ba}\right]\)
Reactions with these very active metals are so vigorous and exothermic that the liberated hydrogen immediately catches fire which may cause accidents. Because of high I.E., Be does not react with water (hot and cold) to produce H2.
2. By the reaction of less active metals like Mg, A1, Zn, etc., with boiling water: Mg, Al, Zn, etc. (less active metals) react with boiling water to produce dihydrogen.
⇒ \(\mathrm{Mg}+\mathrm{H}_2 \mathrm{O} \stackrel{\Delta}{\longrightarrow} \mathrm{Mg}(\mathrm{OH})_2+\mathrm{H}_2 \uparrow\)
⇒ \(2 \mathrm{Al}+6 \mathrm{H}_2 \mathrm{O} \stackrel{\Delta}{\longrightarrow} 2 \mathrm{Al}(\mathrm{OH})_3+3 \mathrm{H}_2 \uparrow\)
3. By the reaction of least active metals like iron and zinc with steam: Least active metals like Fe and Zn react with steam to produce dihydrogen.
⇒ \(3 \mathrm{Fe}+4 \mathrm{H}_2 \mathrm{O} \stackrel{\Delta}{\longrightarrow} \mathrm{Fe}_3 \mathrm{O}_4+4 \mathrm{H}_2 \uparrow\)
⇒ \(\mathrm{Zn}+\mathrm{H}_2 \mathrm{O} \stackrel{\Delta}{\longrightarrow} \mathrm{ZnO}+\mathrm{H}_2 \uparrow\)
By the reaction of strong alkali with metals and certain non-metals: Metals like Al, Sn, Zn, Si, etc., react with strong alkali to liberate dihydrogen.
⇒ \( 2 \mathrm{Al}+2 \mathrm{NaOH}+2 \mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{NaAlO}_2+3 \mathrm{H}_2 \uparrow\)
Sodium aluminate
⇒ \(\mathrm{Sn}+2 \mathrm{NaOH} \rightarrow \mathrm{Na}_2 \mathrm{SnO}_2+\mathrm{H}_2 \uparrow\)
Sodium stannate
⇒ \( \mathrm{Zn}+2 \mathrm{NaOH} \rightarrow \mathrm{Na}_2 \mathrm{ZnO}_2+\mathrm{H}_2 \uparrow\)
Sodium zincate
⇒ \( \mathrm{Si}+2 \mathrm{NaOH}+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Na}_2 \mathrm{SiO}_3+2 \mathrm{H}_2 \uparrow\)
Sodium silicate
By the hydrolysis of ionic hydrides:
⇒ \(\mathrm{CaH}_2+2 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Ca}(\mathrm{OH})_2+2 \mathrm{H}_2 \uparrow\)
⇒ \(\mathrm{LiH}+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{LiOH}+\mathrm{H}_2 \uparrow\)
Calcium hydride (CaH2) is called hydrolith and the method of preparing dihydrogen from it is known as the hydrolith process.
By the electrolysis of molten ionic hydrides: in a molten state, ionic hydrides except LiH conduct electricity with the liberation of dihydrogen at the anode.
⇒ \(\mathrm{CaH}_2(\text { molten }) \rightarrow \mathrm{Ca}^{2+}+2 \mathrm{H}^{-}\)
Cathode: \(\mathrm{Ca}^{2+}+2 e \rightarrow \mathrm{Ca}\)
Anode: \(2 \mathrm{H}^{-} \rightarrow \mathrm{H}_2 \uparrow+2 e\)
| Class 11 Physics | Class 12 Maths | Class 11 Chemistry |
| NEET Foundation | Class 12 Physics | NEET Physics |
Preparation Of Pure Dihydrogen
Pure dihydrogen (>99.95%) can be prepared by the following methods:
1. By the action of pure dilute H2SO4 on Mg -ribbon:
⇒ \(\mathrm{Mg}+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{MgSO}_4+\mathrm{H}_2 \uparrow\)
2. By the hydrolysis of sodium hydride:
⇒ \(\mathrm{NaH}+\mathrm{H}_2 \mathrm{O} \rightarrow\mathrm{NaOH}+\mathrm{H}_2 \uparrow\)
3. By the action of KOH on scrap aluminum (Uyeno’s method):
⇒ \(2 \mathrm{Al}+2 \mathrm{KOH}+2 \mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{KAlO}_2+3 \mathrm{H}_2 \uparrow\)
4. By the electrolysis of a warm dilute solution of barium hydroxide [Ba(OH)2] using orPt-electrode:
Cathode: \(2 \mathrm{H}_2 \mathrm{O}(l)+2 e \rightarrow \mathrm{H}_2(g)+2 \mathrm{OH}^{-}(a q)\)
5. By electrolysis of brine solution:
Cathode: \(2 \mathrm{H}_2 \mathrm{O}(l)+2 e \rightarrow \mathrm{H}_2(g)+2 \mathrm{OH}^{-}(a q)\)
Industrial Preparation Of Dihydrogen
Lane’s process:
This process consists of two stages:
Oxidation stage: Superheated steam is passed over iron filings heated to about 750-800°C. Iron reduces water to dihydrogen and itself gets oxidized to Fe3O4
⇒ \(\begin{gathered} 3 \mathrm{Fe}(s)+\underset{2}{4 \mathrm{H}_2 \mathrm{O}(g) \stackrel{750-800^{\circ} \mathrm{C}}{\longrightarrow}} \mathrm{Fe}_3 \mathrm{O}_4(s)+4 \mathrm{H}_2(g)+\text { heat } \\ \text { steam } \end{gathered}\)
Industrial Methods of Dihydrogen Production
Reduction stage: After the complete oxidation of iron to Fe3O4, the supply of steam is stopped and a steam of water gas (H2 + CO) is passed to reduce Fe3O4 back to iron.
⇒ \(\mathrm{Fe}_3 \mathrm{O}_4+4 \mathrm{H}_2 \rightarrow 3 \mathrm{Fe}+4 \mathrm{H}_2 \mathrm{O} ;\)
⇒ \(\mathrm{Fe}_3 \mathrm{O}_4+4 \mathrm{CO} \rightarrow 3 \mathrm{Fe}+4 \mathrm{CO}_2\)
In the actual process, dihydrogen gas is manufactured from a small amount of iron by passing steam and water gas over it alternately.
Bosch process:
This process involves the steps as follows:
Preparation of watergate /syngas:
1. Water gas (1:1 mixture of CO and H2 ) is produced by passing superheated steam over red hot coke in the presence of Ni catalyst. This process of producing water gas (also called syngas, a term used to indicate a mixture of CO & H2 in any ratio) from coke or coal is called “coal gasification.”
⇒ \(\mathrm{C}(s)+\mathrm{H}_2 \mathrm{O}(g) \stackrel{1000^{\circ} \mathrm{C}}{\mathrm{Ni}} \underbrace{\mathrm{CO}(g)+\mathrm{H}_2(g)}_{\text {Water gas }}-121.3 \mathrm{~kJ}\)
2. Syngas may also be produced by heating hydrocarbon with steam at 1000°C in the presence of a nickel catalyst. This is called steam reforming of hydrocarbon.
⇒ \(\mathrm{C}_n \mathrm{H}_{2 n+2}+n \mathrm{H}_2 \mathrm{O}(\mathrm{g}) \stackrel{1000^{\circ} \mathrm{C}}{\mathrm{Ni}} \underbrace{n \mathrm{CO}(g)+(2 n+1) \mathrm{H}_2}_{\text {Syngas }}\)
Example: \(\mathrm{C}_3 \mathrm{H}_8(g)+3 \mathrm{H}_2 \mathrm{O}(g) \stackrel{1000^{\circ} \mathrm{C}}{\longrightarrow} 3 \mathrm{CO}(g)+7 \mathrm{H}_2(g)\)
Separation of dlhydrogen:
When syngas are passed over steam in the presence of iron chromate or a mixture of ferric oxide and chromium oxide heated at 400°C, CO is oxidized and more hydrogen is produced.

This is called the water gas shift reaction. When the resulting mixture is passed through the water under 30 atm pressure, C2 dissolves leaving behind H2 which is collected.
Electrolysis of water: In this process, electrolysis of a 15-20% NaOH solution is carried out using an iron cathode and nickel-coated iron anode. Pure water is a bad conductor of electricity while water containing a small amount of alkali (or acid) is a good conductor of electricity.
Cathode:\(2 \mathrm{H}_2 \mathrm{O}(l)+2 e \rightarrow 2 \mathrm{H}_2(g)+2 \mathrm{OH}^{-}(a q)\)
Anode:\(2 \mathrm{Cl}^{-}(a q) \rightarrow \mathrm{Cl}_2(g)+2 e\)
Overall reaction:
⇒ \(2 \mathrm{Cl}^{-}(a q)+2 \mathrm{H}_2 \mathrm{O}(l) \rightarrow\mathrm{Cl}_2(g)+\mathrm{H}_2(g)+2 \mathrm{OH}^{-}(a q)\)
From methanol: The process involves the catalytic decomposition of methanol. A 1:1 mixture of vaporized methanol and water is passed over a special type of catalyst ‘base metal chromite type’ at 400°C. The mixture of H2 and CO obtained as a result of the decomposition reaction is made to react with steam at the temperature to give CO2 and more H2 gas.
⇒ \(\mathrm{CH}_3 \mathrm{OH} \underset{\text { Catalyst }}{\stackrel{40{ }^{\circ} \mathrm{C}}{\longrightarrow}} \mathrm{CO}+2 \mathrm{H}_2 ; \mathrm{CO}+\mathrm{H}_2 \mathrm{O} \underset{\text { Catalyst }}{\stackrel{400^{\circ} \mathrm{C}}{\longrightarrow}} \mathrm{CO}_2+\mathrm{H}_2\)
CO2 is separated bypassing the gas mixture through cold water under pressure.
At present, about 77% of industrial hydrogen is produced from petrochemicals, 18% from coal, 4% from the electrolysis of aqueous solutions, and 1% from other sources.
Properties Of Dihydrogen
Physical properties of Dihydrogen
- Dihydrogen is a colorless, odorless, tasteless, and highly inflammable gas.
- It is the lightest gas known. For example, one liter of H2 gas at STP weighs 0.0899 g. Its density is approximately 1/14 -th of that of air.
- It is diatomic gas (r = Cp/Cv = 1.40). The two atoms are joined by a very strong covalent bond (bond dissociation enthalpy = 435.9 kj • mol-1 ).
- Since its molecules are non-polar its solubility in water is extremely low.
- It can be liquefied under high pressure and at low temperatures.
Dihydrogen Uses and Applications in Chemistry
Chemical Properties of Dihydrogen
- The chemical properties of dihydrogen depend mainly on its bond dissociation enthalpy. The bond dissociation enthalpy of the H —H bond (435.88kj • mol-1 ) is higher than that of any single bond between two atoms.
- At 2000K, only 0.081% of it dissociates to form H-atoms. At 5000K, the percentage of dissociation increases to about 95.5%. Hence, the activation energy of the reactions involving H2 is very high and at ordinary temperature is very stable and unreactive.
- Nearly all of its reactions occur at much higher temperatures or under ultraviolet radiations. Atomic hydrogen having electronic configuration Is1 needs one more electron to complete its orbital. Therefore, atomic hydrogen is very reactive and capable of combining with almost all the elements.
It reacts in three different ways:
- By the loss of its single electron to form H+,
- By the gain of one electron to form H– ion and
- By sharing its electrons with other atoms to form single covalent bonds.
Dihydrogen Reactions with Metals and Nonmetals
Combustion: Dihydrogen is a combustible gas but does not support combustion. It burns with blue flame in air or in oxygen and as a result, water is formed. The reaction is highly exothermic.
⇒ \(2 \mathrm{H}_2(g)+\mathrm{O}_2(g) \stackrel{\Delta}{\longrightarrow} 2 \mathrm{H}_2 \mathrm{O}(l), \Delta H=-286 \mathrm{~kJ} \cdot \mathrm{mol}^{-1}\)
Reaction with non-metals:
1. Reaction with halogens to form hydrogen halides (HX):
It combines with fluorine in the dark and at ordinary temperatures, with chlorine in the presence of sunlight, with bromine when heated, and with iodine when heated in the presence of Pt -catalyst to form the corresponding hydrogen halides.
⇒ \(\mathrm{H}_2(\mathrm{~g})+\mathrm{F}_2(\mathrm{~g}) \stackrel{\text { Dark }}{\longrightarrow} 2 \mathrm{HF}(\mathrm{g}) ; \mathrm{H}_2(\mathrm{~g})+\mathrm{Cl}_2(\mathrm{~g}) \stackrel{\text { Sunlight }}{\longrightarrow} 2 \mathrm{HCl}(\mathrm{g})\)
⇒ \(\mathrm{H}_2(g)+\mathrm{Br}_2(g) \stackrel{673 \mathrm{~K}}{\longrightarrow} 2 \mathrm{HBr}(g) ; \mathrm{H}_2(g)+\mathrm{I}_2(g) \underset{\mathrm{Pt}}{\longrightarrow} 2 \mathrm{HI}(g)\)
Therefore, the reactivity of halogens towards dihydrogen decreases in the order: \(\mathrm{F}_2>\mathrm{Cl}_2>\mathrm{Br}_2>\mathrm{I}_2\)
Physical Properties of Dihydrogen: Class 11 Overview
2. Reaction with nitrogen to form ammonia:
⇒ \(3 \mathrm{H}_2(g)+\mathrm{N}_2(g) \underset{\mathrm{Fe}(\mathrm{Mo})}{\stackrel{673 \mathrm{~K} / 200 \mathrm{~atm}}{\longrightarrow}} 2 \mathrm{NH}_3(g)+\text { heat }\)
3. Reaction with sulfur to form hydrogen sulfide:
⇒ \(\mathrm{H}_2(g)+\mathrm{S}(l) \stackrel{700 \mathrm{~K}}{\longrightarrow} \mathrm{H}_2 \mathrm{~S}(g)\)
4. Reaction with carbon to form methane and acetylene:
⇒ \(2 \mathrm{H}_2(g)+\mathrm{C}(s) \stackrel{1275 \mathrm{~K}}{\longrightarrow} \mathrm{CH}_4(g)\)
⇒ \(\mathrm{H}_2(g)+2 \mathrm{C}(s) \underset{3300 \mathrm{~K}}{\stackrel{\text { Electric spark }}{\longrightarrow}} \mathrm{C}_2 \mathrm{H}_2(g)\)
Reaction with metals: Dihydrogen reacts with strongly electropositive metals like Na, K, Ca, etc., at much higher temperatures to form salt-like (ionic) metal hydrides. In these hydrides, the oxidation state of hydrogen is -1. In these reactions, H2 acts as an oxidizing agent.
⇒ \(\begin{array}{r} \stackrel{0}{\mathrm{H}}_2(g)+2 \mathrm{M}(g) \rightarrow 2 \mathrm{MH}^{-1}(s) ; \stackrel{0}{\mathrm{H}}_2(g)+\mathrm{M}^{\prime}(g) \rightarrow \mathrm{M}^{\prime-1} \mathrm{H}_2(s) \\ {\left[\mathrm{M}=\mathrm{Li}, \mathrm{Na}, \mathrm{K} \text { etc. and } \mathrm{M}^{\prime}=\mathrm{Ca}, \text { Ba etc. }\right]} \end{array}\)
Reaction with metal ions and metal oxides:
Dihydrogen reduces some metal ions (metals lying below hydrogen in the activity series) in aqueous solutions to the corresponding metals.
⇒ \(\mathrm{CuO}(s)+\mathrm{H}_2(g) \stackrel{\text { heat }}{\longrightarrow} \mathrm{Cu}(s)+\mathrm{H}_2 \mathrm{O}(l)\)
⇒ \(\mathrm{PbO}(s)+\mathrm{H}_2(g) \stackrel{\text { heat }}{\longrightarrow} \mathrm{Pb}(s)+\mathrm{H}_2 \mathrm{O}(l)\)
Reaction with carbon monoxide to form methanol:
⇒ \(\mathrm{CO}(g)+2 \mathrm{H}_2(g) \underset{\mathrm{ZnO} / \mathrm{Cr}_2 \mathrm{O}_3}{\stackrel{700 \mathrm{~K} / 200 \mathrm{~atm}}{\longrightarrow}} \mathrm{CH}_3 \mathrm{OH}(l)\)
Reaction with organic compounds: Dihydrogen reacts with many organic compounds in the presence of a catalyst to form useful hydrogenated products which are commercially important. For example:
1. When dihydrogen is passed through edible vegetable oils such as soybean oil, groundnut oil, cotton seed oil, etc., at about 200°C under pressure in the presence of finely divided Ni as a catalyst, they undergo hardening and change into edible fats such as vanaspati ghee (Example data) or margarine. In this reaction, the C=C bonds present in glycerides or triglycerides (triesters of glycerol) become saturated.
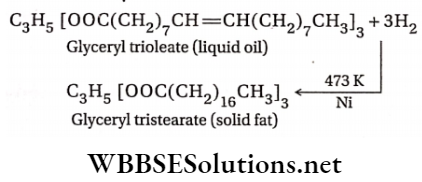
The above process is known as hydrogenation or hardening of oils.
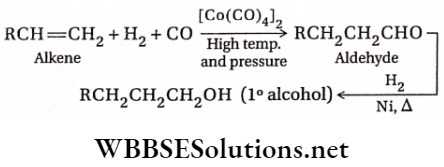
2. Hydroformylation is an important process for the manufacture of aldehydes and alcohols from alkenes. The reaction which involves the addition of a formyl group ( —CHO) and hydrogen atom to a carbon-carbon double bond, is carried out by treating alkenes with CO and H2 at high pressure (10-100 atm) and at temperatures between 40-200°C using transition metals as catalysts. The aldehydes thus obtained further get reduced to alcohols.
Proton (H+) has no chemical existence. Because of higher charge density and higher hydration enthalpy (256 kcal • mol-1), in water, it always remains solvated forming droxoniumion or hydroniumion or oxoniumion \(\left(\mathrm{H}_3 \mathrm{O}^{\oplus}\right)\). This ion is again stabilized by solvation through hydrogen bonding with water molecules. Therefore, in water H+ ions actually exists as [H30(H20)3)+ or [H9O4]+ions.
Allotropy Of Dihydrogen And The Two Active Forms Of Dihydrogen
Hydrogen Production by Electrolysis Explained
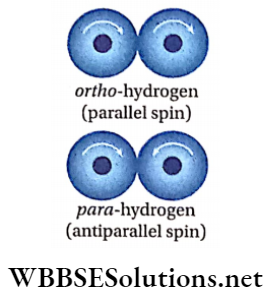
Ortho- And Para-Hydrogen
- A dihydrogen molecule contains two H-atoms. The nuclei of both atoms are spinning about their own axis like a top. Depending upon the direction of spin of the nuclei, the molecules of hydrogen are of two types.
- Dihydrogenin which the spins of both the nuclei are in the same direction (parallel spin) is called ortho-hydrogen and dihydrogen in which the spins of both the nuclei are in the opposite direction (antiparallel spin) is called para-hydrogen.
- These two types of dihydrogen are called nuclear spin isomers. These are also called allotropes of hydrogen. At ordinary temperatures, dihydrogen contains 75% ortho- and 25% para-hydrogen.
- As the temperature is lowered the percentage of ortho-hydrogenin tire mixture decreases while that of the para-hydrogen increases. At very low temperatures (20K), it is possible to obtain pure (-100%) para-hydrogen.
- At high temperatures (>400K), the mixture contains 75% ortho-form. Therefore, the para-form has lower energy as compared to the ortho-form. Both ortho- and para-forms of H2 have the same chemical properties.
- However, due to different nuclear spins and consequently, different internal energies, they have different physical properties like specific heat, thermal conductivity, boiling point, etc.
Deuterium and helium also exist in ortho and para-allotropic forms.
Atomic hydrogen
Atomic hydrogen Definition:
Hydrogen in its atomic state, produced by the dissociation of molecular hydrogen at a very high temperature is called atomic hydrogen.
Safety Guidelines for Handling Dihydrogen
Preparation:
Because of high H—H bond dissociation enthalpy, atomic hydrogen is produced only at a much higher temperature. It is usually obtained by passing dihydrogen at atmospheric pressure through an electric arc struck between two tungsten electrodes. The electric arc produces a temperature around 4000-4500°C.
⇒ \(\mathrm{H}_2(\mathrm{~g}) \stackrel{\text { Electric arc }}{\longrightarrow} 2 \mathrm{H}(\mathrm{g}) ; \Delta H=435.90 \mathrm{~kJ} \cdot \mathrm{mol}^{-1}\)
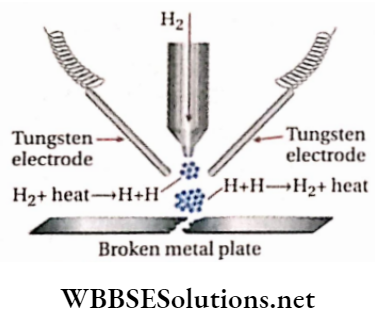
Uses: Atomic hydrogen is very’ reactive (lifetime 0.3 sec). As soon as the hydrogen atoms come in contact with any metallic surface, they immediately get converted into molecular form liberating a large amount of energy (nearly 4000°C), and as a consequence, an extremely hot flame is produced. An atomic hydrogen torch, devised on this principle, is used for welding and cutting purposes
