Chapter 2 Is Matter Around Us Pure Long Answer Type Question And Answers
Question 1. Does starch sol show Tyndall effect?
Answer.
Starch sol show Tyndall effect:
Scattering of light by particles in a colloid is Tyndall effect. Consider a situation, when a strong beam of light is passed through a colloidal solution, its path becomes visible when viewed from a direction at right angle to that of incident light. It is only observed when the size of the particles are larger than the wavelength of visible light.
Usually the particles of size which are in the range of 10–9 m to 10–6 m scatter the visible light. The intensity of Tyndall scattering increases with increase in the size of colloidal particles as well as the concentration.
In this case, as Starch sol is a lyophilic sol and its particles are very small and highly solvated. There is no difference in the refractive indices of dispersion medium (water) and dispersed phase (starch). Hence it shows only very weak or almost no Tyndall effect.
NEET Foundation Chemistry Chapter 2
Question 2. Soluble substances cannot be separated through filtration as the particles of the solute are too small. So which techniques one need to follow to separate solutions?
Answer.
To separate the soluble substance whose solute particles are too small, one can use the following techniques:
Read and Learn More NEET Foundation Long Answer Questions
- Evaporation is when you heat the solution so the solvent evaporates, which will leave the solute in the container as a residue of crystals.
- Distillation is the same as evaporation but the gas (distillate) is collected and cooled to create distilled water.
- Chromatography is a technique that is used to separate mixtures due to their solubility. A spot of the mixture is placed on filter paper and placed in water. The substances in the mixture with higher solubilities will move further up the filter paper.
Question 3. Explain the suitability of copper and aluminium that is used as electrical conducting materials.
Answer.
Suitability of:
- Copper: It is used for the winding of electrical machines. High purity copper is obtained by electrolytic refining. Traces (0.1%) of iron, silicon or phosphorous seriously reduce the conductivity of copper. The conductivity of copper is also decreased when it is hard drawn into wires for use in machines. Annealing is therefore necessary before the material can be used in machines. Hard drawn copper because of its increased mechanical strength compared with annealed copper is used for conductors in low voltage overhead distribution lines. Long span lines of thin cross section require conductors of higher mechanical strength, which is achieved by adding small percentage of cadmium to copper. Copper is used in machine windings because it is easily workable without any likelihood of fracture.
- Aluminium: Conductors are suitable for very high, ambient temperature. Use of aluminium as an electrical material particularly in aircraft industries has a considerable advantages because of the saving in weight. Aluminium because of its lightness is being used for bus bars. The current carrying capacity of aluminium is 75% that of copper and its density being approximately one third that of copper and aluminium bus bar is only about half the weight of copper bus bar of equal current carrying capacity. The steel reinforced aluminium conductor is extensively being used for long span transmission lines.
| Class 11 Physics | Class 12 Maths | Class 11 Chemistry |
| NEET Foundation | Class 12 Physics | NEET Physics |
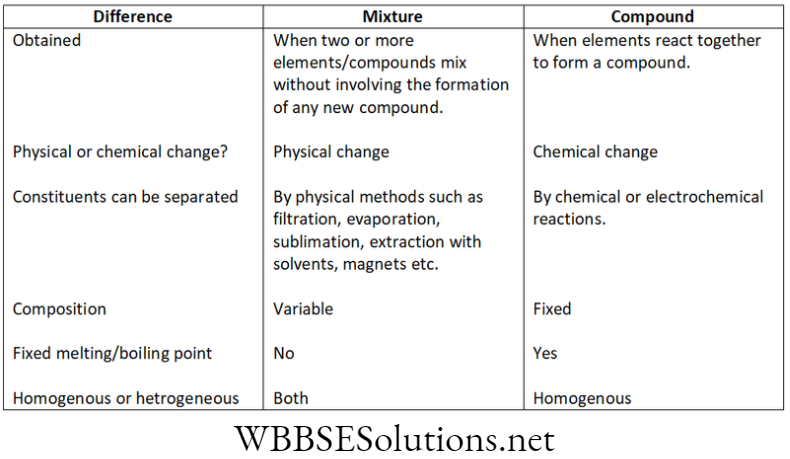
Question 4. Differentiate between compounds and mixtures.
Answer.
Difference between compounds and mixtures:
NEET Foundation Chemistry Chapter 2
Question 5. How will you separate the components of air?
Answer.
Steps involved in separation of the components of air:
- First the air is filtered and the dust particlesare removed.
- The air is then compressed under high pressure in a chamber.
- It is then passed through a water condenserto lower its temperature.
- The compressed air then moves into a separator where carbon dioxide separates out as dry ice.
- The air now becomes cold and turns into aliquid because of repeated compression.
- The liquid air then moves into the distillation column through expansion jet where it is warmed slowly.
- The boiling point of liquid nitrogen is –196°Cso it boils out first to form liquid nitrogen gas.
- Argon is collected next having a boilingpoint of –186°C and finally oxygen having aboiling point of –183°C is collected last.
- The process is called fractional distillation.