Chapter 3 Element Compound And Mixture
If we look around us, we can see a wide range of objects. All these objects are made up of a variety of materials.
The tables and chairs are made of wood, the chalk is made of calcium carbonate, and mugs and buckets are made up of plastic.
The almirah is either made of wood or steel, the glasses and plates are made up of stainless steel or plastics, and some of the cooking utensils such as pressure cookers, etc.
Are made up of aluminium, electrical wires are made up of copper or aluminium and so on. A variety of materials are used to build a house.
Concrete and iron [mild steel is required to make bridges. So we can conclude that whatever we see around us is made up of matter.
A matter has some mass; it occupies some space and we can see, touch, smell and taste it. Some examples of matter are air, water, stones, clouds, sand, plants, wood, food etc.
For example, take a piece of stone and put it on the pan of an electronic weighing balance. The balance will show the weight of the piece of stone.
Read And Learn More: WBBSE Notes For Class 6 School Science
So the piece of stone has a mass. Now take a glass completely filled with water. Now carefully drop the same piece of stone in it.
You will find that some amount of water comes out of the glass. This happens because the stone is not soluble in water.
It occupies some space within the glass and so it displaces some water from the glass.
Hence we can see that the piece of stone has some mass and it occupies some space. The space occupied by the piece of stone is called its volume.
This type of experiment can be repeated with almost all the matters and it can be shown that each matter has mass and each of them occupies some space.
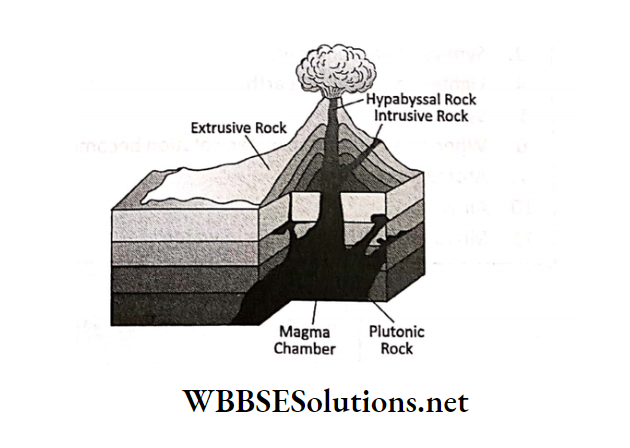
Chapter 3 Element Compound And Mixture Three States Of Matter
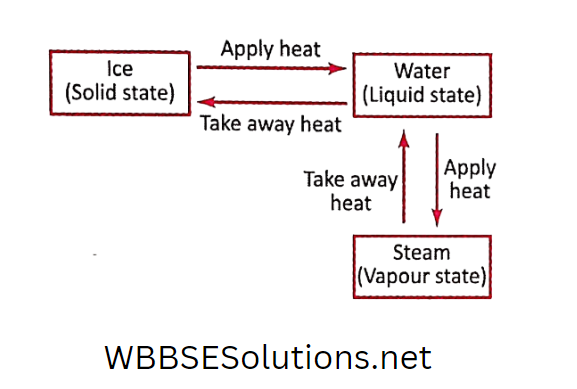
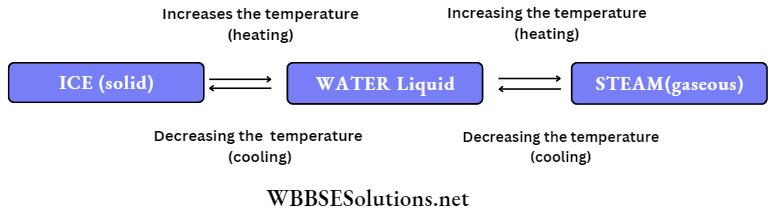
Matter can exist in three different states- solid, liquid and gaseous.
From our everyday experience we know that at ordinary temperatures, plastic is solid and so are iron and aluminium. Hair oil, water, after-shave lotion, petrol, diesel, kerosene, etc.
are all liquid at ordinary temperatures. Air is a mixture of a number of gases. When water is boiled, then steam is formed. Steam is gaseous.
But we should keep in mind that the state of some of the matters can be changed by changing the temperature.
You must have observed that during winter, coconut oil freezes (i.e. solidifies). If we warm it, then it again becomes liquid.
Similarly, water can be transformed into a solid or gaseous state.
Take some water in a plastic container and place it inside the freezer compartment of the refrigerator. Wait for some time.
You will observe that water has transformed into ice. You take out the container containing ice and place it on a table inside the room. After some time you will find that the ice has melted.
Again take some water in a kettle and heat it by placing it on a gas burner. After some time you will see that steam is coming out from it.
This is an example of a transformation of a liquid state (i.e. water) into a gaseous state (i.e. steam).
If we place a stainless steel plate over the emitting steam, the steam will condense on the steel plate in the form of very small water droplets.
From the above experiments, we can very well understand that one state can be converted into the other by changing the temperature.
Chapter 3 Element Compound And Mixture Classification Of Matter Elements Compounds And Mixture
One may wonder how a particular matter can exist in three different states at different temperatures. For that, we should know how matter is formed.
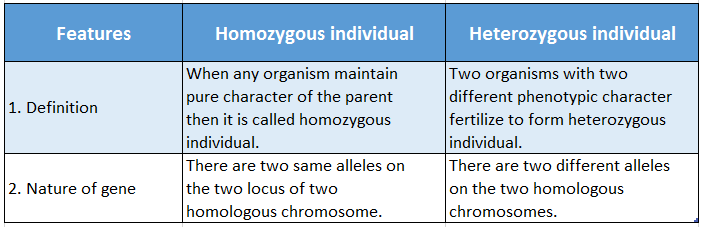
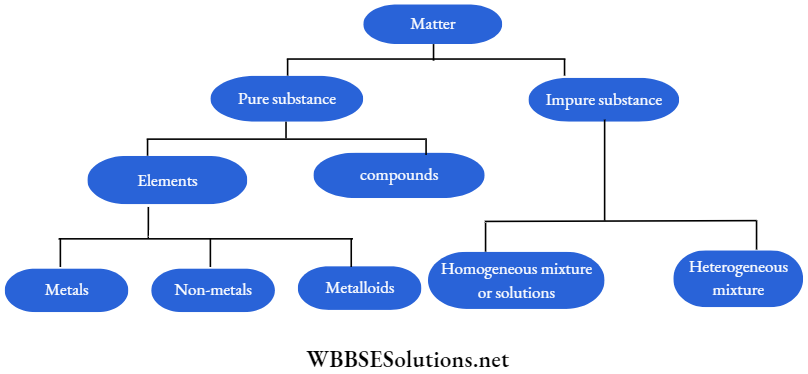
All matter around us is either pure or impure. Pure matter means that it contains only one kind of particle. Thus it is homogeneous in nature.
For example, sugar is a pure substance since it is made of particles of the same kind.
An impure matter contains two or more pure matters mixed in a certain proportion. Thus it contains more than one type of particle in it.
For example, milk is an impure matter since it contains different types of substances like water, fat, protein etc.
Based on the above matter may be classified as shown below:
particles of extremely small size. If we go on dividing such particles further, a time will come when it will be impossible to recognize each of the very, very tiny particles even by a powerful microscope.
Each of the smaller particles is known as atoms and molecules.
The ancient Hindu philosopher and sage, Kanad first proposed the existence of the atom (para Manu), the smallest part of the matter.
Much later, Avogadro proposed the concept of molecules the smallest part of an element or compound that can exist freely.
A molecule is composed of one or more atoms. A molecule of a particular substance possesses all the properties of that substance.
We have just mentioned two words elements and compounds. Let us know a little Every pure matter can be divided into a bit more about them.
Chapter 3 Element Compound And Mixture Elements
An element can be defined as a pure substance made of one kind of atom which can not be further subdivided by any physical or chemical means.
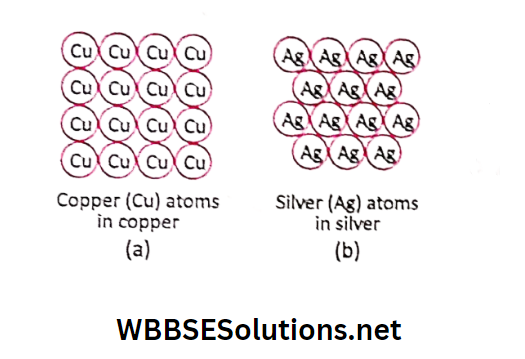
Examples: Copper, gold, silver, oxygen, mercury, chlorine etc. Thus atom is the smallest particle of an element.
It cannot be further broken into two or more simpler substances. For example, copper is made of only copper (Cu) atoms and silver is made of only silver (Ag) atoms.
There are more than 110 elements known to us to date. Among them, 92 are found in nature (i.e. they are naturally occurring).
The rest have been prepared by the scientists in the laboratory.
The naturally occurring elements are available in the earth’s crust, in the atmosphere and in the seas and oceans.
Oxygen (46.4%) and silicon (27.7%) are the two most abundant elements present in Earth’s crust. Besides, aluminium, iron, calcium, sodium, magnesium etc. are also present.
abundant element present in the earth’s atmosphere followed by oxygen.
Very small amounts of other elements such as hydrogen and ‘inert’ gases are also present in the atmosphere.
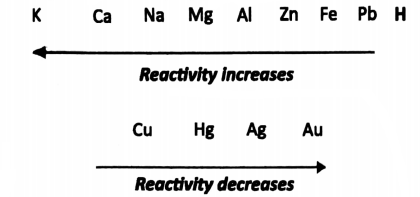
All these elements can be classified into three groups:
- Metals, such as iron, silver, gold, copper, lead, magnesium, aluminium, zinc, mercury, sodium, potassium etc. About 70 of all naturally occurring elements are metals.
- Non-metals, such as carbon, hydrogen, nitrogen, phosphorous, sulphur, chlorine etc. Non-metals have properties almost opposite to those of metals as is indicated by the name. Only 18 of the naturally occurring elements are non-metals.
- There are some elements which show properties of both metals and non-metals. They are called metalloids. Examples of metalloids are arsenic, antimony, bismuth etc.
It is important to know the differences between metals and non-metals. While comparing, we should always keep in mind that exceptions are always there.
Comparison Of The Properties Of Metals And Non-Metals
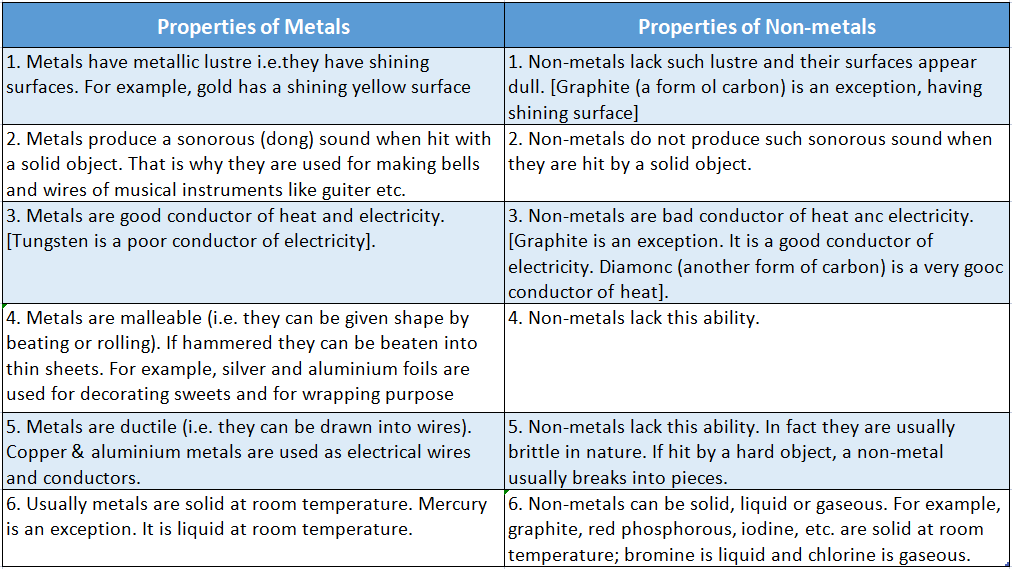
Let us illustrate the above-mentioned properties of metals and non-metals in some detail.
Activity 1: Take a one rupee coin (made of metal) and a piece of charcoal (a form of carbon- a non-metal).
Now drop them from a height onto the concrete floor. When the one rupee coin hits the floor, a sonorous (dong) sound is heard.
This sound is typical for metal. So it is usually referred to as “metallic sound”. But when a piece of charcoal hits the concrete floor, no such sound is heard.
Activity 2: Take a clean stainless steel plate and place it in the sunlight for some time.
The plate shines when sunlight falls upon it. Then if you touch the plate it feels hot. So a metal plate shines in the light and becomes hot quickly.
But this is not the case for any object made up of non-metals. Of course, graphite is an exception.
Activity 3: The surface of a clean, polished metal sheet, say a sheet of aluminium appears to shine in the light, but the surface of charcoal does not. In general, we can conclude that metals have shining surfaces.
Activity 4: Hold one end of a stainless steel spoon over a flame.
Within a very short time, it will become impossible to hold the spoon by hand.
This is because heat is conducted through the metal spoon very quickly from one end to the other.
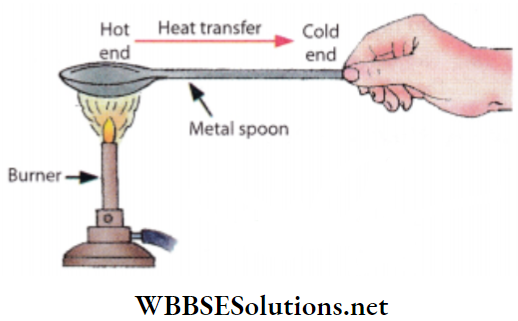
Although the other end of the spoon is not in direct contact with the flame, heat is conducted pretty quickly from one end to the other through the spoon.
Cooling utensils and water boilers etc are usually made up of copper or aluminium metals since these are the best conductors of heat.
In general, we conclude that metals are good conductors of heat. Non-metals are, in general, not a good conductor of heat.
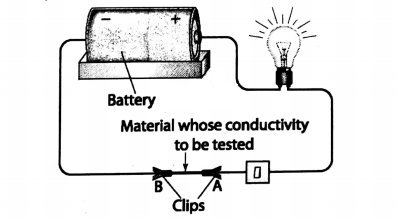
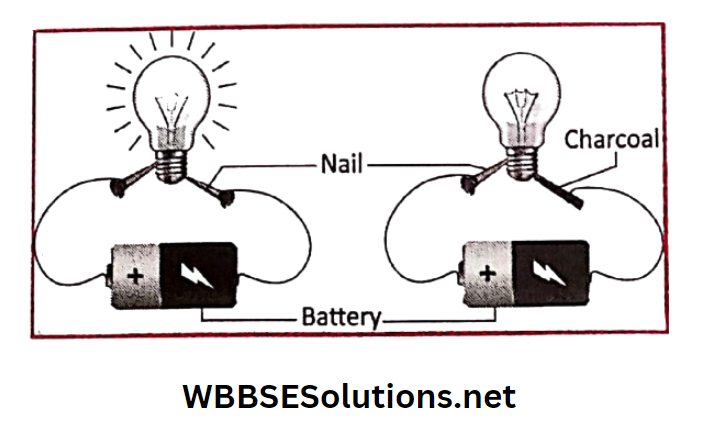
Activity 5: Take a few pieces of copper wire, one battery, a small flashlight bulb (or a small LED bulb), a pair of iron nails and a piece of charcoal.
First make an assembly as shown in with the bulb, battery and copper wires.
Now connect two ends of the iron nails with the copper wire. The bulb will glow, indicating that an electric current is flowing through the iron nail.
When the copper wire is touched with the piece of charcoal, the bulb does not glow. This proves that electricity cannot flow through the charcoal.
Hence, in general, we can say that metals are good conductors of electricity, but non-metals are not.
The metals are also malleable and ductile. If you go to the workshop of a blacksmith, you will see that iron pieces are being heated and hammered to give them different shapes.
This happens because of the malleability and ductility of iron.
Chapter 3 Element Compound And Mixture Compounds
A compound is a pure substance, consisting of two or more elements, which are combined chemically in a definite proportion.
Let us elaborate on the following experiments.
Activity 6: Take a small rubber cork and fix two iron nails in it. Now place it inside a glass beaker. Pour some water on it.
Add one teaspoonful of lime juice to the water. Stir it thoroughly.
With the help of pieces of copper wire, connect the two iron nails with the two opposite ends of a 9-volt battery.
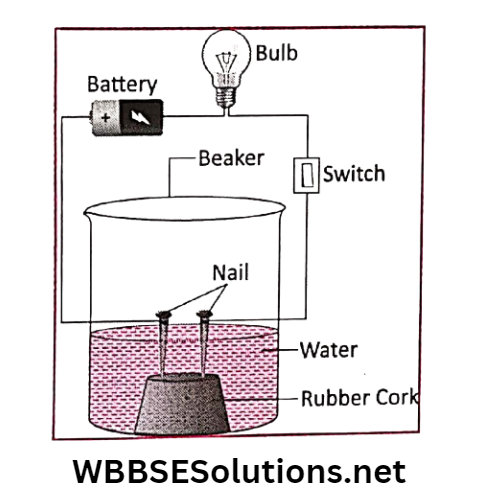
As soon as the iron nails are connected with the two opposite ends of the battery, gas bubbles start evolving from each of the iron nails.
Gas coming out from each of the iron nails can be separately collected and analyzed.
It will be found that hydrogen gas evolves from the iron nail connected with the negative end of the battery and oxygen gas evolves from the iron nail connected with the positive end of the battery.
A word of caution: Never perform this experiment using a connection from the electrical mains of the house or inverter.
You must use a 9-volt battery or 1.5-volt torchlight battery for this purpose.
So you find that when electricity is passed through the acidulated water, it breaks up (or dissociates) to form hydrogen and oxygen.
The phenomena of dissociation of water into hydrogen and oxygen due to the passage of electricity through it is known as the Electrolysis of water.
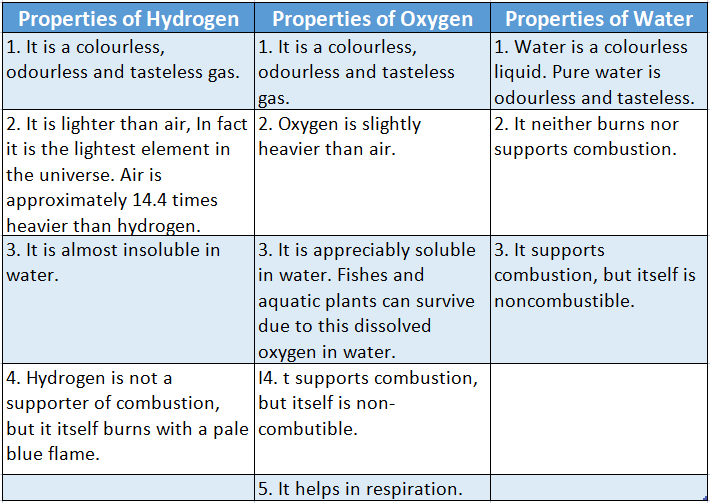
Properties of Hydrogen 1 It is a colourless, odourless and tasteless gas.
2 It is lighter than air, In fact, it is the lightest element in the universe. Air is approximately 14.4 times heavier than hydrogen.
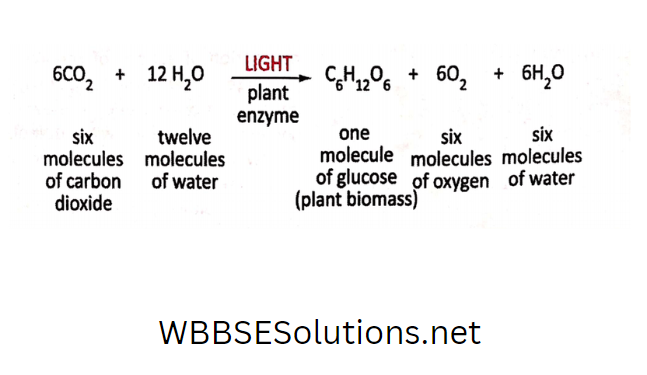
Oxygen and hydrogen are elements. They are gaseous at ordinary temperature and pressure. But when they “chemically” combine, water is formed in a liquid state.
The properties of water are also different from the properties of oxygen and hydrogen.
Some of the properties of hydrogen, oxygen and water have been summarized below in.
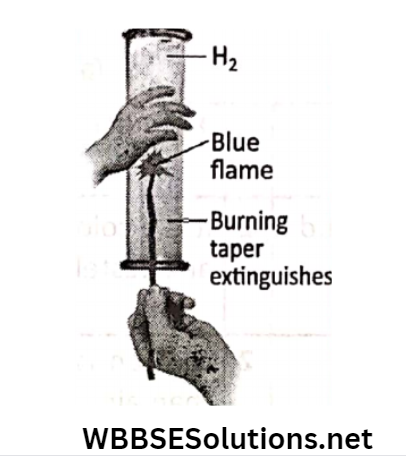
Activity 7: Let us take a gas jar filled with hydrogen. Now a burning taper is introduced in the gas jar.
It will be seen that the flame extinguishes but the gas burns with a “pop” sound with a pale blue inflame.
It proves that hydrogen is not all or supporter of combustion but itself burns (i.e. combustible)
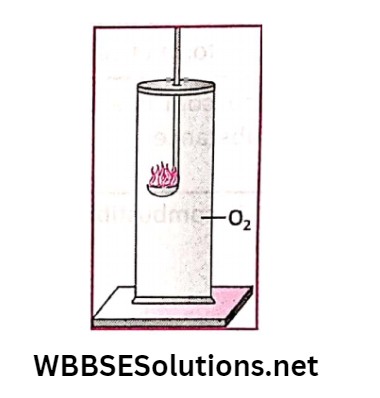
Activity 8: Let us take a gas jar filled with oxygen gas. A glowing splint is slowly introduced into the gas jar.
It is found that the glowing splint rekindles inside the gas jar.
This observation confirms that oxygen gas itself does not burn but it allows other substances to burn, which means it supports the combustion.
From this and it is evident that hydrogen itself burns (i.e. combustible) and is not a supporter of combustion, whereas oxygen does not burn but it supports combustion.
Both these properties are absent in water. In fact, when a fire breaks out accidentally, the first thing we all do is pour water on the fire to extinguish it.
Hence we find that the properties of water are quite different from those of its constituent elements.
Also, if we can analyze water, we will find that each molecule of water is composed of two atoms of hydrogen and one atom of oxygen.
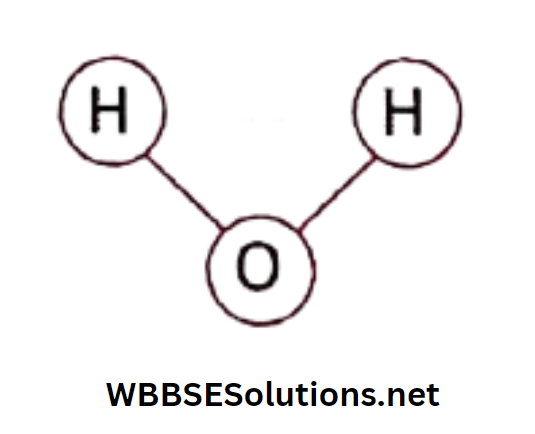
So hydrogen and oxygen combine in a definite proportion to form water. So, we can conclude that water is a compound.
More Examples of Compound
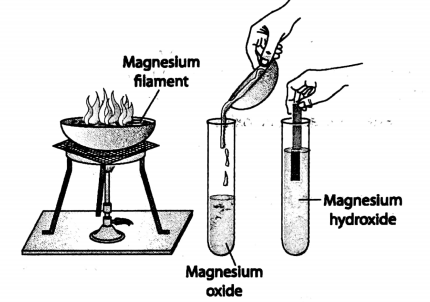
1. When a burning magnesium ribbon is introduced in a gas jar filled with oxygen, it burns very brightly producing blinding white light.
A white powdery substance, called magnesium oxide, is formed due to the burning of magnesium ribbon in oxygen.
Magnesium oxide is a compound formed by a chemical combination of two elements-magnesium and oxygen.
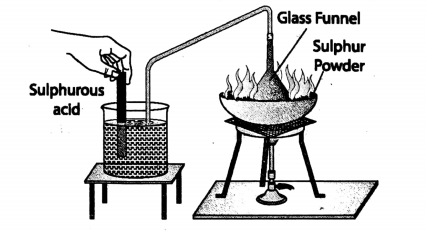
2. When a piece of glowing charcoal (a form of carbon) is introduced in a gas jar filled with oxygen, the charcoal burns more brightly throwing sparks.
Due to this burning, a gaseous compound called carbon dioxide is formed. Its properties are different from that of its constituting elements-carbon and oxygen.

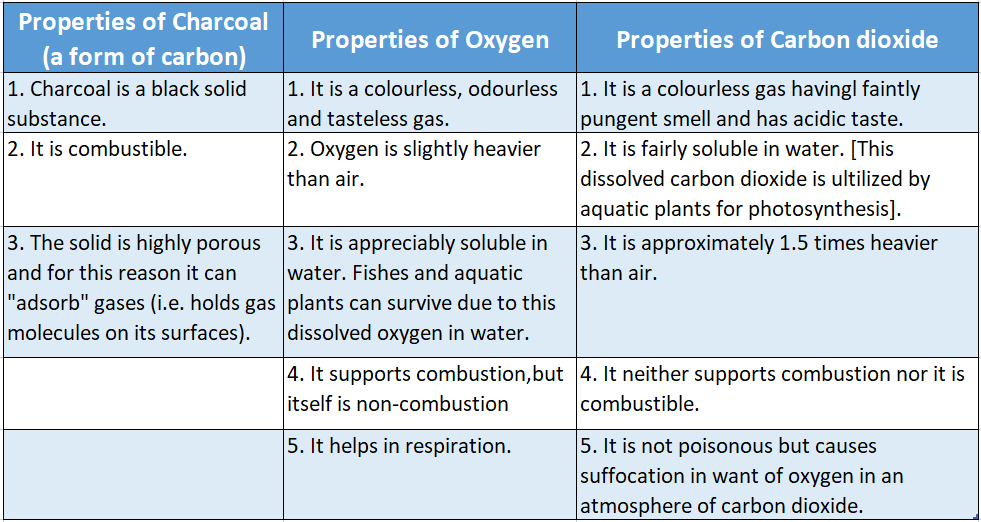
Hence comparing the various properties of carbon dioxide, carbon and oxygen, we can very well understand that in a compound individual properties of its constituent elements are lost.
The characteristic properties of compounds may thus be summarised as:
- A compound is always homogenous in nature.
- In a compound, the constituent elements are present in a definite proportion.
- The properties of a compound are different from those of its constituent elements.
- The constituent elements of a compound om cannot be separated by simple physical processes.
- The formation of a compound is usually accompanied by an evolution of energy in the form of heat and light.
Chapter 3 Element Compound And Mixture Mixture
We have already mentioned that matter can be classified as elements, compounds and mixtures. Let us now understand a little bit about mixtures.
We have mentioned the names of elements such as oxygen, hydrogen, carbon etc. We have mentioned the names of compounds such as carbon dioxide and water.
All these are components of air. In other words, the air is a mixture of nitrogen, oxygen, hydrogen, carbon dioxide, water vapour, etc.
So, scientifically speaking, the air is an impure substance.
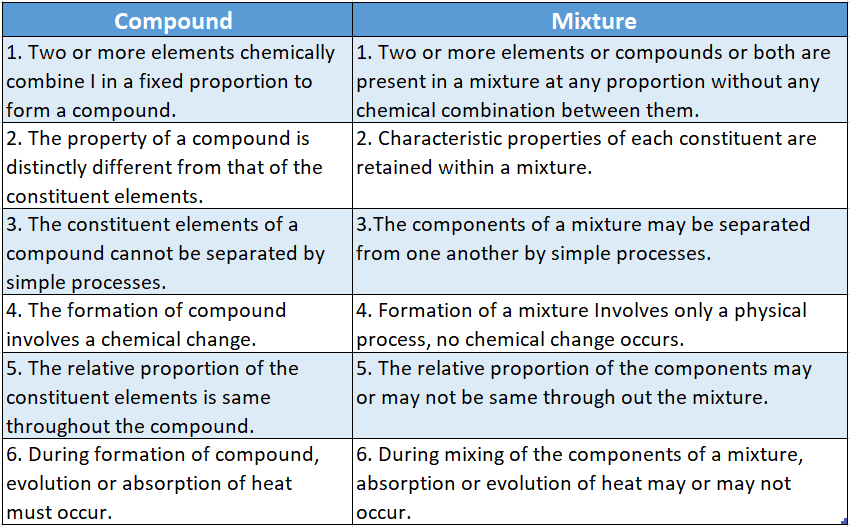
- A mixture is an “impure” substance in the sense that it may contain several elements or compounds or both.
- A mixture is prepared by simply mixing some elements or compounds or both in any proportion by weight or volume but without any chemical combination occurring between them.
- In a mixture, the constituting components remain side by side and each of them retains its individual characteristic properties.
- The constituents of a mixture can be separated by simple processes.
Compounds also contain more than one element but in a particular compound, the constituting elements chemically combine in a definite proportion.
Also, the individual properties of the constituting elements are lost when they combine to form a compound.
Activity 9: Let us take some iron filings and sulphur powder and mix them together in any proportion. Both iron and sulphur are elements.
Now a magnet is brought closer to the mixture. It will be found that iron filings are attracted towards the poles of the magnet.
So, the characteristic To property of iron (i.e. being attracted towards a magnet) is retained when it is simply mixed with sulphur powder.
So, it is a mixture of iron filings and sulphur powder. Using a magnet, one of the constituents of this mixture can be separated from the other.

Activity 10: Let us now take 7 grams of iron filings and 4 grams of sulphur in a porcelain dish. Heat this mixture until it begins to glow.
A new substance, called iron sulphide (it is actually called ferrous sulphide) is formed. Its appearance is distinctly different from both sulphur and iron.
It is a compound of iron and sulphur. It is not attracted by a magnet. So individual properties of constituting elements of a compound are lost.
Also, unlike a mixture, the constituting elements of a compound cannot be easily separated from one another.
A mixture may be prepared by simply mixing more than one element or compound or both in any proportion.
For example, air is a mixture of mainly nitrogen and oxygen. Other compounds such as carbon dioxide, water, hydrogen, etc.
are present in a very small amount. The relative proportion of nitrogen to oxygen varies with altitude.
At higher altitudes, the oxygen level falls and hence climbers and mountaineers experience respiratory trouble.
The amount of water vapour present in the air also fluctuates from place to place and from season to season.
In places, nearer to seas and oceans, the water content in the air is high. Again during the rainy season the water content in the air increases.
Due to the presence of a higher amount of water in the air, wet clothes require much more time to dry during the rainy season.
So, a mixture may be defined as a material which consists of two or more pure substances (elements or compounds) present in any. proportion.
The pure substances forming a mixture are called the components or constituents of the mixture.
No chemical reaction takes place during the formation of a mixture and all the constituents retain their properties in a mixture.
The mixture is abundant around us. Milk is a mixture of water, fat and proteins. Gunpowder is a mixture of sulphur, charcoal and potassium nitrate.
Crude oil is a mixture of several hydrocarbons. Air, blood, seawater, lemon water, soft drinks, smoke, etc. are other examples of mixtures.
Depending on the state of the mixture and the state of the components of the mixture, several types of mixtures are possible. Such types are listed in Table.
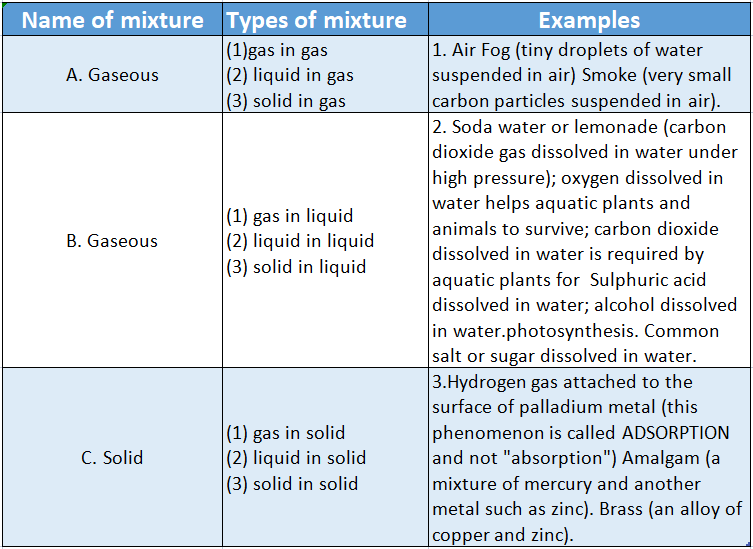
Differences Between Compound And Mixture
Chapter 3 Element Compound And Mixture Types Of Mixtures
Homogeneous and Heterogeneous Mixtures
We have already mentioned that the proportion of different components may or may not vary within a mixture.
Considering the distribution of components, a mixture can be classified as a homogeneous or heterogeneous mixture.
1. Homogeneous mixture:
A homogeneous mixture is one where all the components are uniformly mixed throughout the mixture.
This means that the relative proportion of different components is the same everywhere within the mixture.
For example, let some amount of salt be dissolved in a glass of water. It is stirred very well so that the salt dissolves completely in water.
The salt is now uniformly distributed throughout the water. The water is equally salty throughout. Hence, it is an example of a homogeneous mixture.
“Sharbat” (or a uniform mixture of sugar and water) is also an example of a homogeneous mixture.
2. Heterogeneous mixture:
A heterogeneous mixture is one where the constituting components are not uniformly mixed throughout.
This means the relative proportion of different components varies from place to place within such a mixture.
A mixture of sand and water, a mixture of sand and sugar, muddy water, etc. are examples of heterogeneous mixtures.
Chapter 3 Element Compound And Mixture Solution
Usually, a homogeneous liquid mixture of two or more chemically non-reacting substances is called a solution.
When some amount of sugar is dissolved completely in water, we get a sugar solution.
When some amount of common salt is dissolved completely in water, a salt solution is obtained.
The substance which is dissolved is called the solute, and the substance in which the solute is dissolved is called the solvent.
In the case of sugar solution or salt solution, water is the solvent and sugar or salt is the solute.
In general, we can write, Solution= Solvent+ Solute
Usually, in a solution, the component which is present in a larger proportion is called the solvent and the other component, which is present in a smaller proportion, is called the solute.
The characteristic properties of the solution may be briefly mentioned:
- A solution is homogeneous and transparent in nature.
- The solution may be coloured or colourless.
- The solute particles in a solution easily pass through a filter paper.
- Solute particles in a solution are so small in size that they cannot be seen with the naked eye or even under a simple microscope.
- The properties of solute are retained in a solution.
- The solute particles in a solution do not settle down on keeping.
When solid sugar particles are dissolved in water, they become invisible. Though we cannot see them, we can realize that they are within the solution. Its taste is sweet.
So, the characteristic property of sugar is retained in the
sugar solution. Then why can’t we see them even with a simple microscope? This is because a sugar particle is composed of a huge number of smaller particles.
When dissolved in water, water separates the very, very tiny particles from one another.
Due to this “break up”, the solid sugar particle becomes so small, they spread all over the solution and becomes invisible through our naked eyes or even by simple microscopes.
Chapter 3 Element Compound And Mixture Separation Of Mixture Into Its Components
We have already mentioned that components of a mixture can be separated from one another by simple physical processes.
In our daily lives, we use several techniques to separate the components from a mixture.
For example, milk or curd is churned to separate the butter.
While preparing tea, tea leaves are separated from the liquid by using a strainer.
In villages, farmers use a method called winnowing to separate lighter husk particles from heavier seeds of grains.

If some small pieces of stone are mixed with rice grains, then we remove the pieces of stone by handpicking them.

In our homes, we use a water filter to separate some impurities from water to make it suitable for drinking.
In a flour mill, impurities like husk and stones are removed from wheat by sieving it.
Many of you must have seen that pebbles and stones are being removed from sand by sieving them. Sieves of different sizes holes are used.



the holes should be such that smaller sand grains will pass through the holes of the sieve while the pebbles and stones can’t.

Hence different techniques of separation of constituents of a mixture are used depending upon the characteristic properties of the substances to be separated.
Some methods for separating mixtures are:
- Winnowing
- Sieving
- Hand-picking
- Decantation
- Sublimation
- Filtration
- Crystallization
- Magnetic separation
- Sedimentation, etc.
Below we shall discuss some of the techniques in little more detail.
1. Filtration:
This technique is utilized to separate an insoluble solid from a liquid component.
Principle: Filtration is done by sieving.
The sieve has holes of a particular size. The size of the holes of the sieve should be smaller than the insoluble solid particles which are to be separated from the liquid solution.
The molecules of the liquid are so small that they can pass through the holes of the sieve.
The insoluble solid particles remain on the sieve. Thus the liquid obtained after filtration is pure and devoid of any impurity. Let us illustrate this with an example.
Activity 11: Let us take some amount of muddy water. We want to separate the “mud”, which is an insoluble impurity present in water by filtration.
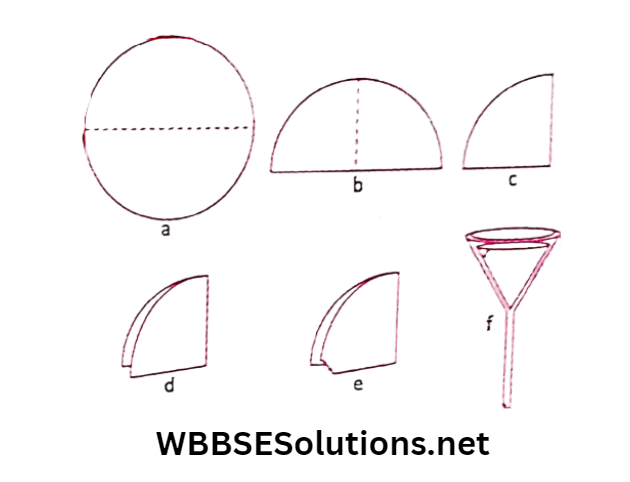
For this, we need a sieve. A filter paper is used for this purpose. Mud particles are small. But a filter paper has even smaller holes.
The shows the steps involved to fold a filter paper in the form of a cone. The cone-shaped folded filter paper is then placed on a funnel.
The muddy water is then poured into the filter paper. The molecules of water pass through the holes of the filter paper but the mud particles can’t. They remain on the filter paper.
The liquid which is coming out through the filter paper is in general called filtrate while the solid particles that remain on the filter paper are called residue.
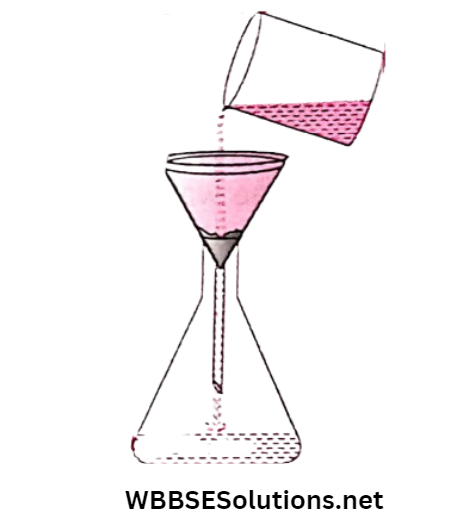
If the solid is soluble in water, as in the case of a sugar solution or salt solution, the size of the dissolved solid becomes so small that even a filter paper with very small holes cannot separate the sugar or salt from the solution.
In modern days, several water filters are available which can separate very small particles from the water using advanced techniques.
But in ancient times, people used to filter water by passing it through gravels of different sizes and layers of sand.
Application: Filtration tank in public water works.
2. Sedimentation and Decantation:
If the size of the insoluble solid impurity in the liquid solution is big enough, as in the case of sand and water, then sand can be separated from water by sedimentation followed by decantation.
Principle:
The mixture containing solid insoluble impurities is allowed to settle for some time. As a result, the bigger and heavier sand particles gradually settle at the bottom of the container.
This process is called sedimentation. The top layer of the liquid is now free from sand particles and is clear.
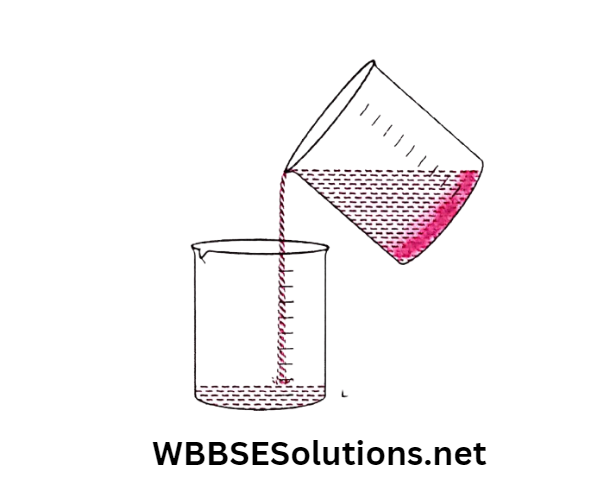
This clear top layer of water can then be poured gently into another container without disturbing the bottom layer of sand particles (decantation).
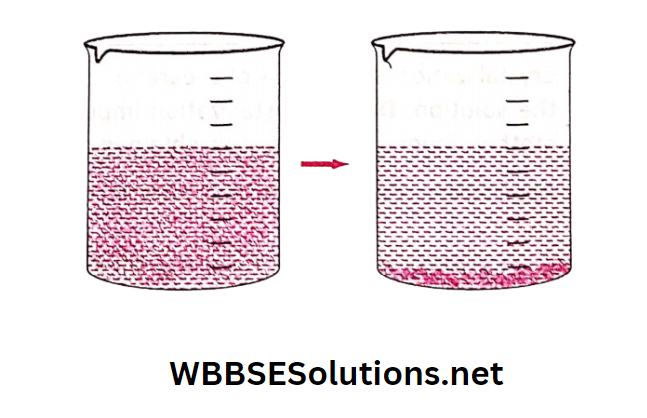
Application: Before cooking, rice and pulses are washed with water and then the water is separated by decantation.
3. Crystallization:
We have just mentioned that salt cannot be separated from salt solution (or sugar cannot be separated from sugar solution) by filtration or sedimentation decantation but since it is a mixture, there must be some simple means to separate salt (or sugar) and water.
Let us go through the undermentioned Activity 12 to learn the technique.
Activity 12: Let us take some amount of salt water in a beaker or any other container. Now boil it for some time.
As a result, some water will vapourize and the solution will become more concentrated. Now allow this concentrated solution to cool down undisturbed.
You will find that some solid salt particles are separated from the mixture. These solid salt particles are called crystals.
The liquid (called mother liquor) can now be slowly decanted and the crystals can be collected.
If we keep the solution for a longer period more water will evaporate and the crystals so formed will be bigger in size.
This process by which crystals of solids are obtained from a solution is called crystallization.
This technique is frequently used by Chemists in the laboratory to separate a particular solid into a pure form.
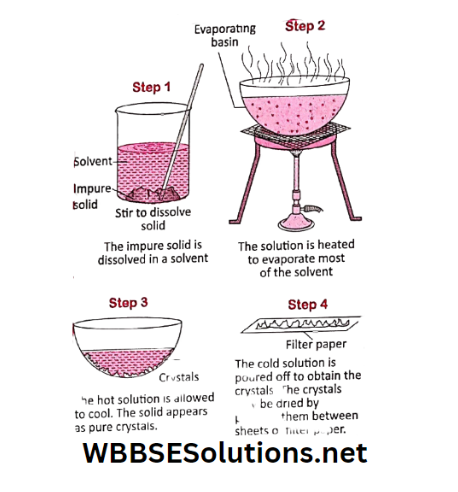
Crystallization is a process of separation of dissolved solid salt from the solution.
During crystallization impurities are retained in the mother liquor and the crystals obtained are of the pure substance.
Application: Purification of salt from seawater, purification of sugar and alums.
4. Separating a Mixture by using Magnet:
The magnetic separation technique is employed in the case of solid-solid mixtures. We know that iron is attracted towards a magnet. Cobalt and nickel are also attracted by magnets.
They are called magnetic substances.
So, when iron (or any other solid magnetic material) is mixed with some other non-magnetic solid materials like sand, then iron can be easily separated by using a magnet.
When a magnet is brought near to such a mixture, only iron particles are attracted and attached to the poles of the magnet. This way iron is easily separated from the sand.

Application: A strong magnet (such as an electromagnet) is used in the industry to separate scrap iron from the heap of waste materials.
Chapter 3 Element Compound And Mixture Symbol Formula And Valency
By now you have got some preliminary ideas about elements, compounds and mixtures. We already know that elements consist of only one kind of atom.
It cannot be further divided. So the smallest particle of an element is an atom.
A compound is a substance, consisting of two or more elements, which are combined chemically in a definite proportion. The smallest particle of a compound is a molecule.
The molecules of a particular compound can be broken down into its constituting atoms representing the elements.
A molecule of a particular compound has all the characteristic properties of that compound. A molecule is formed when two or more atoms combine chemically in a definite proportion.
The number of each atom in a molecule can be determined by analysis in the laboratory. Below we have listed some compounds and the elements present in each of its molecules.
But the names of the elements and compounds are often quite big. Scientists don’t use such big names when they represent elements and compounds.
They have devised some ways for shorthand representation of elements and compounds.
Chapter 3 Element Compound And Mixture Symbols
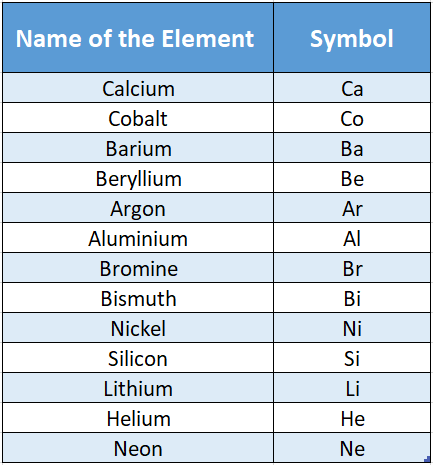
Elements are represented by a symbol which is the abbreviated English name (and in some cases the Latin or German name) of an element.
The symbol is given in different ways. For example, some of the elements are represented by the first letter of the name of the element.
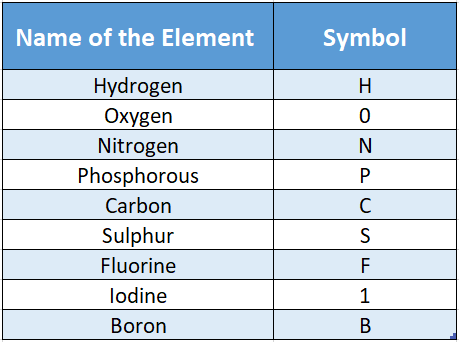
But there are only 26 letters in the English alphabet and there are more than 110 elements discovered so far.
So you can clearly understand that using only the first letter of an element will not do. Two or more elements can have the same first letter.
For example, carbon, cobalt, and calcium all begin with the letter “C”. So, only the first letter of the element is not enough to conclusively represent an element.
In the case of barium and beryllium, both have the same first letter (i.e. “B”); the first two letters are used to represent the two elements.
So some elements are represented by the first two letters of the name of the element. Some examples are given.
Chapter 3 Element Compound And Mixture Formula
When two or more atoms combine chemically in a definite proportion, they form molecules. Atoms usually cannot exist in a free state, but molecules can exist in a free state.
But atoms of inert gases [such as helium (He), neon (Ne), argon (Ar) etc. and metals such as gold (Au), platinum (Pt) etc. can exist in a free state.
Molecules of elements or compounds are formed by the combination of the same or different atoms.
It is the smallest unit (or particle) of a substance that can independently exist.
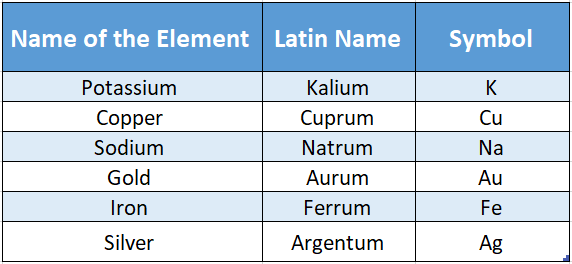
Using the first two letters of an element particular element. For example, thinking of sometimes is not sufficient to represent the first two letters (i.e. “M” and “A”).
In the case of manganese and magnesium-both have the same chromium and chlorine same problem persists.
So in this case, instead of the first two letters, the first and third letters have been used.
Sometimes, to represent some elements, their Latin names have been used. Examples are mentioned below:
Significance of the Symbol:
- It denotes the name of an element.
- A symbol also represents one atom of that element. So “H” means one hydrogen atom, and “2Cl” means two chlorine atoms.
- It represents the atomic weight of an element. For example, O stands for 16 parts by weight of oxygen element.
A chemical formula represents symbolically the composition of one molecule of a compound or an element.
The formula of an element is written by its symbol with a number placed to its right and a little below it.
The number indicates how many atoms of the element are contained in one molecule of it.
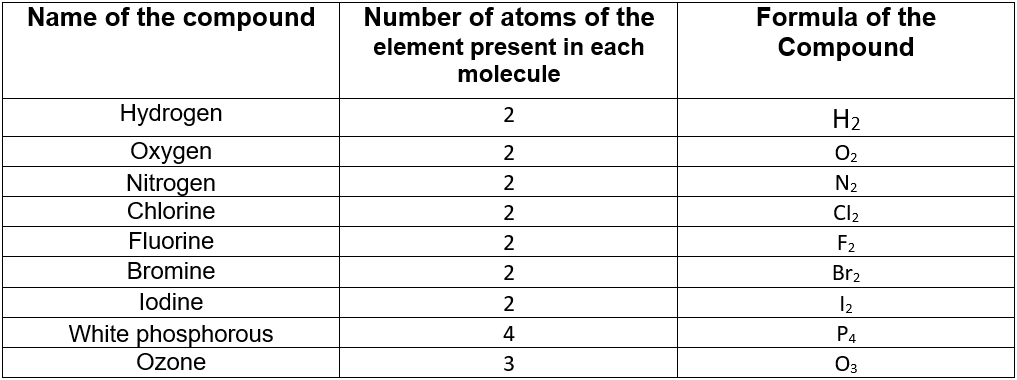
Let us illustrate this with the formula of elemental molecules of hydrogen.
The hydrogen molecule is made up of two hydrogen atoms. So a molecule of hydrogen is represented as “H2“.
You can see that the symbol of hydrogen (i.e. “H”) is written first and the number of hydrogen atoms in a hydrogen molecule (i.e. “2”) is written as a subscript after the symbol of hydrogen.
In a similar way, the formula of some common compounds may be represented as follows. All these compounds have only one type of element.
The number of atoms present in a molecule is called the atomicity of that molecule.
So atomicity of H2, N2, and O2, is 2; the atomicity of ozone is 3 and that of white phosphorous is 4.
one atom of an element is present in a molecule, then only its symbol is written and is not followed by the subscript ” 1″. So the formula of carbon dioxide is CO2 (and not C1O2).
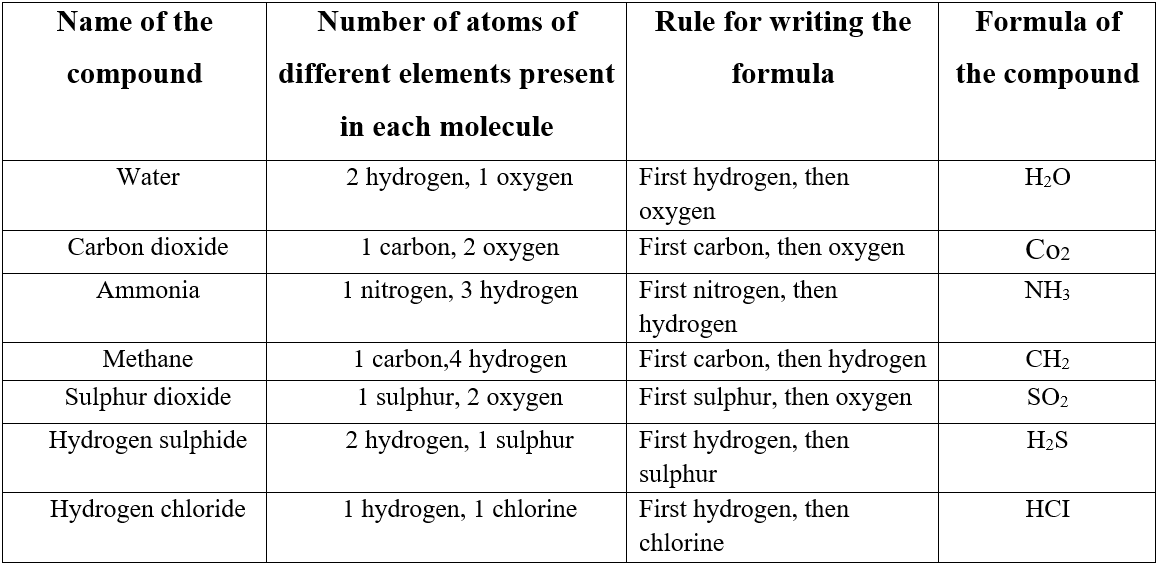
But not all the molecules contain only one type of atom. Most of the compounds contain two or more different types of atoms.
For, example, a molecule of carbon dioxide contains two different elements-carbon and oxygen. Each molecule of carbon dioxide has one carbon atom and two oxygen atoms.
According to the internationally accepted rule, carbon should be written first followed by oxygen. So it should be represented by the formula C2O2.
When only Below we have provided some more examples.
While writing the names of the compound prefixes indicating the number of a particular atom are used before the name of that element.
Mono is used for 1, bi or di is used for 2, tri is used for 3, tetra is used for 4, Penta is used for 5, Hexa is used for 6, and so on. Some examples are given below.
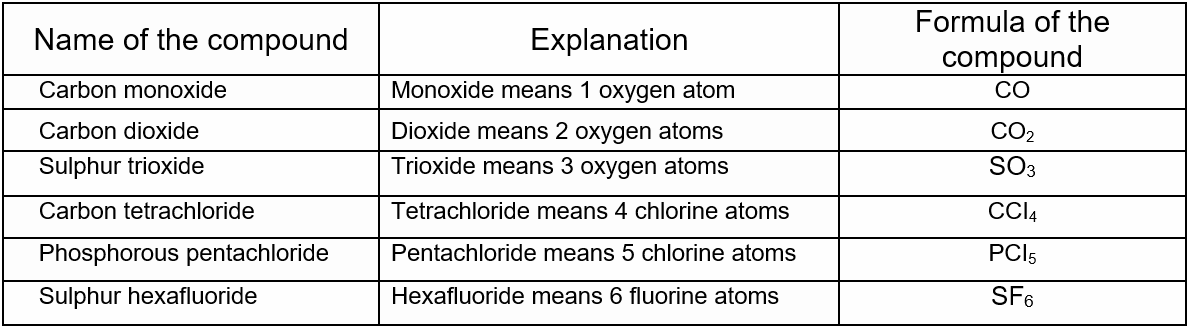
The formula of a compound, therefore, expresses its composition as well as the number of atoms of each of its components.
The formula Na2CO3 shows that the elements composing the molecule are sodium, carbon and oxygen and the corresponding number of atoms of the said elements are 2, 1 and 3. Therefore, the atomicity of Na2CO3 is 6.
Significance of formula:
- It states the name of a compound or an element. It also represents one molecule of a compound or an element.
- A formula expresses the names as well.
- The formula of an elemental molecule tells the number of atoms present in one molecule of it.
- A formula expresses the molecular weight of an element or compound.
- From a formula, it can be known which elements form the compound and in what proportion of their weights they exist.
- The formula of a gaseous element or compound can give information about its volume and pressure.
Chapter 3 Element Compound And Mixture Valency
Atoms of various elements combine with each other to form the molecules of compounds.
We now know the formula of hydrogen sulphide. It is H2S.
So in this case one sulphur atom is combined with two hydrogen atoms in a molecule. Similarly, in methane (CH4), one carbon atom is combined with 4 hydrogen atoms.
In ammonia (NH3) one nitrogen atom is combined with 3 hydrogen atoms.
The valency of an element means its combining capacity with another element with which it chemically combines to form one or more compounds.
This combining capacity of an element is measured by the number of hydrogen atoms with which one atom of the element combines the number of atoms of the elements present in one molecule of a compound.
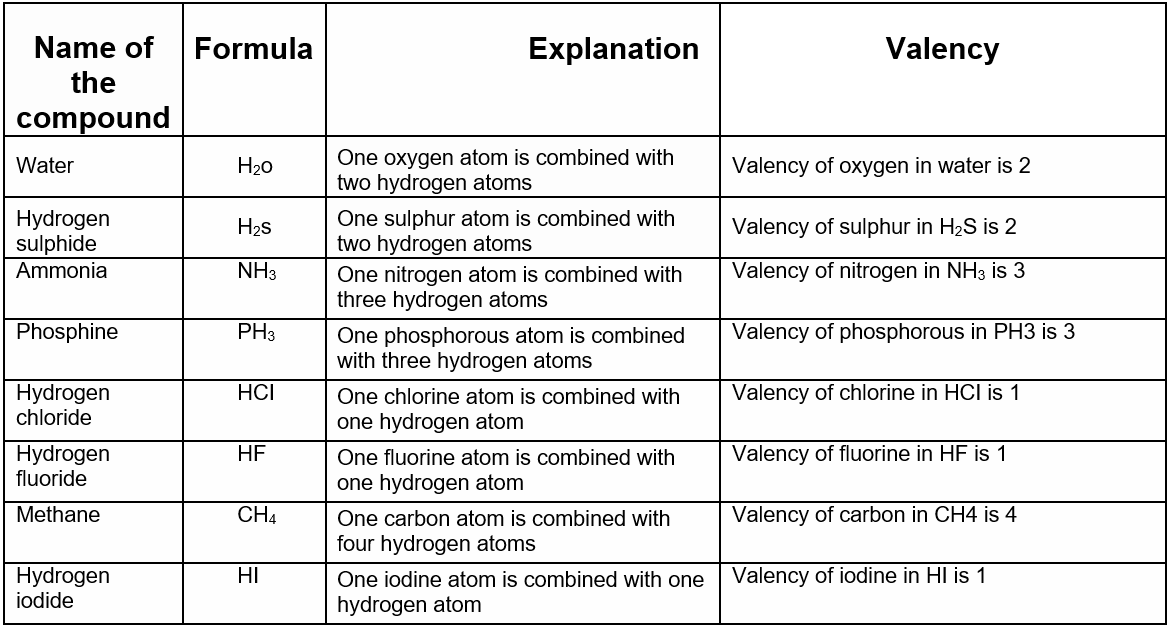
The number of hydrogen atoms that combine with one atom of a particular element to form a compound is called the valency of that element in that particular compound.
The valency of the element is determined by assuming the valency of hydrogen to be unity, ie. 1. This is called the valency with respect to hydrogen.
It may so happen that the same element combines with different numbers of hydrogen atoms forming another compound.
So in that case the valency of that element will be different.
We will not elaborate on this at this stage. Below we have listed the valencies of some elements with proper explanation.
You should remember the valency of different elements. This will help you to write the formula of a compound. You will learn more about this in higher classes.