Chapter 3 Element Compound And Mixture Review Questions Environment Review Questions MCQs
Question 1. Which is not an element?
- Copper
- Ammonia
- Gold
- Carbon
Answer: 2. Ammonia
Question 2. The symbol of sodium was chosen from its Latin name
- Natrum
- Hydrargyrum
- Laplacian
- Kalium
Answer: 1. Natrum
Read And Learn More: WBBSE Solutions For Class 6 School Science
Question 3. The Latin name of silver is
- Plumbum
- Argentum
- Stannum
- Ferrum
Answer: 2. Argentum
Question 4. The German name Wolfram stands for
- Tungsten
- Tin
- Copper
- Iron
Answer: 1. Tungsten
WBBSE Class 6 Elements and Compounds MCQs
Question 5. Which statement is correct in the case of a mixture of water and sugar?
- They can be separated out through filtration
- They can be separated by winnowing
- Water is a solvent and sugar is solute
- Water is a solute and sugar is solvent
Answer: 3. Water is a solvent and sugar is solute
Question 6. Which of the following is non-metal but a good conductor of heat?
- Copper
- Mercury
- Graphite
- Boron
Answer: 3. Graphite
Question 7. Atomicity of Ca(OH), is
- 5
- 3
- 4
- 6
Answer: 1. 5
Question 8. The elements which are present in a molecule of sugar are
- Na, O, H
- 5
- C, N, O
- C, H
Answer: 2. 5
Question 9. One molecule of hydrogen sulphide contains
- One hydrogen atom and one sulphur atom
- One hydrogen atom and two sulphur atoms
- Two hydrogen atoms and one sulphur atom
- None of these
Answer: 3. Two hydrogen atoms and one sulphur atom
Question 10. An example of a metal which is liquid at room temperature is
- Mercury
- Aluminium
- Bromine
- Iodine
Answer: 1. Mercury
Question 11. The symbol of manganese is
- Mg
- Ma
- Mn
- Mo
Answer: 3. Mn
WBBSE Class 6 Science Question Answer
Question 12. Which one of the following mixtures is a solution?
- Chalk powder in water
- Oil in water
- Common salt in an aqueous medium
- Soil
Answer: 3. Common salt in an aqueous medium
Question 13. Identify the wrong statement.
- The number of atoms present in a molecule is called the atomicity of the molecule
- Hydrogen supports combustion
- Oxygen is non-combustible but supports combustion
- The valency of nitrogen in NH3 is 3
Answer: 2. Hydrogen supports combustion
Question 14. Ca is the symbol of
- Carbon
- Calcium
- Cadmium
- Cobalt
Answer: 3. Cadmium
Question 15. The process used to separate a mixture of sand
- Filtration
- Crystallization
- Magnetic separation
- None of these
Answer: 1. Filtration
Question 16. Which one of the following is not an element?
- Copper
- Graphite
- Phosphine
- Uranium
Answer: 2. Graphite
Question 17. Which one of the following supports combustion?
- Hydrogen
- Oxygen
- Carbon dioxide
- Nitrogen
Answer: 2. Oxygen
Question 18. Hydrogen and oxygen combine to form
- Water
- Methane
- Salt
- Alcohol
Answer: 1. Water
Common MCQs on Properties of Compounds
Question 19. The substance which is attracted by a magnet is
- Copper
- Sand
- Iron
- Silica
Answer: 3. Iron
Question 20. Which of the following is not a compound?
- Water
- Ammonia
- Diamond
- Phosphine
- None of these
Answer: 3. Diamond
Question 21. The symbol of potassium is
- P
- K
- Po
- None of these
Answer: 2. K
Question 22. 5N2 means
- 5 atoms of nitrogen
- 5 molecules of nitrogen
- 10 atoms of nitrogen
- 10 molecules of nitrogen
Answer: 2. 5 molecules of nitrogen
Question 23. Arsenic is a
- Metal
- Non-metal
- Metalloid
- Mixture
Answer: 3. Metalloid
Question 24. When electricity is passed through the water, the two gases obtained are
- Carbon and oxygen
- Oxygen and hydrogen
- Hydrogen and nitrogen
- Carbon and hydrogen
Answer: 2. Oxygen and hydrogen
Question 25. Carbon is a
- Metal
- Non-Metal
- Metalloid
- Compound
Answer: 2. Non-Metal
Practice MCQs on Separation Techniques
Question 26. The lightest element is and water is
- Hydrogen
- Helium
- Lithium
- Aluminium
Answer: 1. Hydrogen
Question 27. The symbol of silver is
- Si
- Ag
- Au
- S
Answer: 2. Ag
Question 28. Which one is not attracted by a magnet?
- Cobalt
- Copper
- Nickel
- Iron
Answer: 2. Copper
Question 29. The formula of hydrogen sulphide is
- HS
- H2S
- HS2
- H2S2
Answer: 2. H2S
Question 30. A liquid non-metal at room temperature is
- Mercury
- Sodium
- Bromine
- Galium
Answer: 3. Bromine
Question 31. CCI4 is
- Carbon trichloride
- Carbon tetrachloride
- Carbon pentachloride
- Carbon hexachloride
Answer: 2. Carbon tetrachloride
Question 32. A polyatomic element is
- S8
- H2
- C
- Does not exist
Answer: 1. Sg
Question 33. An example of an elemental molecule among the following is
- NH3
- CaO
- H2SO4
- CI2
Answer: 4. CI2
Class 6 WBBSE Science Question Answer
Question 34. The smallest particle of an element which may or may not have independent existence is called
- An atom
- Molecule
- A compound
- An ion
Answer: 1. An atom
Question 35. Which among the following is a pure substance?
- Dilute sulphuric acid
- Aqueous NaCl
- Vinegar
- Molten NaCl
Answer: 4. Molten NaCl
Chapter 3 Element Compound And Mixture Fill In The Blanks
Question 1. Antimony is a __________
Answer: Metalloid
Question 2. __________ metal is the best conductor of electricity.
Answer: Silver
Question 3. The symbol of beryllium is __________
Answer: Be
Question 4. A mixture of iron fillings and table salt can be separated by __________
Answer: Magnet
Question 5. The symbol of sodium is __________
Answer: Na
Class 6 WBBSE Science Question Answer
Question 6. __________ is a metal which is liquid at room temperature.
Answer: Mercury
Question 7. The formula of ammonia is __________
Answer: NH3,
Question 8. When magnesium filament is burnt in oxygen, __________ coloured substance is formed.
Answer: White
Question 9. The Latin name of silver is __________
Answer: Argentum
Question 10. In a solution, __________ amount.
Answer: Solvent
Question 11. The atomicity of Ca(OCI)CI is __________
Answer: 4
Question 12. Diamond is a non-metal and it is __________ conductor of heat.
Answer: Good
Question 13. The liquid obtained after filtration is called __________
Answer: Filtrate
Question 14. In the case of a sugar solution (sharbat), the solvent is __________
Answer: Water
Question 15. Chlorine (element) is represented by __________
Answer: CI
Question 16. __________ is represented as PH3
Answer: Phosphine
Question 17. The formula of carbon tetrachloride is __________
Answer: CCI4
Question 18. The atomicity of chlorine is 2. The formula of chlorine (molecule) is __________
Answer: CCI2
Class 6 WBBSE Science Question Answer
Question 19. Formula of __________ SF4 is
Answer: Sulphur tetrafluoride
Question 20. The formula is __________ CH4
Answer: Methane
Question 21. Solution = __________ + __________
Answer: Solvent, Solute
Question 22. The Latin name of gold is __________
Answer: Aurum
Chapter 3 Element, Compound And Mixture Identify as True or False
Question 1. Iron is a compound.
Answer: False
Question 2. Metal is a good conductor of heat and electricity.
Answer: True
Question 3. One molecule of chlorine contains two atoms of chlorine.
Answer: True
Question 4. Carbon dioxide is a compound.
Answer: True
Question 5. Air is a compound.
Answer: False
Question 6. A solution is a mixture.
Answer: True
Question 7. The valency of oxygen in water is 2.
Answer: True
Question 8. The atomicity of methane is 5.
Answer: True
Question 9. The atomicity of nitrogen dioxide is 2.
Answer: False
Question 10. In a solution, a solute is present in a smaller amount compared to the solvent.
Answer: True
Question 11. Diamond is a non-metal.
Answer: True
Question 12. A valency is always a whole number.
Answer: True
Question 13. The valency of nitrogen in ammonia is 3.
Answer: True
Chapter 3 Element, Compound And Mixture Match the Columns
Question 1.
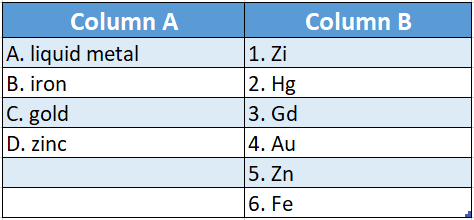
Answer: A-2,B-6,C-4,D-5
Question 2.
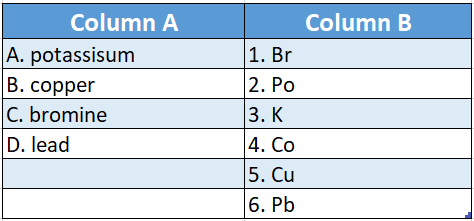
Answer: A-3,B-6,C-1,D-6
Question 3.
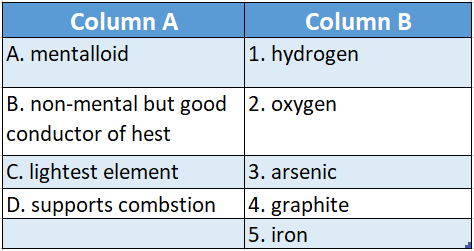
Answer: A-3,B-4,C-1,D-2
Question 4.
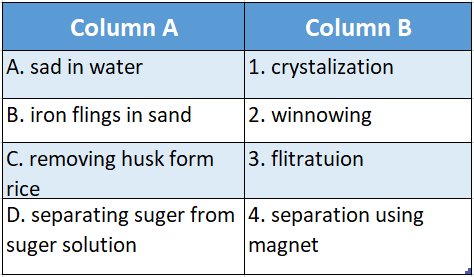
Answer: A-3,B-4,C-2,D-1
Question 5.
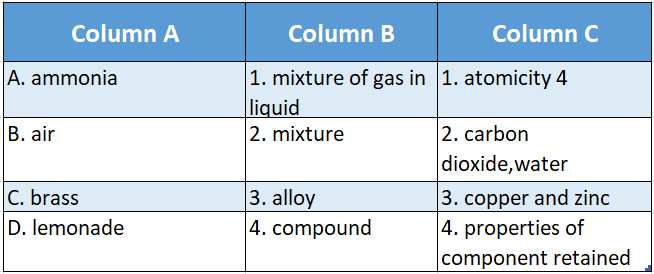
Answer: 1-D-1,2-B-4,3-C-3,4-A-2
Chapter 3 Element Compound And Mixture Answer In Words Or A Sentence
Question 1. One molecule of phosphorous trichloride has only one phosphorous atom. What is the formula of phosphorous trichloride?
Answer: The formula of phosphorus trichloride is PCI3.
Question 2. Write the symbols of phosphorous and potassium.
Answer: The symbol of phosphorous is P; the symbol of potassium is K.
Question 3. Name two elements for each of which both the symbol and the formula are the same. MonatomicAnswer: molecules like Na (Sodium), and K (Potassium) are such elements.
Class 6 WBBSE Science Question Answer
Question 4. What are the elements present in one molecule of carbon dioxide? Mention their number also.
Answer: Each molecule of carbon dioxide (CO2) contains one carbon atom and two oxygen atoms.
Question 5. Write the name of the compound: PCI5.
Answer: Phosphorous pentachloride.
Question 6. How can we obtain pure copper sulphate from the impure sample?
Answer: Using filtration and crystallization techniques.
Question 7. Name the process of separation of sand and camphor from their mixture.
Answer: Sublimation is used to separate them.
Question 8. Write the formula of sulphur hexafluoride.
Answer: SF6
Question 9. Name the elements present in a molecule of sugar.
Answer: Each molecule of sugar contains carbon, hydrogen and oxygen.
Question 10. What is the valency of carbon in methane?
Answer: The valency of carbon in methane is 4.
Question 11. What is the symbol of argon?
Answer: The symbol of argon is Ar.
Important Questions Related to Elements and Compounds
Question 12. What is the formula of oxygen gas?
Answer: The formula of oxygen gas is O2.
Question 13. Name a zero-valent element.
Answer: Inert gas Argon (Ar) is a zero-valent element.
Question 14. Which elements are present in water?
Answer: Hydrogen and oxygen are present in water (H2O).
Question 15. Write the name of a non-metal.
Answer: Nitrogen is a non-metal
Question 16. Sugar and salt are dissolved together in the water. Identify the solvent and solute.
Answer: Here water is the solvent; sugar and salt are the solutes.
Question 17. Give an example of a mixture.
Answer: Air is an example of a mixture.
Question 18. Write the formula of common salt.
Answer: The formula of common salt is NaCI.
Class 6 Science Question Answer WBBSE
Question 19. Identify heterogeneous mixture from the following: Air, liquor Ammonia, muddy water, and an Aqueous solution of copper sulphate.
Answer: Muddy water.
Question 20. Write the formula of the following: hydrogen sulphide and phosphine.
Answer: The formula of hydrogen sulphide and phosphine are HS and PH, respectively.
Question 21. What is the symbol of iodine?
Answer: The symbol of iodine (element) is I.
Question 22. Which one is the lightest metal?
Answer: Lithium is the lightest metal.
Question 23. Name two elements which possess both the characteristic properties of metal and non-metal.
Answer: Arsenic and bismuth are two elements which possess both the characteristic properties of metal and non-metal.
Question 24. What is the atomicity of H2SO4?
Answer: The atomicity of H2SO4 is 7.
Question 25. Identify the non-metal(s) from the following: potassium, helium, chromium, and iodine.
Answer: Helium and iodine are non-metals.
Question 26. Name the compound formed when magnesium filament is burnt in oxygen.
Answer: When magnesium filament is burnt in oxygen, magnesium oxide (MgO) is formed.
Question 27. The formula of carbon dioxide is CO2. What can you conclude from this?
Answer: We can say that each molecule of carbon dioxide contains one carbon atom and two oxygen atoms. The atomicity of CO2 is 3.
Question 28. Give an example of a mixture where a gas is dissolved in a liquid.
Answer: Soda water or lemonade is a mixture where carbon dioxide gas is dissolved in water at high pressure.
Question 29. Mention one property of oxygen.
Answer: Oxygen is not combustible but supports combustion.
Question 30. Give the names of elements present in the compound called baking powder.
Answer: Baking powder or Sodium hydrogen carbonate (NaHCO3) contains sodium, hydrogen, carbon and oxygen.
Chapter 3 Element Compound And Mixture Short Answer Type Questions
Question 1. Water is a compound-explain why?
Answer: Water is a pure substance composed of two constituent elements. The constituent elements of water can not be separated by simple processes.
When electricity is passed through water, hydrogen and oxygen gases are produced following a definite volume and mass ratio.
Their properties are different from water. Hence water is not a mixture. It is a compound.
Class 6 Science Question Answer WBBSE
Question 2. What is a compound? Give example.
Answer: A compound is a pure substance, consisting of two or more elements, which are combined chemically in a definite proportion. Water, carbon dioxide etc. are compounds.
Question 3. What is the difference between Cl and CI2?
Answer: Cl means an atom of chlorine in an uncombined state. Chlorine element is represented by Cl. CI2 is the formula of chlorine molecule.
It implies that in each chlorine molecule, two chlorine atoms are present (diatomic molecule).
Question 4. If lighted which will burn quickly-a big lump of coal or crushed coal of the same weight? Give
reasons.
Answer: Among the two, crushed coal will burn quickly. The surface area of crushed coal is much more than that of a lump of coal of the same weight.
More surface area makes it easy to combine with oxygen and thus making the burning process faster.
Question 5. When dissolved in water, sugar is no longer seen. Why?
Answer: A particle of sugar is composed of a very, very large number of smaller particles.
When dissolved in water, water breaks them apart and the smaller particles are so small that they are not visible through our naked eyes.
Short Answers on Elements and Mixtures
Question 6. Write two properties of hydrogen.
Answer: Two properties of hydrogen:
1. It is lighter than air.
2. Hydrogen is not a supporter of combustion, but it itself burns with a pale blue flame.
Question 7. How can you separate sugar from a sugar solution? What is the name of this process?
Answer: Sugar dissolves in water to form a homogeneous mixture. Let us take some amount of sugar solution in a beaker and boil it for some time.
As a result, some water will vapourize and the solution will become more concentrated.
If this concentrated solution is allowed to cool down undisturbed, solid sugar particles are separated from the solution. This process is known as crystallization.
Question 8. Mention the processes of separation of the constituting components from the following mixtures: (i) muddy water, and (ii) a mixture of sand and iron.
Answer:
1. The components of muddy water can be separated by using filtration. The solution is to be filtered through a filter paper.
The water will pass through the filter paper but the solid, insoluble mud particles will remain within the paper. So water is filtrate and mud is residue.
2. A mixture of sand and iron can be separated from one another by using a magnet.
When a magnet is brought near the mixture, it attracts the iron particles only. So the mixture becomes free from iron particles.
Question 9. What do you mean by valency? How can it be determined? Give a suitable example.
Answer: The number of hydrogen atoms that combine with one atom of a particular element to form a compound is called the valency of that element in that particular compound. The valency of hydrogen is assumed to be unity,1.
The formula of hydrogen sulphide is HS. In this case, one sulphur atom is combined with two hydrogen atoms in a molecule. So valency of sulphur in hydrogen sulphide is 2.
Class 6 Science Question Answer WBBSE
Question 10. Write the symbols of the following elements: nitrogen, beryllium, manganese, and gold.
Answer: Nitrogen: N; beryllium: Be; manganese: Mn; gold: Au.
Question 11. Name the elements present in each of the following compounds: sugar, salt, marble rock, and ice.
Answer:
Sugar: carbon, hydrogen, oxygen
Salt: sodium and chlorine
Marble rock: calcium, carbon, oxygen Ice: hydrogen, oxygen.
Examples of Real-Life Mixtures
Question 12. Which of the following are compounds and which of the following are elements? Charcoal, salt, copper, sugar.
Answer: Compound: salt, sugar Element: copper, charcoal.
Question 13. Write the symbols of magnesium and helium.
Answer: The symbol of magnesium is Mg: and the symbol of helium is He.
Question 14. Write two general properties of metal. Or, How can you recognize a metal?
Answer: Metals have the following characteristics:
1. Metals have metallic lustre i.e. they have shining surfaces. Metals produce a sonorous (dong) sound when hit with a solid object.
2. Metals are usually good conductors of heat and electricity.
Question 15. In how many ways matter can be classified on the basis of their state? Name them.
Answer: A matter can be classified as solid, liquid or gas on the basis of its state.
Question 16. Define symbol and formula.
Answer: Symbol is the short form or abbreviated English name (and in some cases the Latin or German name) of an element.
The formula is a representation of a substance (elements or compounds) by means of symbols. It also denotes the number of atoms of each element present in one molecule of the substance.
Question 17. Write the symbol formula of the following: oxygen gas, carbon, carbon monoxide, ammonia, iron, and white phosphorous.
Answer: Oxygen gas: O2 Carbon: C, Carbon monoxide: CO, Ammonia: NH3, Iron: Fe,
White phosphorous: P4
Question 18. The formula of water is H2O. What information do you get from this?
Answer: From this formula, we find that two hydrogen atoms combine with one oxygen atom in a definite weight ratio. H2O means one triatomic molecule of water.
Question 19. Give an example of one liquid metal and a non-metal which is a good conductor of electricity.
Answer: An example of a metal which is liquid at room temperature: Mercury.
Example of a non-metal which is a good conductor prime of electricity: Graphite.
Conceptual Questions on Atomic Structure and Compounds
Question 20. Write a short note on valency.
Answer: The number of hydrogen atoms that combine with one atom of a particular element to form a compound is called the valency of that element in that particular compound.
The valency of hydrogen is assumed to be unity, ie. 1.
The formula of hydrogen sulphide is H2S. So in this case one sulphur atom is combined with two hydrogen atoms in a molecule.
Similarly, in methane (CH4), one carbon atom is combined with 4 hydrogen atoms.
In ammonia (NH3) one nitrogen atom is combined with three hydrogen atoms.
So valency of sulphur in hydrogen sulphide is 2; the valency of carbon in methane is 4; the valency of nitrogen in ammonia is 3.
Class 6 Science Question Answer WBBSE
Question 21. Write a short note on filtration.
Answer: This is a technique of separation of insoluble solute from a liquid solution. This is done by sieving.
The sieve has holes of a particular size. The size of the holes of the sieve should be smaller than the insoluble solid parties which are to be separated from the liquid solution.
The molecules of a liquid are so small that they can pass through the holes of the sieve. In the laboratory, we use filter paper as sieves.
The liquid which passes through the holes is called filtrate and the insoluble solute particles which remain on the filter paper are called residue. For the purification of water, a filter is used.
Question 22. Write the formula of phosphorous pentachloride and methane.
Answer: The formula of phosphorous pentachloride and methane are PCI5, and CH1, respectively.
Question 23. Write the symbol of the following elements: magnesium, chromium, silver, and boron.
Answer: The symbol of magnesium, chromium, silver and boron are Mg, Cr, Ag and B, respectively.
Question 24. What do you mean by crystallization?
Answer: Crystallization is a method of separation of a soluble solute from a liquid solution.
In this method, the liquid solution is boiled for some time so that most of the liquid solvent evaporates and the concentration of solute in the solution increases.
When this is kept undisturbed for some time, solid particles of solute separate from the solution.
Question 25. Write about the atomicity of S8.
Answer: In S, there are 8 atoms of sulphur constituting a molecule. Hence the atomicity is 8.
Question 26. What is the difference between 2H and H2?
Answer: The hydrogen element is represented by the symbol H. 2H means two atoms of hydrogen in an uncombined state.
H2 represents one molecule of hydrogen. Each molecule of hydrogen contains two atoms of hydrogen. So its atomicity is 2.
Real-Life Scenarios Involving Homogeneous and Heterogeneous Mixtures
Question 27. On a piece of white paper, you are given a mixture of tea and iron powder. How can you separate them?
Answer: Since one of the two components is a magnetic substance, they can be separated by using a magnet.
If a magnet is brought near the mixture, only iron powder is attracted to the magnet.
So the mixture becomes free from iron powder.
Question 28. What is the atomicity of white phosphorous, sodium, ozone and iodine?
Answer: Write the chemical formula of molecules of these elements.
The atomicity of white phosphorous, sodium, ozone and iodine are 4, 1, 3 and 2, respectively. Chemical formula: White phosphorus-P4Sodium-Na, Ozone-03, Lodine-I2
Question 29. Classify the following as metal or non-metal: gold, sulphur, zinc, copper, nitrogen, and graphite.
Answer: Metals: gold, zinc, copper Non-metals: sulphur, nitrogen, graphite.
Question 30. Air contains hydrogen and oxygen. When water is dissociated we get hydrogen and oxygen. Are both of them a mixture? Explain.
Answer: In air properties of both hydrogen and oxygen are retained. Their proportion varies from place to place. So air is a mixture.
But the properties of water are different from that of hydrogen and oxygen. Hydrogen and oxygen combine in a definite proportion to produce water.
We can not obtain gaseous hydrogen and oxygen from water by simple processes. So water is a compound.
Question 31. List two methods by which we can obtain pure salt from seawater. Which method is better and why?
Answer: Evaporation and crystallisation.
Crystallisation is the better method. It has the following advantages over the evaporation technique :
- The salt obtained after evaporation is contaminated with other impurities but the crystals obtained are of pure substance only.
- Some solids decompose on heating to dryness. This does not happen in crystallisation.
Class 6 Science Question Answer WBBSE
Question 32. Which separation technique would you apply for the separation of the following: (a) small pieces of metals from the engine oil of a car, (b) tea leaves from tea,(c) chalk powder from water, (d) stone dust from water
Answer:
1. Filtration
2. Filtration
3. Filtration
4. Sedimentation and Decantation
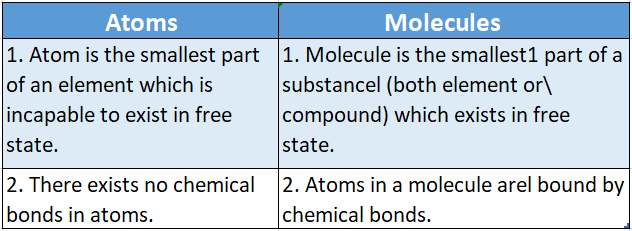
Question 33. Mention two differences between atoms and said molecules.
Answer:
Mixtures: Dust storm (mixture of dust and gases), tap water (mixture of water and impurities) and cooking gas (mixture of hydrocarbons like propane and butane).
Question 34. Identify compounds from the following: Marble, Dust storm, distilled water, tap water, and Cooking gas. Give reasons.
Compounds: Marble calcium carbonate (CaCO), Distilled water (H2O)
Chapter 3 Element Compound And Mixture Qlong Answer Type Questions
Question 1. Describe a process of separation of water and mud from muddy water.
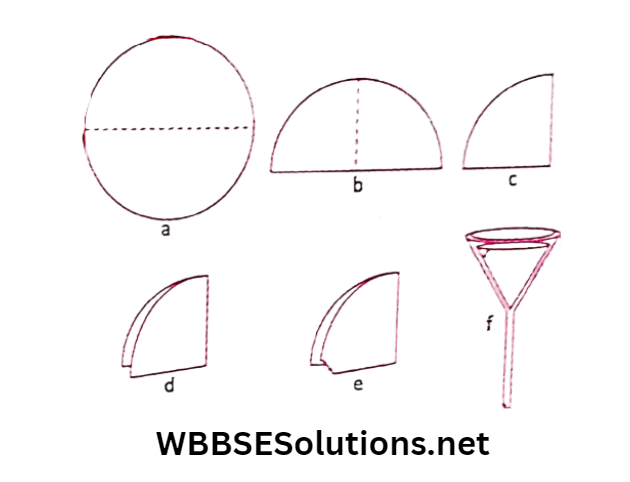
Answer: Activity 11: Let us take some amount of muddy water. We want to separate the “mud”, which is an insoluble impurity present in water by filtration.
For this, we need a sieve. A filter paper is used for this purpose. Mud particles are small. But a filter paper has even smaller holes.
The shows the steps involved to fold a filter paper in the form of a cone. The cone-shaped folded filter paper is then placed on a funnel.
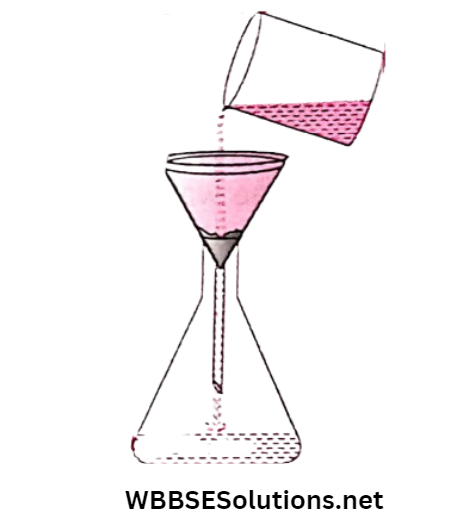
The muddy water is then poured into the filter paper. The molecules of water pass through the holes of the filter paper but the mud particles can’t. They remain on the filter paper.
The liquid which is coming out through the filter paper is in general called filtrate while the solid particles that remain on the filter paper are called residue.
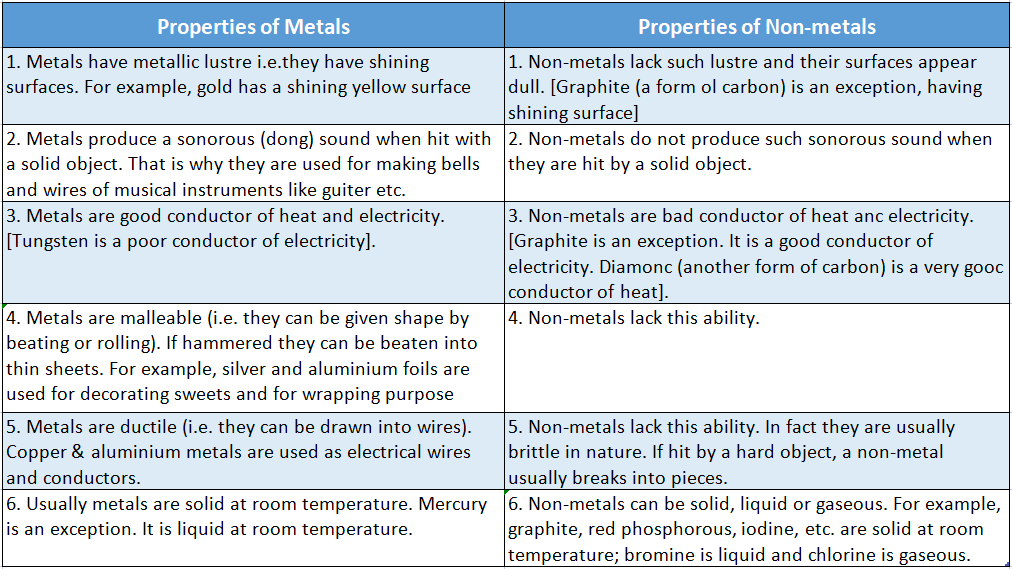
Question 2. Write three differences between metal and non-metal.
Answer:
Question 3. Write the symbol of hydrogen and oxygen. Write the formula of the compound they form by combining with each other. Write the valency of oxygen in this compound. The symbol of hydrogen is H
Answer: The symbol of oxygen is O
The formula of the compound they form by combining with each other is water.
Its formula is H2O. Here one oxygen is combined with two hydrogen atoms. So the valency of oxygen in water is 2.
Question 4. In relation to filtration, what do you mean by filtrate and residue? Which of the following mixtures can be separated into constituent components by filtration: sugar solution; oil and a pigment which is soluble in oil; the mixture of water and sand?
Answer: The filtration technique is used to separate insoluble solid solute from a liquid solution. This is done using sieves having a large number of holes.
The size of the holes is such that when such a solution is poured over the sieve, the solvent molecules can easily pass through.
School Science is called filtrate, while the bigger solute particles cannot pass through the holes and they remain on the sieve. They are called residues.
A mixture of water and sand can be separated from their mixture by filtration. This technique can be used for solutions where an insoluble, solid solute is present in a liquid.
For sugar solution, sugar is completely dissolved in water. For oil and a pigment which is soluble in oil, this technique cannot be used as the pigment is soluble in the solvent (oil).
Question 5. Name and write the symbol of one tetra-atomic molecule. Give the name and symbol of an element which can exhibit atomicity of both 2 and 3.
A metal ‘M’ forms a hydride having a chemical formula of MH2. What can you infer about the valency of the metal?
Answer: White phosphorus (P4) is a tetra atomic molecule. Oxygen (O) shows atomicity of 2 (in O2) and 3 (in O3).
The valency of hydrogen is assumed to be unity, ie. 1. Since the metal M combines with two numbers of hydrogen atoms, hence the valency of the metal is 2 in MH2.
Question 6. An element is sonorous and highly ductile. Under which category would you classify this element?
Name a non-metal which is known to form the largest number of compounds. Name a non-metal which is an absolute essential for combustion to happen.
Answer: Metals are sonorous and highly ductile. Hence the element must be metal.
Carbon forms the largest number of compounds.
Question 7. When a metal (A) is burnt in an atmosphere of it in an atmosphere of a colourless and odourless gas, a white powder (B) is formed. The gas is essential to carry out respiration. Identify A, B and the gas.
Answer: The metal
(1) is magnesium (Mg) and the gas is Oxygen (O2).
Oxygen is colourless and odourless and it is essential for respiration. These compounds are classified under organic compounds. oxygen, it forms a white powdery substance
(2) called magnesium oxide (MgO).
Question 8. What will happen when current is passed through two iron nails placed inside acidulated water taken in a beaker? Which gases will evolve from the nails connected to the battery terminals?
Answer: When electricity is passed through acidulated water (water with lime juice), water undergoes electrolytic dissociation to form hydrogen and oxygen.
This is called the electrolysis of water. It shows that the compound called water (H2O) is composed of two constituent elements of hydrogen (H2) and oxygen (O2).
Oxygen is the non-metal which is required for combustion.
planning brs When magnesium is burnt in an atmosphere Hydrogen gas shall evolve from the iron nail connected to the negative terminal of the Un battery (acting as cathode) and oxygen gas shall evolve from the iron nail connected to the positive terminal of the battery (acting as anode).
