NEET Foundation Notes For Chemistry Chapter 1 Matter In Our Surrounding
Matter
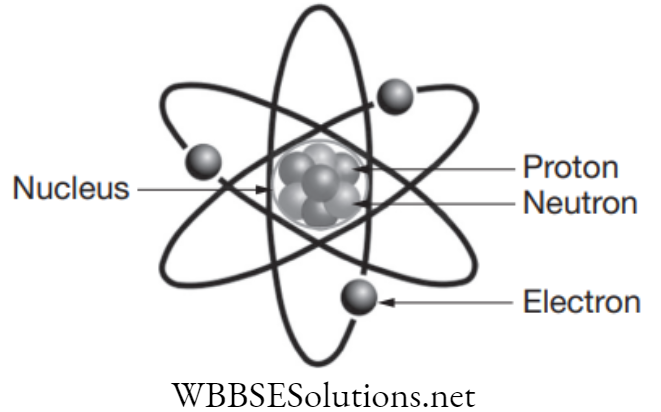
Matter is anything that has mass and takes up space. Matter consists of various types of particles like atoms, molecules and ions, which are further divided into electrons, protons and neutrons. Atoms are considered to be the basic unit of matter which may or may not exist independently.
They have their own chemical properties. Combination of atoms creates molecules. Combination of molecules and/or atoms forms compounds.
Read and Learn More: NEET Foundation Notes
The earth and all that it was meant to constitute arose from five core elements, which Indian philosophy recognizes as ‘panchatatva’. The word ‘panchatatva’ originates from Sanskrit, where ‘panch’ stands for five and ‘tatva’ indicates elements. Everything on this planet is composed of five basic elements, namely, Akash (space), Vayu (air), Jal (water), Agni (fire) and Prithvi (Earth).
It is thus true that all natural life including plants, animals and humanity at large are combination of these five elements.
NEET Foundation Notes For Chemistry Chapter 1 Matter In Our Surrounding
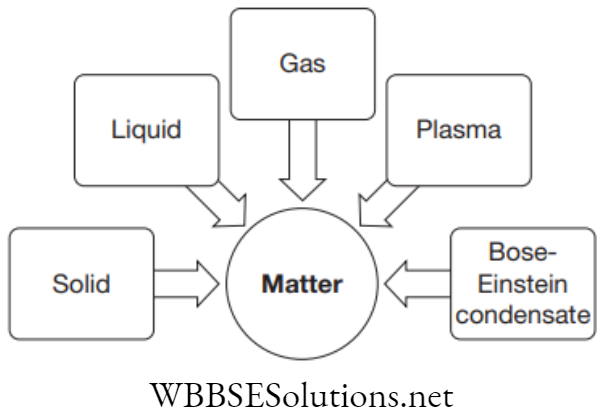
Matter exists in several states. The commonly known states are solid, liquid and gas. Besides these, some other states of matter also exist viz. Plasma and Bose-Einstein condensate.
On a fundamental level these are the elements that make matter as they have mass and volume. But photons on the other hand are not considered matter as it lacks mass and volume. A photon is defined as a bundle (or quantum) of electromagnetic (or light) energy. Photons are always in motion and, in a vacuum, have a constant speed of light to all observers.
Matter can be defined as any substance which has mass and occupies space. All solids, liquids and gases in the surroundings of earth are made up of matter. According to several researches, matter is made up of tiny particles which are bonded together. These particles cannot be seen but as a whole matter can be seen, touched or felt.
Example: a book, a car, a letter, a hand set, a piece of wood, a tree, a bag, etc.
Mass is the amount of matter a substance is made up of. It is the scientific measure of the amount of the constituents of an object. It’s SI unit is kilograms (kg) and often expressed in grams (g) as well.
The mass of a substance remains constant irrespective of its location in the universe. It means that the mass is not affected by gravitation or any other types of forces.
| Class 11 Physics | Class 12 Maths | Class 11 Chemistry |
| NEET Foundation | Class 12 Physics | NEET Physics |
Matter In Our Surroundings
Volume is the three-dimensional space occupied by matter. It’s SI unit is cubic metres (m3), but can also be expressed in litres (L), millilitres (mL) etc. Volume of the solids can be measured by measuring their height, length and width.
Similarly, the volume of the liquids and gas can be measured by the space that they occupy in a marked vessel.
Physical Nature of Matter
As per old school theories, nature of matter has two views:
- One believed that matter can be broken into unlimited pieces as it is continuous. Greek philosophers Plato and Aristotle belong to this school of thought.
- The other set believed that the process of subdivision is limited. Matter comes to a stage where the tiny particles cannot be subdivided. They believed matter has particulate nature i.e., it is made up of tiny particles. The smallest indivisible particle of matter was called ‘atom’ from the Greek word ‘atomos’ for ‘indivisible’.
The modern view of atom was given by John Dalton in 1803. He believed in two types of constituent particles atoms and compound atoms (presently known as molecules).
Matter is made up of atoms and all chemical properties of matter can be explained on its basis. Molecules are important in explaining physical properties of matter.
Characteristics of Matter
The basic characteristics of matter are discussed below:
- Continuous movement of particles.
- Intermolecular attraction between particles.
- Space between particles.
On the basis of above mentioned characteristics, matter is classified into three states namely: solid, liquid and gas.
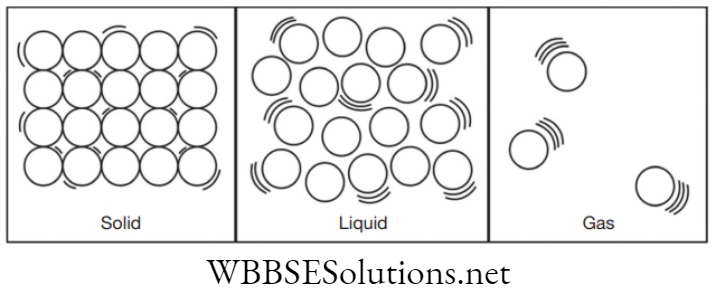
Solid
- Intermolecular forces of attraction are strong between particles.
- Their pattern is predetermined (lattice).
- Due to strong forces of attraction even on vibration, atoms cannot change position therefore, have fixed volume and shape.
- Due to strong intermolecular forces, the kinetic energy is very low.
- The process of diffusion is either absent or extremely low, if present.
- Due to close arrangement of particles, the matter is very dense.
- Due to very limited intermolecular space, the compressibility is almost absent.
Liquid
- Intermolecular forces of attraction are moderate.
- Pattern is not pre-determined; they take up the shape of the container in which they are stored.
- Due to weak forces of attraction, liquid particles slide past each other.
- The kinetic energy is more than the solid.
- The density is relatively less.
- Diffusion takes place at higher rates than solids.
- Due to more intermolecular space, compressibility is higher than solids.
Gas
- Intermolecular forces of attraction in gases is negligible.
- Particles are far apart from each other and their movement is quick and rapid.
- Due to large intermolecular space they collide with each other and bounce in all directions.
- Weak intermolecular force of attraction results in very high kinetic energy.
- Process of diffusion is very fast.
- Density is very low due to large intermolecular space.
- Presence of large intermolecular space makes the gas highly compressible.
- Difference between solid, liquid and gas
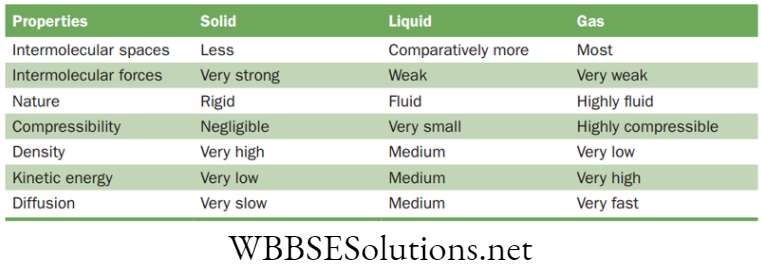
NEET Foundation Notes For Chemistry Chapter 1 Matter In Our Surrounding States of Matter
Matter can be classified mainly in two ways as discussed below.
By its physical state – solid, liquid, or gas,
By its chemical composition – element, compound or mixture.
Physical State of Matter
Matter can exist in three states – solid, liquid and gas. These three states of matter have different properties. Water is the best example of a substance that naturally exists in all the three states – solid (ice), liquid (water), gas (vapour).
Intermolecular forces hold the molecules together while the thermal energy functions in the opposite direction, i.e., to move them away from each other. It is the sum of the opposite forces, i.e., intermolecular and thermal which determines whether the matter is solid, liquid or gas.
If intermolecular force is greater than thermal force then the matter will be in solid state. By increasing thermal energy, state of matter may change, i.e., it may convert solid into liquid or liquid into gas.
Solid State of Matter
A solid has a definite size and shape which does not change its state without any external condition. Example: A piece of wood, a stone, etc. But on application of external forces, the solid changes shape. Example: With continuous hammering, gold is shaped into thin sheets.
In solids, particles are very close to each other due to strong intermolecular force which keeps these particles in a fixed position. Due to this factor, solids are hard and rigid and cannot be compressed. When the atoms are forced to come closer, the intermolecular force becomes repulsive.
With an increase in thermal energy, the kinetic energy of the molecules increases resulting in conversion of solid into liquid. The temperature at which the conversion of solid state to liquid state takes place is called melting point.

Liquid State of Matter
Water is the best example of liquid. Liquid has a definite volume but does not have a definite shape. Example: Mustard oil and kerosene oil, etc. A liquid has a definite volume.
The particles in liquid are not as densely packed as in solids due to weak intermolecular forces between them. The particles do not stay at a fixed positions like solids and move around due to the space available. The particles move away due to weak intermolecular forces and get attracted to other molecules that approach them in movement.
The particles can slide off each other as they can break away from each other and get attracted while approaching the other molecules. Similarly as in solid, liquid molecules also repulse on the attempt of getting the molecules closer by applying pressure. Due to this, pressure does not have much effect on volume of liquids.

Gaseous State of Matter
Gas is not physically seen but is present in the surroundings. The gas takes up the whole volume of the container, irrespective of its size. Intermolecular force in gases is the least of all states due to which the particles move freely, moving far apart giving it no shape. Due to the space in molecules, on application of pressure they can be brought closer.
This is why gases are highly compressible. It can be compressed much more than liquids and solids, but to an extent. Volume of gas is also affected by temperature. If the temperature increases, the volume of the gas also increases. Example: Wind, CNG, oxygen cylinder.

With advanced technology scientists are accepting five states of matter: solid, liquid, gas, plasma and Bose–Einstein condensate.
1. Plasma: The particles in plasma are super energetic and super excited. They are in form of ionised gases. Example: The fluorescent tube and neon sign bulbs.
2. Bose–Einstein condensate: If a very light density gas (1/100000) is cooled at super low temperatures a new matter is found. This matter is considered to be 5th state of matter. This matter was found by Cornell, Ketterle and Wieman in 2001 (received Nobel Prize for the same).
The whole discovery is based on some calculations which were performed by the Indian physicist Satyendra Nath Bose in 1920. Albert Einstein predicted a new state of matter by building upon Bose’s calculation. So, the newly discovered 5th matter is called Bose–Einstein condensate (BEC).
Brownian Motion
Brownian motion or the Brownian movement was given by Scottish botanist Robert
Brown (1827). Brownian motion is the random movement of particles suspended in a fluid (liquid or gas) resulting from their collision with the fast-moving atoms or molecules in the gas or liquid.
In a given medium, a number of particles are subjected to Brownian motion with no choice of direction for the random movement, then in a particular span of time the particles will spread uniformly throughout the medium.
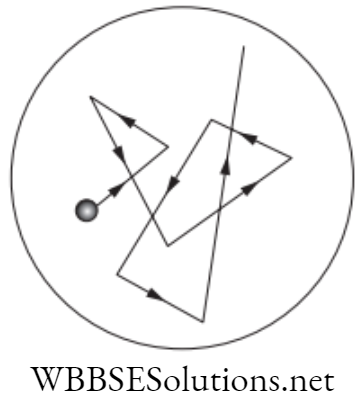
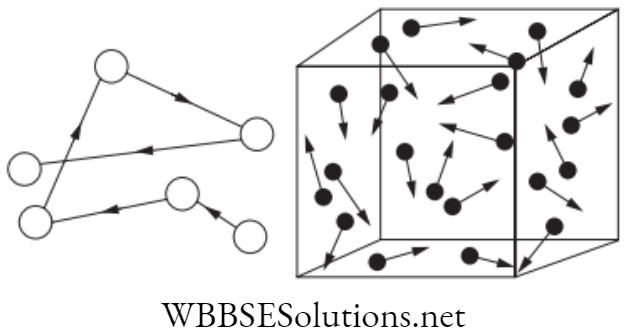
If X and Y are two adjacent regions and X has twice as particles than Y, then the probability of particles moving from X to Y is double than the probability of particles moving from Y to X.
The process by which the particles move from high density to low density to spread evenly in whole region is called diffusion. Examples: Pollutants through the atmosphere, diffusion of calcium through bone tissue in living organisms.
Example:
- When the crystal of potassium permanganate is placed in a beaker of water, the water slowly turns purple on its own, without stirring.
- This mixing of water and the crystals of potassium permanganate takes on its own as both potassium permanganate crystal and water are made up of tiny particles.
- When the crystals of potassium permanganate are put in water, then its particles start separating from one another.
- These purple colour particles of potassium permanganate get evenly spread throughout the water, thus making the whole water look purple.
- The whole water looks purple, as on dissolving, the particles of potassium permanganate get into the spaces among the particles of water.
NEET Foundation Notes For Chemistry Chapter 1 Matter In Our Surrounding Matter Changes Its State
Substances convert from one state to another with temperature and surrounding pressure working as catalysts. If the pressure is constant then temperature remains as the only determining factor. For example, the atmospheric pressure. The temperature is increased to a point where the change takes place and then it stays constant till the conversion is complete.
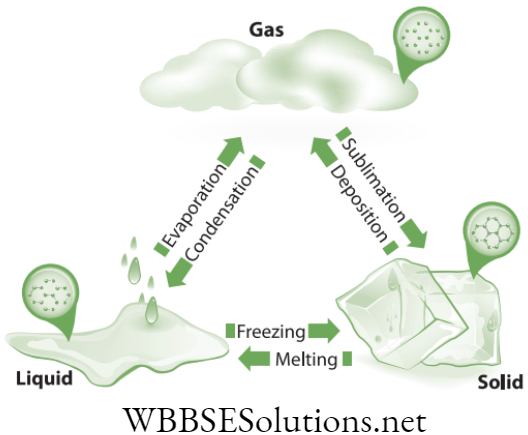
On heat application, the state changes from solid to liquid to gas except few which directly converts from solid to gas. On cooling, the conversion of state is from gas to liquid to solid except few which directly converts from gas to solid.
Processes of change of state
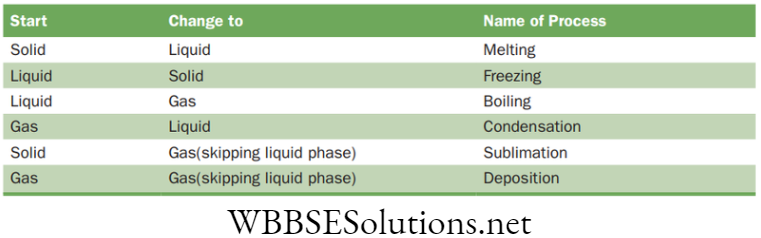
Processes of Change of State
- Melting: When the solid is heated, the particles receive thermal energy which results in faster vibration in them. With increase in temperature, the vibrations keep on increasing. At a particular temperature the vibrations increase so much that they break their ordered structure. At this point the solid converts into liquid. The process is called melting and the temperature is called melting point.
- Freezing: It is the opposite process of melting. When the temperature of a liquid is decreased, the vibrations in the particles become restricted. At a particular temperature the vibrations become so restricted that the matter gets confined in a fixed order. At this point the liquid converts into solid. The process is called freezing and the temperature is called freezing point.
- Boiling: When the liquid is heated the particles start moving faster. At one point the movement becomes so fast that they break away from each other. The liquid converts into gas. The process is called boiling and the temperature is called as boiling point. However, the conversion of liquid into gas may start long before reaching the boiling point. This process is called as evaporation.
- Condensation: It is the opposite of evaporation. In this process the temperature of gas is decreased which results in decrease in the movement of particles. At one point the movement becomes so restricted that the particles join together converting gas into liquid. The process is called condensation and the temperature is called dew point.
- Sublimation: There is a point of temperature and pressure at which a substance can exist in solid liquid and gas stages. It is known as ‘Triple Point’. Below this point the solid directly converts into gas skipping the liquid phase with increase in temperature.
- Deposition: It is the process in which the gas directly converts into solid skipping the liquid phase. It opposite of sublimation. When the condensation takes place at below freezing point the gas directly converts into solid. Frosting is a common example of this.
Effect of Change of Temperature
On exposure to heat a solid expands. The expansion is small. On receiving thermal energy the particles vibrate more rapidly in their position and take up more space. On providing more thermal energy the particles become more energetic and leave their determined positions leading to change of state, i.e., solids melts into liquid.
On giving thermal energy to liquid it converts to a gas. This takes place because kinetic energy of the particles becomes so high that they can overcome the intermolecular force within the liquid. Therefore, liquid is converted into gas (vapour).
There are three most prevalent scales to measure temperature in our surroundings. These are Fahrenheit, Celsius and Kelvin. Fahrenheit scale is based on 32 degree for freezing point and 212 degree for boiling point of water. Celsius is based on 0 degree for freezing point and 100 degree for boiling point.
Kelvin scale is based on 0 degree for absolute zero. Kelvin is related with Celsius scale as it also has a difference of 100 degrees between freezing point and boiling point of water. These scales can be converted between each other as follows:
K = 273.15 + ˚C ˚C = (5/9) × (˚F – 32) ˚F = (9/5) × (˚C + 32)
Measures of scaling temperature
Latent Heat
When the water is vaporised from the surface, it needs energy to convert from liquid state to gaseous state. The water absorbs this heat energy from the environment. This energy does not heat up the water but is used in converting from liquid to gas.
This heat which is required to change the state of matter, is called Latent heat. Due to absorption of this heat energy, the surface becomes cool.
Example: When a person who is sweating due to high temperature stands near a fan, the air circulated through fan evaporates the moisture from the skin and feels a cooling effect in this process.
It does not cause temperature change as it is used for changing the state of substance. At molecular level it can be understood as the energy which is required to overcome the intermolecular attraction and is very essential in order to change the state.
For example, when water boils, the temperature of water remains constant at 100°C. The additional heat is absorbed in converting the liquid into vapour and is carried by the vapour. This energy is released during condensation.
Types of Latent Heat
- Latent heat of fusion
- Latent heat of vaporization
1. Latent heat of fusion: The amount of heat energy that is required to change 1 kg of solid into liquid at atmospheric pressure at its melting point is called latent heat of vaporisation. The latent heat of fusion of ice is 3.34 × 105 Joules per kilogram.
2. Latent heat of vaporization: The amount of heat which is required for converting 1 kilogram of the liquid (at its boiling point) to vapour or gas, without any change in temperature. When the temperature of the solid substance is measured on heating, the following graph is obtained:
The temperature change with energy input. In this, phase changes are indicated by flat regions where heat energy used to overcome attractive forces between molecules.
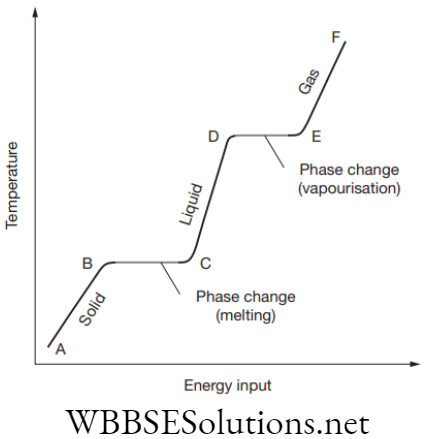
Explanation
- At point A, the substance is in its solid phase, heating it, brings the temperature up to its melting point but the material still remains a solid at point B.
- As it is heated further, the energy from the heat source breaks the bond which holds the atoms in place. This takes place from B to C.
- At point C the entire solid has been transformed into the liquid phase.
- From now onwards energy which is added goes into the kinetic energy of the particles in raising the temperature (C to D).
- At point D the temperature has reached its boiling point but it is still in the liquid phase.
- In D to E, thermal energy is overcoming the bonds and the particles have enough kinetic energy to escape from the liquid.
- The substance now is entering the gas phase.
- Beyond E, if you further heat the substance under pressure it can raise the temperature still further. This is how a pressure cooker works.
Specific Latent Heat
The heat energy required to boil or melt a particular substance is known as specific latent heat of that substance. It is measured in terms of amount of energy required to change the state of one kilogram of substance.
Example: Specific latent heat of water is 334 kilojoule/kg for melting and 2260 kJ/kg for boiling.
Effect of Change of Pressure
Gas can be compressed by applying pressure because of large intermolecular space and weak intermolecular force.
When thermal energy is provided to a gas, kinetic energy of the particles increases. They start moving more freely and at much higher speed. On the pressure being constant the intermolecular distance also increases and the volume of the gas increases.
At a fixed temperature pure solid turns to liquid. This particular temperature is called melting point of that particular solid substance. Liquid on cooling converts into solid at a particular temperature.
This temperature is called freezing point of that particular liquid substance. The temperature at which a liquid boils and is converted into a gas is boiling point of the liquid.
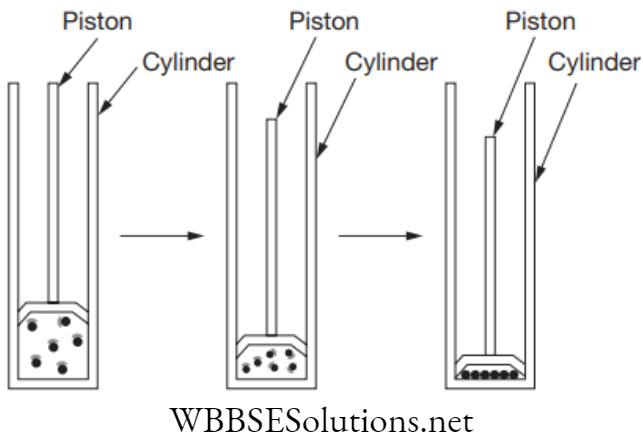
NEET Foundation Notes For Chemistry Chapter 1 Matter In Our Surrounding Evaporation
It is a phenomenon of changing liquid into vapour at any temperature below its boiling point, i.e., water changes from a liquid to a gas or vapour form. Water boils at 100°C, but it begins to evaporate at 32°C. As the temperature increases, the rate of evaporation also increases.
The rate of evaporation depends on the temperature and it also depends on the amount of water present to evaporate.
Factor Effecting Evaporation
- Temperature: Evaporation is affected by temperature, higher the temperature of the liquid then higher is the rate of the molecules to escape from the surface
- Humidity of surrounding air: Humidity is inversely proportional to evaporation. Higher the humidity, lower is the evaporation.
- Boiling point: If the boiling point of the liquid is lower the evaporation starts at relatively lower temperatures due to weak inter-particle interaction.
- Surface area of liquid: Large surface area enables more number of molecules to be exposed for evaporation. This results in faster evaporation.
- Movement of air: Higher air movement results in faster evaporation as the moisturised air mass is quickly replaced by another dry air mass.
- Pressure: Atmospheric pressure and rate of evaporation are inversely proportional to each other. So, if the atmospheric pressure decreases the rate of evaporation increases.
NEET Foundation Notes For Chemistry Chapter 1 Matter In Our Surrounding Fill In The Blanks
Question 1. __________ is the densest state of matter.
Answer. Solid
Question 2. Solids have tendency to maintain shape because they are _________.
Answer. Rigid
Question 3. Gases have neither definite shape nor definite ____________.
Answer. Volume
Question 4. Conversion of gas to liquids is called __________.
Answer. Condensation
Question 5. The freezing point of pure water is _________.
Answer. 0°C
Question 6. The boiling point of alcohol is 1000C. In Kelvin scale it is equal to ___________.
Answer. 373K
Question 7. The temperature at which vapour changes into liquids is called ___________.
Answer. Liquefication point
Question 8. ___________ is an example of subliming solid.
Answer. Ammonium chloride
Question 9. ___________ exert pressure on the walls of the container.
Answer. Gases
Question 10. Matter is made up of ______________.
Answer. Particles
NEET Foundation Notes For Chemistry Chapter 1 Matter In Our Surrounding Match the Column
Question 1. Match the physical quantities with the SI units:
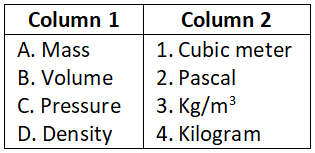
Select the correct option:
- A-1, B-3, C-2, D-4
- A-3, B-2, C-4, D-1
- A-3, B-1, C-2, D-4
- A-4, B-1, C-2, D-3
Answer. 4. A-4, B-1, C-2, D-3
Question 2. Match the following
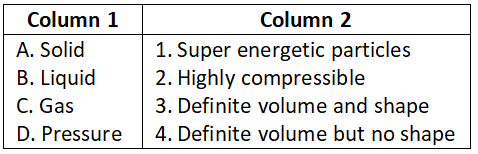
Select the correct option:
- A-3, B-4, C-2, D-1
- A-3, B-2, C-4, D-1
- A-3, B-1, C-2, D-4
- A-4, B-2, C-1, D-3
Answer. 1. A-3, B-4, C-2, D-1
Question 3. Match the following

Select the correct option:
- A-1, B-3, C-2, D-4
- A-4, B-1, C-2, D-3
- A-1, B-2, C-3, D-4
- A-3, B-1, C-2, D-4
Answer. 3. A-1, B-2, C-3, D-4
NEET Foundation Notes For Chemistry Chapter 1 Matter In Our Surrounding Assertion Reason
For the following questions, the options will remain as follows:
- Both A and R are correct and R is the explanation of A
- Both A and R are correct, but R is not the logical explanation of A
- A is correct but R is incorrect
- A is incorrect but R is correct
Question 1. Assertion: People usually prefer desert coolers on a hot, dry day.
Reason: Low humidity increases the rate of evaporation of water.
Answer. 1. Both A and R are correct and R is the explanation of A
Question 2. Assertion: Solid does not fill its container completely.
Reason: The particles of solid are very closely packed.
Answer. 2. Both A and R are correct, but R is not the logical explanation of A
Question 3. Assertion: We should wear cotton clothes in summers.
Reason: Cotton is less expensive.
Answer. 3. A is correct but R is incorrect
NEET Foundation Notes For Chemistry Chapter 1 Matter In Our Surrounding Comprehension Passage
Read the passage and answer the questions:
Latent Heat
The term latent means hidden. The latent heat of a substance is the amount of heat absorbed by a unit mass of the substance to change its state without change of temperature. Its SI unit is Joules per kilogram. Latent heat is of two types:
- Latent heat of fusion
- Latent heat of vaporization
The Latent heat of fusion of a solid is the quantity of heat in Joules required to convert 1 kg of solid (at its melting point) to liquid, with no change in temperature.
The Latent heat of vaporization of a liquid is the quantity of heat in Joules required to convert 1 kg of the liquid (at its boiling point) to gas with no change in temperature.
Specific Heat
It is the amount of heat which is required to raise the temperature of a unit mass of the substance by 1°C.
The symbol is C or S. The specific heat varies slightly with temperature. This is due to the change which occurs in the structure of molecules in a substance with change in temperature. The unit of specific heat is Joules per kilogram per degree Celsius
