Chapter 4 Structure Of Atom Long Answer Type Question And Answers
Question 1. What happens when a Sodium atom becomes a Sodium Ion?
Answer.
Sodium atom becomes a Sodium Ion:
A sodium atom has 1 electron in its outer shell. It is in group 1 of the periodic table. When sodium reacts with non-metals (for example chlorine) it will lose its outer electron. Its outer shell will then have no electrons. It is as though the outer shell has vanished. The next shell in is full. This full inner shell becomes the new full outer shell.
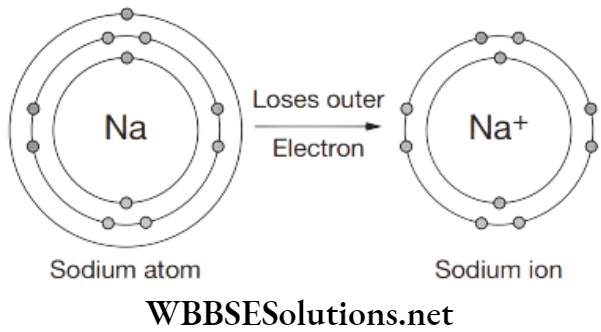
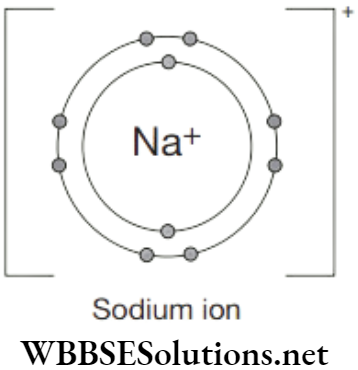
The sodium atom loses its outer electron to become a sodium ion. The sodium ion still has 11 protons (11 positive charges) but now only 10 electrons (10 negative charges). The sodium ion has an extra positive charge, shown by the + sign. All group 1 metals will form a 1+ ion when they react with non-metals.
The charge on the ion can also be shown as and the electron structure written as [2, 8]+ The charge on the sodium ion will make it react and form ionic bonds with other oppositely charged ions.
NEET Foundation Chemistry Chapter 4
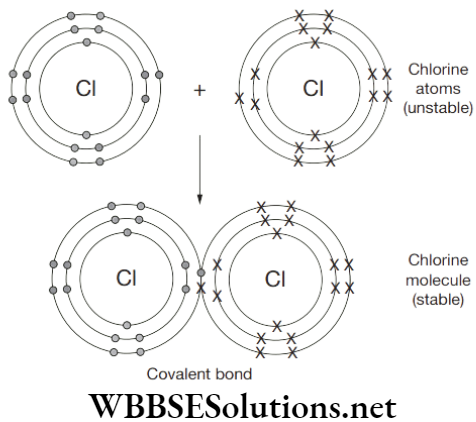
Question 2. Draw a Dot diagram for a Chlorine Molecule.
Answer.
Dot diagram for a Chlorine Molecule:
Chlorine is a non-metal. A chlorine atom has 7 electrons in its outer shell. Chlorine is in group 7 of the periodic table. Two chlorine atoms will each share one electron to get a full outer shell and form a stable Cl2 molecule.
Read and Learn More NEET Foundation Long Answer Questions
See a picture of the shared electrons making a covalent bond in a chlorine molecule. Chlorine is a simple molecule.
NEET Foundation Chemistry Chapter 4
| Class 11 Physics | Class 12 Maths | Class 11 Chemistry |
| NEET Foundation | Class 12 Physics | NEET Physics |
By sharing the two electrons where the shells touch each chlorine atom can count 8 electrons in its outer shell. These full outer shells with their shared electrons are now stable and the Cl2 molecule will not react further with other chlorine atoms. One pair of shared electrons form a single covalent bond.
There are no ions present (no + or − charges) in chlorine gas because the electrons are shared, not transferred from one atom to another. Chlorine does form hydrogen ions when it is dissolved in water to become chloric acid.
Question 3. Which particles are found in the Nucleus?
Answer.
Particles found in the Nucleus:
By sharing the two electrons where the shells touch each chlorine atom can count 8 electrons in its outer shell. These full outer shells with their shared electrons are now stable and the Cl2 molecule will not react further with other chlorine atoms. One pair of shared electrons form a single covalent bond.
There are no ions present (no + or − charges) in chlorine gas because the electrons are shared, not transferred from one atom to another. Chlorine does form hydrogen ions when it is dissolved in water to become chloric acid.
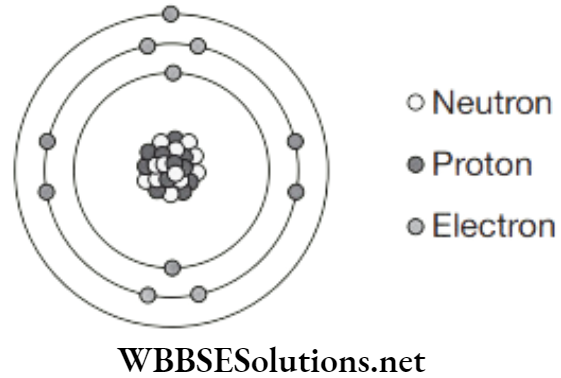
The electron structure is 2, 8, 1. Each proton has an electrical charge of +1. Each electron has an electrical charge of −1. The neutron has no charge (it is neutral). An atom has the same number of protons and electrons so the overall charge is zero (it is neutral).
The mass of a neutron and a proton are the same. An electron is very much smaller, about 1 ÷ 2000 times the size of a proton although it has an equal and opposite electrical charge. The electrons, although tiny, take up most of the space of an atom.
This means that most of the space that an atom fills contains hardly any mass. An atom is mostly empty space with nearly all the mass centred at the nucleus.
Question 4. How isotopes are indicated?
Answer.
To Indicate Isotopes
- List the mass number of an element after itsname or element symbol. For example, an isotope with 6 protons and 6 neutrons is carbon-12 or C-12. An isotope with 6 protons and 7 neutrons is carbon-13 or C-16.
- The mass number may be given in the upperleft side of an element symbol. For example, the isotopes of hydrogen may be written as: 11H, 21H, 31H
NEET Foundation Chemistry Chapter 4
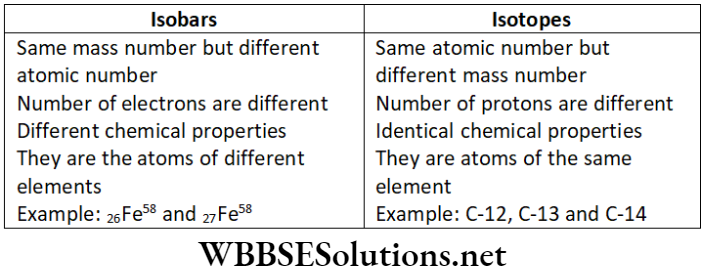
Question 5. Differentiate between isobars and isotopes.
Answer.
Difference between isobars and Isotopes