NEET Foundation Chemistry Notes For Chapter 4 Structure Of Atom
Notes Of Structure Of Atom
As we have learnt in previous chapters, Matter is anything that occupies space and has mass and is made up of tiny particles called as atom. Different types of matter exists because of the different atoms that consists them. Now the question that comes to the mind is that: (i) how these atoms are different from each other? (2) Is it true that the atoms are indivisible, or they can be further divided?
These answers will be provided it the following chapter, where we will learn about the sub-atomic particles and how they were discovered. This chapter will also entail the reason behind the different properties of atoms due to which the matter is varied.
At the end of the 19th century, scientists were facing difficulties to reveal the actual structure of atom and were not able to explain the properties associated with them. There were series of experiments that elucidated the structure of atom.
Atoms are not divisible was first indicated by the study of static electricity and the conditions under which they are conducted.
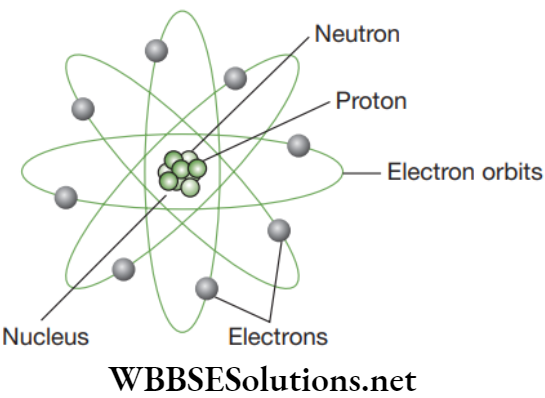
NEET Foundation Chemistry Notes For Chapter 4 Structure Of Atom Charged Particles in Matter
There are almost 120 known elements in the periodic table. The atoms of different elements have different numbers of electrons, protons, and neutrons. Every element is unique and has an atomic number. That number tells you the number of protons in every atom of the element. The atomic number is also called the proton number.
Read and Learn More: NEET Foundation Notes
Atom is labeled with a “+”, “−”, or a “0.” Those symbols refer to the charge of the particle. Charges are also found in tiny particles of matter.
The electron always has a “−”, or negative charge. The proton always has a “+”, or positive charge. If the charge of an entire atom is “0”, or neutral, there are equal numbers of electrons and protons. The third particle is the neutron. It has a neutral charge, also known as a charge of zero.
Electrons
Electrons are negatively charged particles, which were discovered by J. J. Thomson in cathode ray experiment. The term electron was coined by GJ Stoney.
How the Electrons were Discovered?
Discovery of electrons
J. J. Thomson constructed a glass tube from which the air was pumped out and a high electrical voltage among the two electrodes which was placed at either end of the tube was is applied.
He detected that a stream of particle coming out from the negatively charged electrode called cathode to positively charged electrode called anode. This ray is called cathode ray, whole construction is called cathode ray tube and the particles were called electrons.
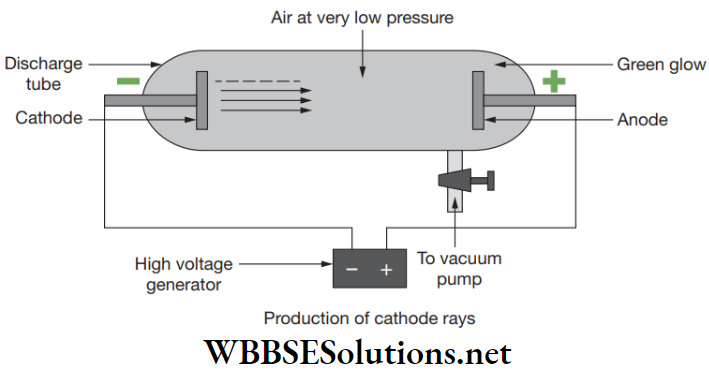
Atomic Structure Notes
Protons
E Goldstein, in 1886 found that anode emits positively charged particles called protons in an anode ray experiment. These positively charged radiations are produced in discharge tube from the anode called canal rays. In 1909, Rutherford discovered proton in his famous gold foil experiment.
| Class 11 Physics | Class 12 Maths | Class 11 Chemistry |
| NEET Foundation | Class 12 Physics | NEET Physics |
Neutrons
Neutrons were discovered by James Chadwick in 1932 and are neutrally charged particles. It stays inside the nucleus of an atom except hydrogen.
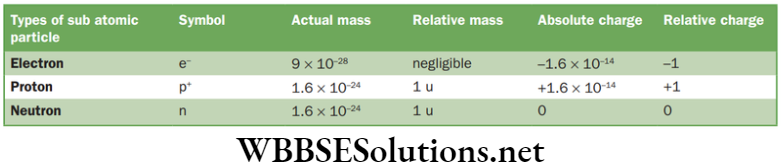
Comparison of three sub-atomic particles
How to Determine the Number of Proton, Electron and Neutron?
Gather Information
Find some information about your element, such as its atomic number (located in the upper left corner) and atomic weight (located on the bottom).
Consider an example for Krypton:
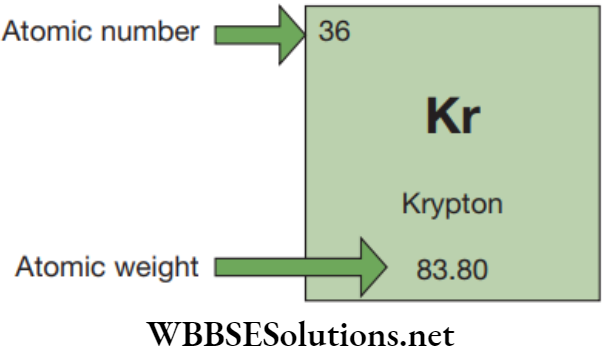
Find the Number of Protons
The atomic number is the number of protons in an atom of an element. In our example, Krypton’s atomic number is 36, so it has 36 protons in its nucleus.
Find the Number of Electrons
Atoms must have equal numbers of protons and electrons. So, atom of Krypton must contain 36 electrons since it contains 36 protons.
Find the Number of Neutrons
The atomic weight measures the total number of particles present in an atom’s nucleus. As the nucleus is made up of protons and neutrons. So,
Mass Number = (Number of Protons) + (Number of Neutrons)
For Krypton, this equation becomes:
84 = (Number of Protons) + (Number of Neutrons)
84 = 36 + (Number of Neutrons) {Number of proton is calculated above}
Number of neutrons = 84 − 36
Number of neutrons = 48
NEET Foundation Chemistry Notes For Chapter 4 Structure of an Atom
Atomic models mainly explain the structure of an atom and also gave us an idea about how subatomic particles behave.
Thomson Model or Water Melon or Plum Pudding Model
In 1897, Thompson proposed that the structure of an atom is similar to that of a Christmas pudding. In this, atom is a positively charged sphere in which the electrons are embedded and the magnitude of positive and negative charge is same inside an atom so the net charge inside an atom is zero.
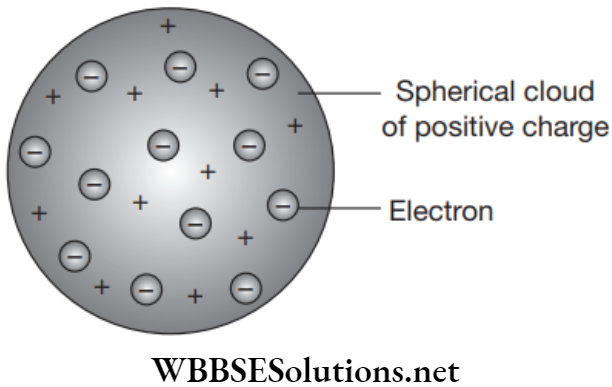
Thomson Model Limitations
- It could not explain the result of scattering experiment performed by Rutherford.
- It did not give any experimental evidence in its support.
Rutherford’s Model or Planetary Model
In gold foil experiment, Rutherford bombarded a beam of alpha particles on an ultrathin gold foil of thickness about 1000 atoms. He uses alpha particles, as these are doubly charged helium ions which moves fast and has a considerable amount of energy.
Rutherford’s Model Method
Fast moving alpha particles were bombarded on thin gold foil and after passing to the foil they hit the screen.
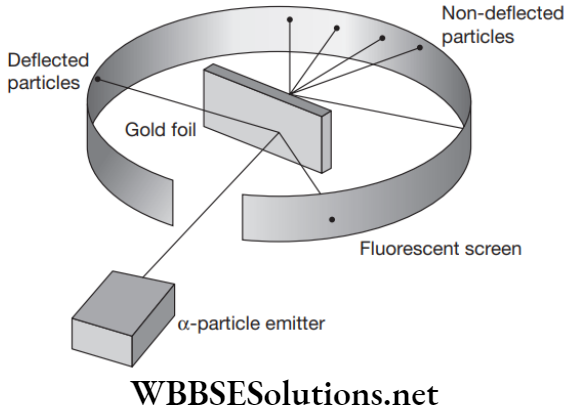
Rutherford’s Model Observations
- Most of the alpha particles pass through the foil without getting deflected, which means that most of the space inside the atom is empty.
- Some of the alpha particles were deflected by small angle, which means that the positive charge of the atom occupies very little space.
- Some of the alpha particles rebound back, which means the entire positive charge and mass of the atom is concentrated in a very small volume inside an atom.
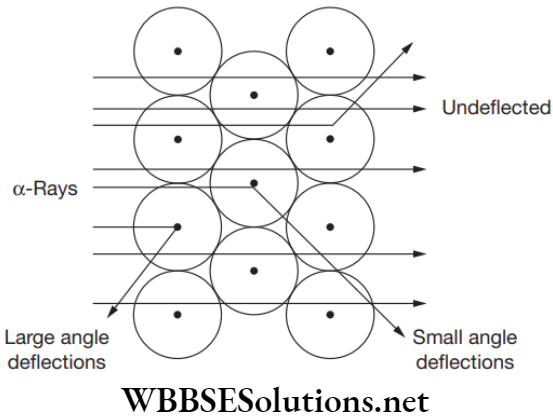
Rutherford’s Model Conclusions
- All the positively charged particles are present in small space inside the atom called nucleus.
- Electrons revolve around the nucleus.
- Most of the space inside an atom is empty.
- Total positive charge in nucleus is same as total negative charge on all electrons of atom, so the net charge of an atom is zero.
Rutherford’s Model Limitations
- Rutherford proposed that the electrons revolve around the nucleus in fixed paths called orbits. But according to Maxwell, an accelerated charged particle such as electron always emits an electromagnetic radiation and this radiation would carry energy from the motion of the electron which would come at the cost of shrinking of orbits. So, the electrons would collapse in the nucleus.
- Rutherford did not say anything about the arrangement of electrons in an atom.
Bohr’s Atomic Model
In 1913, Neil Bohr proposed a model of atomic structure.
He proposed:
- Electrons revolve around a positively charged nucleus in a certain orbit.
- The whole mass of the atom is concentrated in the nucleus.
- Electrons while revolving in an orbit do not radiate energy.
- Orbits or shell are called energy levels, and are represented by the letter K, L, M, N … or by numbers n = 1, 2, 3 …
- Energy level is associated with the definite amount of energy.
- Energy changes when an electron jumps from one energy level to another.
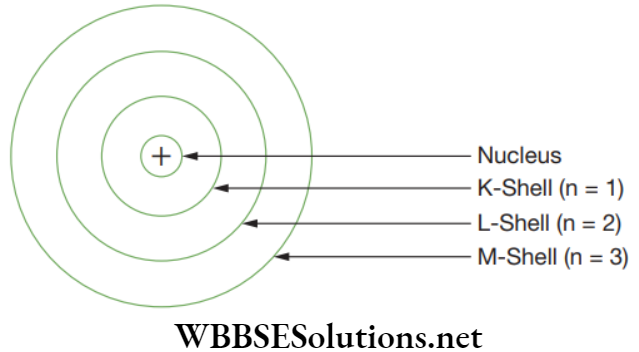
With his model, Bohr explained how electrons jump from one orbit to another either by emitting or absorbing the energy in fixed quanta. Like, if an electron jumps from one orbit which is closer to the nucleus, it must emit energy which is equal to the difference of the energies of the two orbits.
Similarly, when an electron jumps to a larger orbit, it absorbs a light equal in energy to the difference in orbits.
Bohr’s orbits are called stationary states because the energies of orbits in which the electrons revolve are fixed.
Bohr’s Atomic Model Photoelectric Effect
There is immediate ejection of electrons from the surface of metal when light beam of certain frequency strikes on it. This is known as the photoelectric effect.
Heisenberg’s Uncertainty Principle
This principle states that it is impossible to decide both simultaneously and accurately position and momentum of a microscopic moving particle.
Quantum Numbers
They are used to specify the orbitals and the electrons. We will also discuss the principal quantum number, azimuthal quantum number, spin and magnetic quantum number.
Pauli’s Exclusion Principle
It is not possible for an atom to have all 4 quantum numbers same for two electrons.
Hunds’ Rule of Maximum Multiplicity
The pairing of orbitals of the atom is started only when each orbital has occupied one electron.
Schrödinger Wave Equation
The Schrödinger wave equation is used to find the probability of presence of electron. This place where probability of finding electron is highest is known as orbital.
NEET Foundation Chemistry Notes For Chapter 4 Structure Of Atom Track Your learning Question And Answers
Question 1. The is immediate ejection of electrons from the surface of metal when light beam of certain frequency strikes on it is known as the ____________.
Answer. Photoelectric effect
Question 2. The Schrödinger wave equation is used to find the probability of presence of electron. (True/False)
Answer. True
Question 3. The place where probability of finding electron is highest is known as ____________.
Answer. Orbital
Question 4. ____________ are used to specify the orbitals and the electrons.
Answer. Quantum numbers
Question 5. Electrons while revolving in an orbit radiate energy. (True/False)
Answer. False
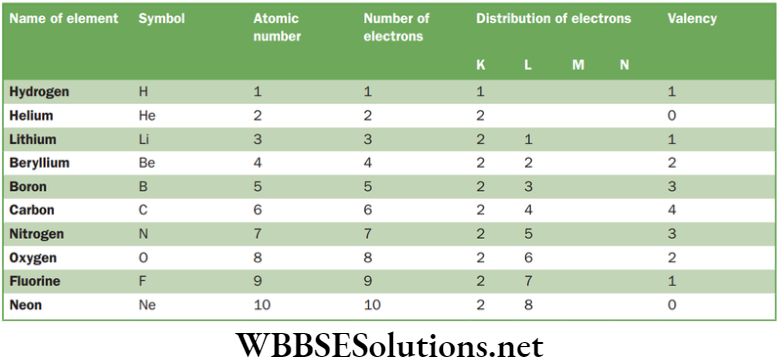
Chapter 4 Structure Of Atom Distribution of Electrons in Different Orbits
Electronic configuration is an arrangement of electrons in various shells of an atom of the element.
Electronic configurations describe electrons as each moves independently in an orbital. Mathematically, configurations is described by Slater determinants or configuration state functions.
According to the laws of quantum mechanics, if systems have only one electron, then its energy is associated with each electronic configuration and, on certain conditions, electrons are able to move from one configuration to another either by emitting or absorbing the quantum of energy, in form of photon.
Maximum number of electron which can be accommodated in any energy level is 2n2, where n = 1, 2, 3 … The maximum number of electrons which can be placed in an orbit is 8. Stepwise filling of shells is followed, like unless and until earlier shell is filled then only it can accommodate another shell.
Filling of orbits takes place from inside to outside. Maximum number of electrons in a given shell are:
- K-shell, n = 1: Maximum electrons = 2n2, 2(1)2 = 2
- L-shell, n = 2: Maximum electrons = 2n2, 2(2)2 = 8
- M-shell, n = 3: Maximum electrons = 2n2, 2(3)2 = 18
- N-shell, n = 4: Maximum electrons = 2n2, 2(4)2 = 32
Examples:
Electronic Configuration of Hydrogen (H)
Atomic number of hydrogen = 1
So, number of electrons = 1
Maximum number of electrons in 1st orbit = 2
Since, hydrogen has only one electron, so, it will reside in 1st orbit i.e in K-shell
Thus, electronic configuration of hydrogen:
Hydrogen and number of orbit present in hydrogen = 1
Electronic Configuration of Lithium (Li)
Atomic number of Lithium = 3
So, number of electrons = 3
Since the maximum number of electrons in 1st orbit, i.e., in K-shell= 2, so, after accommodating 2 electrons in 1st orbit, the third electron will go in 2nd orbit, i.e., in L-shell
Thus, electronic configuration of lithium is: Lithium and number of orbit in Lithium atom = 3.
Electronic Configuration of Calcium (Ca)
Atomic number of calcium = 20
So, number of electrons = 20
Electronic configuration of calcium is: Calcium and number of orbit in calcium = 4
NEET Foundation Chemistry Notes For Chapter 4 Structure Of Atom Valency
It is the number of electrons which an atom must either give away or take in order to attain a stable electronic configuration. Valence electrons are the electrons present in the outermost orbit of an atom, thus determining the valency of an atom. Atom can obtain a stable configuration, either by:
- Losing an electron
- Gaining an electron
- Sharing an electron
Valency = 8-valence electrons
Example:
Sulphur: It has 16 electrons.
Electronic configuration: n = 1, or K=1: 2 electrons
n = 2, or L = 2: 8 electrons
n = 3, or M = 3: 6 electrons
Its electronic configuration is 2,8,6. It has six electrons in its outermost orbit, so it requires two more electrons to complete its outermost orbit (M-shell). So it either takes two electrons to other atom or share electrons from another atom just to complete its octet.
Magnesium: It has 12 electrons.
Electronic configuration: n = 1, or K=1: 2 electrons
n = 2, or L = 2: 8 electrons
n = 3, or M = 3: 2 electrons
Magnesium molecule has an electronic configuration 2,8,2. It has two electrons in its outermost orbit, so it requires six more electrons to complete its outermost orbit (M-shell). So it will either donate its two electrons to other atom or share electrons from another atom just to complete its octet.
Valency of some elements
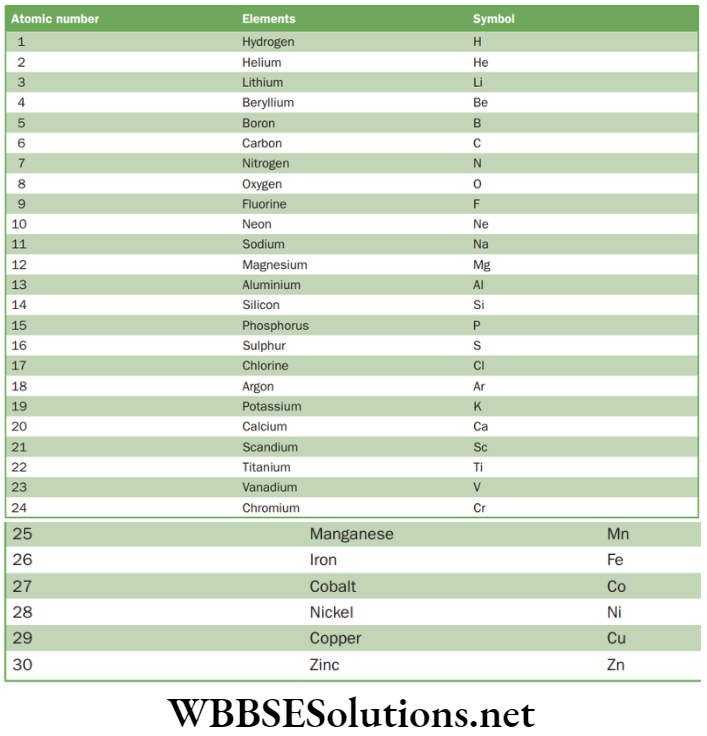
NEET Foundation Chemistry Notes For Chapter 4 Structure Of Atom Atomic Number and Mass Number
Atomic Number
It is represented by Z. It is the number of protons which are present in the nucleus of an atom. The conventional symbol Z comes from the German word Atom zahl which means atomic number.
It uniquely identifies a chemical element, it’s an uncharged atom, and an atomic number is also equal to the number of electrons.
Atomic number = number of proton
As number of proton is equal to number of electron in an atom, so:
Atomic number = number of proton = number of electron
Example, carbon’s atomic number (Z) is 6 as it has 6 protons. The number of neutrons may vary to produce isotopes, which are atoms of the same element that have different numbers of neutrons. The number of electrons can also be different in atoms of the same element, thus producing ions. For example, iron, Fe, can exist in its neutral state, or in the +2 and +3 ionic states.
Atomic number of some elements
How to calculate atomic number?
To calculate the atomic number for krypton: 3684Kr
Number of Protons = Atomic Number = 36
Number of Electrons = Number of Protons = Atomic Number = 36
Mass Number or Nucleon Number
It is the total number of neutrons and proton present in an atom. It is represented by A.
Mass number = Number of protons + number of neutron
To calculate the number of neutrons in an atom
- Number of Neutrons = Mass Number (A) − Atomic Number (Z)or,
- Number of Neutrons (in an atom) = Nucleon Number (A) − Proton Number (Z)
The atomic number, Z, must not be confused with the mass number, A, as mass number is the number of nucleons i.e., the total number of protons and neutrons present in the nucleus of an atom. The number of neutrons, N, is known as the neutron number of the atom.
Thus, A = Z + N.
Where: A = Mass number
Z = Atomic number
N = Number of neutrons.
Protons and neutrons have approximately the same mass and the mass defect of nucleon binding is small as compared to the nucleon mass, the atomic mass of any atom. This approximation of mass is used to calculate the number of neutrons in an element by simply subtracting the number of protons from the mass number.
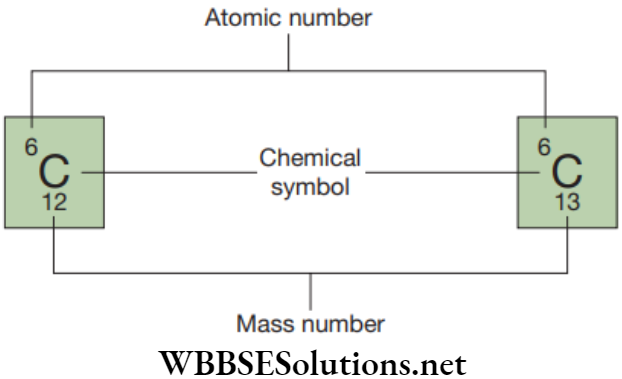
Example
Carbon:
Mass number is 12
Number of proton = 6 and number of neutron = 6.
Carbon has an atomic number of six and two stable isotopes with mass numbers of twelve and thirteen, respectively. So, its average atomic mass is 12.01.
NEET Foundation Chemistry Notes For Chapter 4 Structure Of Atom Fill in the Blanks
Question 1. The atomic number of an atom having 11 electrons is ____________.
Answer. 11
Question 2. Electrons are ____________ charged particles.
Answer. Negatively
Question 3. ____________ are the smallest of the three particles that make up atoms.
Answer. Electrons
Question 4. There are almost ____________ known elements in the periodic table.
Answer. 120
Question 5. Neutrons were discovered by ____________.
Answer. James Chadwick
Question 6. Mass of an electron is ____________ gram.
Answer. 9 x 10-28
Question 7. ____________ mainly explain the structure of an atom.
Answer. Atomic models
Question 8. Alpha particles are doubly charged ____________.
Answer. Helium ions
Question 9. Electrons revolve around a positively charged nucleus in a certain ____________.
Answer. Orbit
Question 10. The chemical properties of isotopes of a single element are nearly ____________.
Answer. Identical
Question 11. ____________ is the number of protons which are present in the nucleus of an atom.
Answer. Atomic number
Question 12. Mass number is the number of ________.
Answer. Nucleons
Question 13. Electronic configurations describe ________ as each move independently in an orbital.
Answer. Electrons
Question 14. Mathematically, configurations is described by _________.
Answer. Slater determinants
Question 15. Filling of orbits takes place from __________.
Answer. Inside to Outside
Question 16. ____________ = 8 valence electrons.
Answer. Valency
Question 17. ____________ is the number of electrons which an atom must either give away or take to attain a stable electronic configuration.
Answer. Valency
Question 18. ____________ has an electronic configuration of 2,8,6.
Answer. Sulphur
Question 19. Valency of carbon is 4. (True/False)
Answer. True
Question 20. __________ are the atoms having same mass number but different atomic number.
Answer. Isobars
Question 21. Isotones are the atoms that have the same neutron number but different number of proton. (True/False)
Answer. True
Question 22. _________ are different forms of a single element.
Answer. Isotopes
NEET Foundation Chemistry Notes For Chapter 4 Structure Of Atom True Or False
Question 1. Atomic number = number of proton = number of electron. (True/False)
Answer. True
Question 2. The number of electrons may vary in an atom to produce isotopes. (True/False)
Answer. False
Question 3. Protons and neutrons have approximately the same mass. (True/False)
Answer. True
NEET Foundation Chemistry Notes For Chapter 4 Structure Of Atom Match the Column
Question 1. Match the following and choose the correct code:
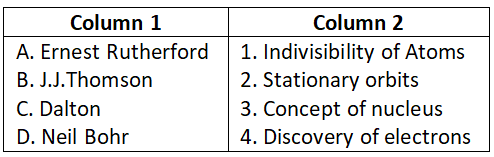
Select the correct option:
- A-4, B-1, C-3, D-2
- A-2, B-4, C-3, D-1
- A-3, B-4, C-1, D-2
- A-2, B-1, C-3, D-4
Answer. 3. A-3, B-4, C-1, D-2
Question 2. Match the following and choose the correct code:
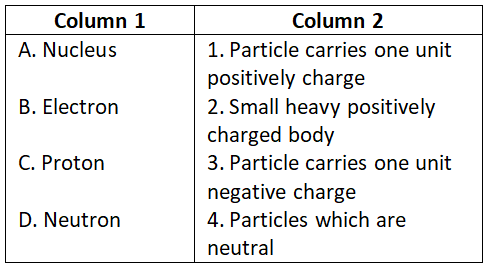
Select the correct option:
- A-2, B-1, C-3, D-4
- A-2, B-3, C-1, D-4
- A-1, B-3, C-4, D-2
- A-3, B-1, C-4, D-2
Answer. 2. A-2, B-3, C-1, D-4
Question 3. Match the following and choose the correct code:
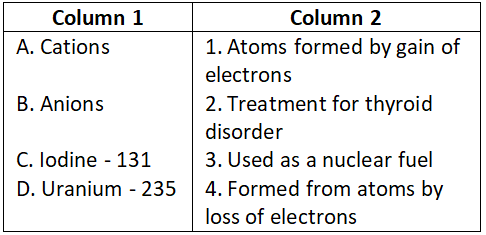
Select the correct option:
- A-3, B-1, C-4, D-2
- A-2, B-3, C-1, D-4
- A-2, B-3, C-4, D-1
- A-4, B-1, C-2, D-3
Answer. 4. A-4, B-1, C-2, D-3
NEET Foundation Chemistry Notes For Chapter 4 Structure Of Atom Assertion Reasoning
For the following questions the options will remain the following:
- Both A and R are correct and R is correct explanation of A.
- Both A and R are correct but R is not a logical explanation of A.
- A is correct but R is incorrect.
- R is correct but A is incorrect.
Question 1. Assertion: Cathode rays glow in the entire tube at 1 mm pressure
Reason: The colour emitted depends upon the nature of the gas taken in the tube. If neon gas is taken, the light emitted is reddish orange.
Answer. 2. Both A and R are correct but R is not a logical explanation of A.
Question 2. Assertion: One unit positive charge corresponds to one proton.
Reason: The number of units of positive charge on the nucleus of an atom is equal to the number of protons present in nucleus.
Answer. 1. Both A and R are correct and R is correct explanation of A.
Question 3. Assertion: An atom is positively charged sphere in which electrons are embedded.
Reason: The protons are electrically neutral.
Answer. 3. A is correct but R is incorrect.
NEET Foundation Chemistry Notes For Chapter 4 Structure Of Atom Comprehension Passage
As per Thomson’s model of the atom, an atom has both negative and positive charges which are equal in number and magnitude. So, they balance each other as a result of which atom as a whole is eletrically neutral. On the basis of Rutherford’s model of an atom, protons are present in the nucleus of an atom.
If α-particle scattering experiment is carried out using a foil of any metal as thin as gold foil used by Rutherford, there would be no change in observations. But since other metals are not so malleable so, such a thin foil is difficult to obtain. If we use a thick foil, then more α-particles would bounce back and no idea about the location of positive mass in the atom would be available with such a certainty.
The three sub-atomic particles of an atom are protons, electrons and neutrons. An electron is a negatively charged particle, whereas a proton is a positively charged particle. The magnitude of their charges is equal. Therefore, an atom containing one electron and one proton will not carry any charge. Thus, it will be a neutral atom.
If the number of electrons in the outermost shell of the atom of an element is less than or equal to 4, then the valency of the element is equal to the number of electrons in the outermost shell. On the other hand, if the number of electrons in the outermost shell of the atom of an element is greater than 4, then the valency of that element is determined by subtracting the number of electrons in the outermost shell from 8.
The distribution of electrons in chlorine, sulphur, and magnesium atoms are 2, 8, 7; 2, 8, 6 and 2, 8, 2 respectively. The valency of an element is the combining capacity of that element. The valency of an element is determined by the number of valence electrons present in the atom of that element.
Question 1. What will be the valency of the element in the outermost shell of the atom of an element is less than or equal to 4?
- More than the number of electrons.
- Equal to the number of electron.
- Less than the number of electrons.
- No one of above
Answer. 2. Equal to the number of electron.
Question 2. On the basis of Rutherford’s model of an atom where does protons presents?
- In the nucleus of an atom.
- In the neutron of an atom.
- All the protons and neutrons of the atom are contained in the nucleus.
- No one of above
Answer. 1. In the nucleus of an atom.
Question 3. What will happen if α-particle scattering experiment is carried out using a thick foil?
- Particles will cross the foil.
- Particles won’t cross the foil.
- Particles will bounce back.
- Nothing will happen.
Answer. 3. Particles will bounce back.
Question 4. As per Thomson’s model of the atom, what kind of charge an atom consists?
- Positive
- Negative
- Both negative and positive
- No charge
Answer. 3. Both negative and positive
Question 5. How the valency of an element is determined?
- Number of electrons present in the atom of that element.
- Atom containing one electron and one proton.
- The total number of electrons in a carbon atom.
- Atomic number is equal to the number of protons.
Answer. 1. Number of electrons present in the atom of that element.
Question 5. The maximum number of electrons which can be placed in an orbit is:
- 8
- 7
- 5
- 4
Answer. 1. 8
Question 6. Which of the following exhibit similar chemical behavior?
- Isotopes
- Isotones
- Isobars
- All the above
Answer. 1. Isotopes
