Unit 5 Motion Of System Of Particles And Rigid Body Chapter 2 Rotation Of Rigid Bodies
System Of Particles Motion
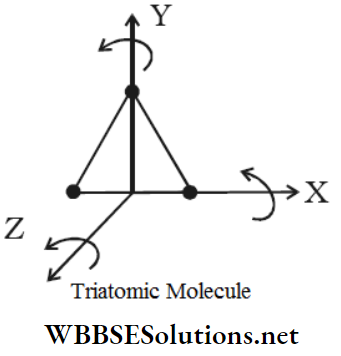
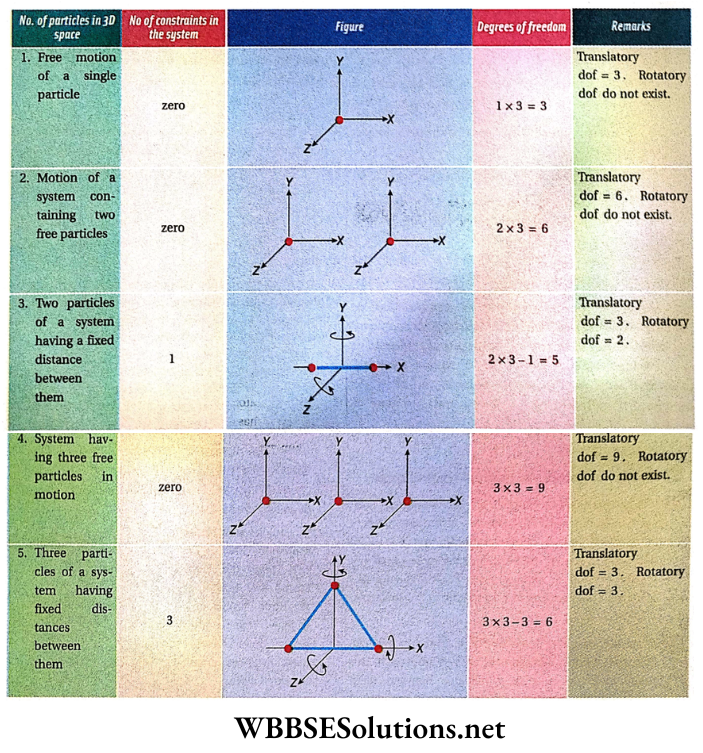
Rotation Of Rigid Bodies Introduction: A rigid body is a continuous distribution of particles of definite masses within an extended volume; these particles do not change their relative positions with respect to one another while rotating about a fixed axis.
Let us consider a rigid body R. The constituent particles of the body are A, B, C, … For a pure rotational motion of this rigid body, each constituent particle undergoes a circular motion. The characteristic of these circular motions is that the centres of all the circles lie on a single fixed straight line.

System of particles motion
This line is normal to the plane of each of the circular paths and is called the axis of rotation of the rigid body.
Rotation Of Rigid Bodies Definition: The axis of rotation is defined as a fixed straight line that passes normally through the centre of the circular path followed by a rotating particle, or by any constituent particle of a rotating rigid body. The axis of rotation may pass through the body itself or may lie entirely outside the body.

WBCHSE Class 11 Physics Rotation Of Rigid Bodies System Of Particles Motion
Couple Torque
Couple Definition: Two equal, parallel and opposite forces, having different lines of action, acting simultaneously on a body, constitute a couple.

A pair of parallel and opposite forces F, act on a body at points A and B. Then, (F, F) is a couple acting on the body. The perpendicular distance between the lines of action of the two forces is called the arm of the couple. A couple tends to set up a rotational motion.
Torque Definition: The tendency of rotational motion, set up in a body by a couple, is called the moment of the couple or torque. The torque is given by the product of one of the forces of the pair and the arm of the couple.
The direction of the torque is given by the direction of advance of a right-handed screw when turned in the direction of rotation. Turning on or off a water tap, screwing or unscrewing the cap of a bottle, using a screwdriver, etc., are associated with torques applied with our fingers thereby setting up a rotational motion.
Torque Vector Form: Torque is a vector quantity. The vector representation for the relation between torque and force is \(\vec{\tau}=\vec{r} \times \vec{F}\)
Torque and the vector product τ = rF sinø
Torque is the vector product between the force vector \(\vec{F}\) and vector \(\vec{r}\). \(\vec{\tau}=\vec{r} \times \vec{F}\)

Unit And Dimension Of Torque:
CGS System: dyn · cm
SI: N · m
Dimension of torque = dimension of force x dimension of length = MLT-2 x L = ML2T-2
System of particles motion
Torque And Pure Rotation: Torque due to a couple can produce rotational motion only. The resultant of the two forces applied at points A and B is zero, i.e., F – F = 0. As the resultant force is zero, no change occurs in its translational motion. But their lines of action are separate. So, only rotation is set up in this case. Such a rotation without translation is called pure rotation.
Moments Of The Two Forces Of A Couple: A point o is taken on the line BC as shown. Moment of force F applied at point A with respect to O = Fx CO. Also, force F at point B sets up the moment about O as FxBO. Hence, the algebraic sum of these two moments = F x CO + F x BO = F(CO+BO) = F x BC. But F x BC is the torque generated by the couple.
The algebraic sum of moments of the two forces of a couple, about a point, is equal to the moment of the couple (often called torque) about that point.
Moment Of Force And Torque Are Identical: From the above discussion, it is clear that torque alone can produce pure rotational motion. However, it is often observed that pure rotation can also be produced by a single force only. For example, a door can be opened by applying a single force on the door panel.
However, even in this case, an equal and opposite reaction force on the door panel is developed at the hinges. This reaction, along with the applied force, constitutes a couple and exerts a torque. Hence, moment of a force and torque are two identical physical quantities.
WBCHSE Class 11 Physics System Of Particles Motion
Work Done By A Couple: We know that two forces of equal magnitude constitute a couple. When a couple produces rotation in a body, the sum of the work done by the two forces of the couple is the measure of the work done by the couple. Suppose a couple (F, F) is acting on a body.

AB is the arm of this couple. So, the moment of the couple or torque, τ = F x AB.
Suppose the body is rotated by an angle θ under the influence of the couple about point O. As a result, point A is shifted to A1 and B to B1. If θ is very small, then we can assume that the arcs AA1 and BB1 are almost straight lines.
Now, AA1 = AO · θ and BB1 = BO · θ
Work done by the force acting on the point A, W1 = F · AA1 = F · AO · θ
Similarly, work done by the force acting on the point B, W2 = F · BB1 = F · BO · θ
So, the total work done by the couple,
W = W1 + W2 = F · AO · θ + F · BO · θ
= F · (AO+BO) · θ = F · AB · θ = τ · θ
= torque x angular displacement
Hence, the amount of work done does not depend on the position of the axis of rotation. For one complete rotation of the body, the angular displacement is 2π. So, for n complete rotations the angular displacement will be 2πn.
Hence, for n complete rotations, the work done by the couple, W = 2πx torque
WBCHSE Class 11 Physics System Of Particles Motion
Relation Between Torque And Angular Acceleration
Moment Of Interia Or Rotational Interia: When a force is applied to a body, a linear acceleration is produced in that body. Similarly, when a torque is applied to a body, an angular acceleration is produced in it. So it can be said that torque plays the same role in rotational motion as that of force in the case of linear motion. Hence, torque is the rotational analogue of force.

Suppose the body PQR is revolving with a uniform angular acceleration about the axis AB. The body is assumed to be made up of innumerable point masses m1, m2, m3, …, etc. These point masses are at distances r1, r2, r3, … etc. respectively from the axis of rotation AB. In the case of pure rotation, the axis of rotation remains fixed and the angular acceleration of each point mass remains the same.
But due to the difference in distances of the point masses from the axis of rotation, their linear accelerations are different. If the linear acceleration of the particle m1 is a1, then a1 = r1α and the force acting on it is F1 = m1a1 – m1r1α.
The moment of force F1 about the axis of rotation, G1 = force x perpendicular distance of the particle from the axis of rotation
⇒ \(F_1 r_1=m_1 r_1^2 \alpha\)
In this way, the moment of force can be found for every particle. The couple or torque acting on the entire rigid body is the algebraic sum of the moments of the forces acting on individual particles.
Hence, torque \(\tau=G_1+G_2+\cdots=m_1 r_1^2 \alpha+m_2 r_2^2 \alpha+\cdots\)
= \(\left(m_1 r_1^2+m_2 r_2^2+\cdots\right) \times \alpha=\sum_i m_i r_i^2\)
[mi is the mass of the i-th particle and ri is its perpendicular distance from the axis of rotation] = Iα …(1)
Here, \(I=\sum_i m_i r_i^2\)….(2)
= moment of inertia of the body about the axis of rotation
So, \(I=\frac{\tau}{\alpha}\)
i.e., moment of inertia = \(\frac{\text { torque }}{\text { angular acceleration }}\)
System of particles motion
Definition Moment Of Inertia: A body about an axis of rotation is defined as the torque acting on the body divided by the corresponding angular acceleration thus generated about the same axis of rotation.
In calculus, equation (2) can be represented as I = \(\int r^2 d m\)…(3)
Unit And Dimension Of Moment Of Inertia:
CGS System: g · cm²
SI: kg · m²
Dimension of moment of inertia = dimension of mass x (dimension of distance)² = ML²
WBCHSE Class 11 Physics System Of Particles Motion
Some Important Points About Moment Of Inertia:
1. Moment of inertia not only depends on the mass of a body but also depends on the perpendicular distance of the particles constituting the body from the axis of rotation, i.e., on the distribution of mass of the body.
2. In case of translational motion, force = mass x acceleration (F = ma)
Again, in case of rotational motion, torque = moment of inertia x angular acceleration (τ = lα)
Hence, the equation τ = lα is the rotational analogue of the equation F = ma. Moreover, we know that rotational analogues of force and linear acceleration are torque and angular acceleration, respectively.
So, comparing the above two equations, we can say that the rotational analogue of the mass of a body is its moment of inertia. Hence, the moment of inertia in rotational motion plays the same role as the mass in the case of translational motion.
3. The moment of inertia of a rigid body about a specific axis does not depend on the total mass \(\left(M=\sum_i m_i\right)\) of the body but on the distribution of mass of the constituent particles i.e., \(\sum_i m_i r_i^2\) of the body.
As the distribution of masses from the axis of rotation changes, the moment of inertia is due to the change of the axis of rotation in its position. Except in those cases, the moment of inertia of the rigid body about a specific axis of rotation. It can safely be assumed to be a scalar quantity.
Concept Of Moment Of Inertia: it has been said that the moment of inertia in rotational motion plays the same role as the mass in translational motion. It is evident from the following discussion.
- We know that the mass of a body in translational motion can be called its translational inertia. This is because mass is nothing but the hindrance that is generated in a body to resist any change in its translational motion.
- In the case of rotational motion, a body is compelled to change its state of motion when an external torque (rotational analogue of force) acts on it.
- In the absence of external torque, the body either remains at rest or executes uniform circular motion. It means that the moment of inertia of a body can be called its rotational inertia.
- It resists any change in the rotational motion of the body. To sum up it can be said that the relation between moment of force (torque) and moment of inertia is similar to the relation between force and mass.
- It is clear that the more the moment of inertia of a body about an axis, the more the torque necessary to rotate the body about that axis or to stop the body from rotating.
Two Important Theorems Regarding Moment Of Inertia: A regular-shaped body usually has some axis of symmetry. When the body rotates about such an axis, it undergoes just a spinning motion; during this spin, the entire body remains confined in the same region of space. A few examples of such axes of symmetry are:
- Circular Ring Or Circular Disc: The axis passing through the centre of the circle and perpendicular to its plane is the axis of symmetry.
- Sphere: Any diameter is an axis of symmetry.
- Right Circular Cylinder: The axis passing through the centres of the two circular faces is the axis of symmetry.
Now, it should be mentioned that the symmetry axis is not the only possible axis of rotation of a rigid body; a body may rotate about any other axis as well.
- For example, the diurnal motion of the earth (a sphere) is about its diameter, which is of course an axis of symmetry. In addition, the Earth rotates around the distant sun the axis of rotation passing through the sun is certainly not an axis of symmetry of the Earth. Earth has a different moment of inertia about that axis also.
- From the above discussion, it is evident that a rigid body may rotate about many possible axes. Fortunately, it is not necessary to tabulate the formulae for moments of inertia corresponding to all those axes.
- The following two theorems help us to find the moments of inertia of a body about some special axes of rotation, provided that the expression for the moment of inertia about a symmetry axis is known beforehand.
1. Parallel-Axes Theorem: This theorem is applicable for a body of any shape.
The moment of inertia (I) of a rigid body about any axis is equal to the sum of its moment of inertia (Icm) about a parallel axis through its centre of mass and the product of the mass (M) of the body with the square of the perpendicular distance (d) between the two axes.

The mathematical form of the theorem, I = Icm + Md² …(1)
WBCHSE Class 11 Physics System Of Particles Motion
Parallel-Axes Theorem Explanation: Let a body is composed of an infinite number of straight-line segments parallel to the z-axis. The masses of the segments are m1, m2, m3,….. The part at which the body intercepts the xy-plane is shown.
Let, the total mass of the body M is concentrated at that intersection and mass m1 is at a distance r from the z-axis
Now, the moment of inertia of \(m_1\) about z-axis, \(I_1=m_1 r^2=m_1\left(x_1^2+y_1^2\right)\)
= \(m_1\left\{\left(x_{\mathrm{cm}}+x_1^{\prime}\right)^2+\left(y_{\mathrm{cm}}+y_1^{\prime}\right)^2\right\}\)
= \(m_1\left(x_{\mathrm{cm}}^2+y_{\mathrm{cm}}^2\right)+2 m_1\left(x_{\mathrm{cm}} x^{\prime}+y_{\mathrm{cm}} y^{\prime}\right)\) + \(m_1\left(x_1^{\prime 2}+y_1^{\prime 2}\right)\)
Therefore, the moment of inertia of the whole body about the z-axis,
I = \(\left(x_{\mathrm{cm}}^2+y_{\mathrm{cm}}^2\right) \sum m_i+2 x_{\mathrm{cm}} \sum_i m_i x_i^{\prime}\)
+ \(2 y_{\mathrm{cm}} \sum_i m_i y_i^{\prime}+\sum_i m_i\left(x_i^{\prime 2}+y_i^{\prime 2}\right) \cdots(2)\)…(2)
[where \(m_i=\) mass of the i th particle]

Now, \(\left(x_{\mathrm{cm}}^2+y_{\mathrm{cm}}^2\right)=d^2 and \sum m_i=M\).
Again \(\frac{\sum_i m_i x_i^{\prime}}{\sum_i m_i}\) and \(\frac{\sum_i m_i y_i^{\prime}}{\sum_i m_i}\) are x and y-coordinates respectively of the mean position of the particles of mass \(m_i\) about the centre of mass of the body.
∴ \(\frac{\sum_i m_i x_i^{\prime}}{\sum_i m_i}=0=\frac{\sum_i m_i y_i^{\prime}}{\sum_i m_i}\)
Hence, \(\sum_i m_i x_i{ }^{\prime}=0=\sum_i m_i y_i{ }^{\prime}\)
The last term of equation (2) is the moment of inertia of the body about A B.
Hence, \(\sum_i m_i\left(x_i^{\prime 2}+y_i^{\prime 2}\right)=I_{\mathrm{cm}}\)
∴ I = \(I_{\mathrm{cm}}+M d^2\)
System of particles motion
2. Perpendicular-Axes Theorem: The moment of inertia (IZ) of a plane lamina about an axis perpendicular to its plane is equal to the sum of the moments of inertia (Ix + Iy) of the lamina about two mutually perpendicular axes lying on the plane of the lamina and intersecting each other at the point through where the perpendicular axis passes.
The mathematical form of the theorem, Ix + Iy = Iz ….(3)
WBCHSE Class 11 Physics System Of Particles Motion
Perpendicular-Axes Theorem Explanation: Let the plane lamina be composed of an infinite number of particles and the masses of the particles are m1, m2, m3,…… Let the distance of the particle of mass m1 from the axes x, y and z be y1, x1 and d1 respectively.

Now, the moments of inertia of the particle of mass m1 about x, y and z-axes, \(I_{x_1}=m y_1^2, I_{y_1}=m_1 x_1^2, I_{z_1}=m_1 d_1^2\)
Therefore, the moments of inertia of the whole lamina about x, y and z-axes,
⇒ \(I_x=\sum_i m_i y_i^2, I_y=\sum_i m_i x_i^2, I_z=\sum_i m_i d_i^2\)
∴ \(I_x+I_y=\sum_i m_i\left(x_i^2+y_i^2\right)=\sum_i m_i d_i^2=I_z\)
It is to be noted that this theorem of perpendicular axes is applicable only for plane sheets of small thicknesses.
Determination Of Moment Of Inertia Of Some UniForm Symmetrical Objects:
1. Moment Of Inertia Of A Uniform Rod About The Perpendicular Axis To Its Length Passing Through Its Centre Of Mass: Let PQ be a uniform rod of mass m and length l. The centre of mass is at the midpoint O of the rod.
Considering O as the origin (0,0) and the x-axis along the length of the rod, the position coordinates of the points P and Q are (-\(\frac{1}{2}\), o) and (\(\frac{1}{2}\), o) respectively. The moment of inertia about the axis CD passing through the point O and perpendicular to the rod is to be determined.
Mass per unit length of the rod = \(\frac{m}{l}\)
Let us consider a small segment dx which is at a distance x from point O.

So, the mass of length dx = (\(\frac{m}{l}\)dx)
Moment of inertia of this small segment dx about CD = (\(\frac{m}{l}\)dx)x²
Hence, the moment of inertia of the whole rod about CD,
⇒ \(I_{C D}=\int_{-l / 2}^{L / 2} \frac{m}{l} x^2 d x=\frac{m}{l}\left[\frac{x^3}{3}\right]_{-\frac{l}{2}}^{\frac{l}{2}}\)
= \(\frac{m}{3 l}\left[\left(\frac{l}{2}\right)^3-\left(-\frac{l}{2}\right)^3\right]\)
= \(\left(\frac{m}{3 l} \cdot \frac{3}{4}\right)=\frac{1}{12} m l^2\)
WBCHSE Class 11 Physics System Of Particles Motion
2. Moment Of Inertia Of A Uniform Rod About The Perpendicular Axis To Its Length Passing Through One End Of The Rod (Application Of Parallel-Axes Theorem): Suppose, the mass of the rod = m, length of the rod = l. Moment of inertia of A the rod about the axis CD passing through its centre of mass and perpendicular to its length, ICD = \(\frac{1}{12}\)ml²

Now by the parallel axes theorem we can write,
⇒ \(I_{A B}=I_{C D}+m\left(\frac{l}{2}\right)^2\)
= \(\frac{1}{12} m l^2+\frac{1}{4} m l^2=\frac{1}{3} m l^2\)….(1)
3. Moment Of Inertia Of A Uniform Rectangular Lamina About An Axis Parallel To Its Length And Breadth Passing Through Its Centre Of Mass: Suppose, the mass of the lamina = m, length = l, breadth = b. The centre of mass of the lamina is O. The moment of inertia of the lamina about CD parallel to its breadth and passing through O is to be determined.
The mass per unit area of the rectangular lamina = \(\frac{m}{l b}\). Let us imagine a small rectangular strip of width dr at a distance r from CD

Area of this strip = bdr
Mass of this strip = bdr = \(\frac{m}{l b}\)bdr = \(\frac{m}{l}\)dr
Therefore, moment of inertia of the whole lamina about the axis parallel to its breadth and passing through the centre of mass,
Similarly, moment of inertia of the lamina about an axis parallel to its length and passing through the centre of mass, Iy = \(\frac{1}{12}\)mb²
System of particles motion
4. Moment Of Inertia Of A Uniform Rectangular Lamina About An Axis Perpendicular To Its Plane Passing Through Its Centre Of Mass (Application Of Perpendicular-Axes Theorem): Suppose, the mass of the lamina = m; length of the lamina = l; breadth of the lamina = b
Suppose O be the centre of mass of the lamina. OX and OY are the two axes lying on the plane of the lamina, mutually perpendicular to each other. The axis OZ is perpendicular to the plane of the lamina.
WBCHSE Class 11 Physics System Of Particles Motion
We know, the moment of inertia of the lamina about an axis passing through its centre of mass and parallel to its length, Ix = \(\frac{1}{12}\)mb²

The moment of inertia of the lamina about an axis passing through its centre of mass and parallel to its breadth, Iy = \(\frac{1}{12}\)ml²
Now, by the perpendicular-axes theorem we can write, \(I_z =I_x+I_y=\frac{1}{12} m b^2+\frac{1}{12} m R^2\)
= \(\frac{1}{12} m\left(b^2+R^2\right)\)….(2)
5. Moment Of Inertia Of A Ring About An Axis Passing Through Its Centre And Perpendicular To The Plane Of The Ring: The mass of the circular ring is m and the radius of the ring is r, whose centre is O. The moment of inertia about AB passing through the point O and perpendicular to the plane of the ring to be calculated.
Let us imagine a small element of length dx on the circumference of the ring.

Mass per unit length of the ring = \(\frac{m}{2 \pi r}\)
Mass of that small element = \(\frac{m}{2 \pi r}\) dx
The moment of inertia of the element of length dx about AB = \(\left(\frac{m}{2 \pi r} d x\right) r^2=\frac{m r}{2 \pi} d x\)
∴ The moment of inertia of the ring about AB, \(I=\int_0^{2 \pi r} \frac{m r}{2 \pi} d x=\frac{m r}{2 \pi}[2 \pi r-0]=m r^2\)
6. Moment Of Inertia Of A Ring About Its Diameter (Application Of Perpendicular Axes Theorem): Let AB and CD be the axes along two mutually perpendicular diameters of the ring.

Now, by the theorem of perpendicular axes we can write, a moment of inertia of the ring about the axis AB + moment of inertia of the ring about the axis CD = moment of inertia of the ring about an axis through the centre of the ring O) and perpendicular to its plane,
i.e., IAB + ICD = mr² [where m = mass of the ring, r = radius of the ring]
For symmetry of the ring, IAB + ICD = I (say)
∴ I + I = mr² or, I = \(\frac{m r^2}{2}\) …..(3)
So, moment of inertia of a ring about its diameter = \(\frac{m r^2}{2}\)
WBCHSE Class 11 Physics Rotation Of Rigid Bodies
7. Moment Of Inertia Of A Circular Disc About An Axis Passing Through Its Centre And Perpendicular To The Plane Of The Disc: P is a circular disc of mass m and radius r with centre O. The moment of inertia about AB passing through the point O and perpendicular to the plane of the disc is to be calculated.

Mass per unit area of the disc = \(\frac{m}{\pi r^2}\)
Let us imagine an annular ring of width dx at a distance x (x < r) from the centre of the disc.
Area of this annular ring = \(\left(\frac{m}{\pi r^2}\right) 2 \pi x d x=\frac{2 m}{r^2} x d x\)
Therefore, a moment of inertia of this annular ring about
AB = \(\left(\frac{2 m}{r^2} x d x\right) x^2=\frac{2 m}{r^2} x^3 d x\)
Hence, moment of inertia of the whole disc about AB,
I = \(\int_0^r \frac{2 m}{r^2} x^3 d x=\frac{2 m}{r^2}\left[\frac{x^4}{4}\right]_0^r\)
= \(\frac{2 m}{4 r^2}\left[r^4-0\right]=\frac{m r^2}{2}\)
8. Moment Of Inertia Of A Circular Disc About Its Diameter (Application Of Perpendicular-Axes Theorem): Since the disc is symmetrical with respect to all diameters, its moment of inertia about every diameter is the same.
System of particles motion
Let AB and CD be the axes along two mutually perpendicular diameters of the circular disc.

Now, by the perpendicular-axes theorem, we can write, a moment of inertia of the disc about the axis AB + moment of inertia of the disc about the axis CD = moment of inertia of the disc about an axis through the centre of the disc O and perpendicular to its plane,
i.e., IAB + ICD = \(\frac{m r^2}{2}\)
[where, m = mass of the circular disc, r = radius of the circular disc]
For symmetry of the disc IAB + ICD = I (say)
∴ I+I= \(\frac{m r^2}{2}\)
or, \(I=\frac{m r^2}{4}\)……(4)
So, moment of inertia of a circular disc about its diameter = \(\frac{3 r^2}{4}\)
WBCHSE Class 11 Physics Rotation Of Rigid Bodies
9. Moment Of Inertia Of A Circular Disc About A Tangent In The Plane Of The Disc (Application Of Parallel-Axes Theorem): Let CD be a tangent in the plane of the circular disc and AB be an axis along the diameter parallel to CD.
Let the mass of the disc be m and its radius is r.

By parallel-axes theorem, we can write, a moment of inertia of the disc about CD = moment of inertia of the disc about AB+mr²
i. e., \(I_{C D}=I_{A B}+m r^2\)
= \(\frac{m r^2}{4}+m r^2\)
= \(\frac{5}{4} m r^2\)…(5)
So, the moment of inertia of a circular disc about a tangent on the plane of the disc = \(\frac{5}{4}\)mr².
10. Moment Of Inertia Of A Circular Disc About A Tangent Perpendicular To The Plane Of The Disc (Application Of Parallel-Axes Theorem): Let CD be a tangent to the circular disc perpendicular to its plane and AB be an axis passing through the centre O of the disc and parallel to CD.

Let the mass of the disc be m and its radius is r.
By parallel-axes theorem, we can write, a moment of inertia of the disc about CD = moment of inertia of the disc about AB+mr²
i.e., \(I_{C D}=I_{A B}+m r^2\)
= \(\frac{m r^2}{2}+m r^2\)
= \(\frac{3}{2} m r^2\)….(6)
So, the moment of inertia of a circular disc about a tangent perpendicular to its plane = \(\frac{3}{2}\)mr².
System of particles motion

WBCHSE Class 11 Physics Rotation Of Rigid Bodies

WBCHSE Class 11 Physics Rotation Of Rigid Bodies

Radius Of Gyration: Notice from the above table, that in all cases moment of inertia of an extended body rotating about a specific axis depends not on total mass but on the mass distribution of the body from that very axis.
We shall now find a measuring way in which the mass of a rotating rigid body is related to the moment of inertia. For this, a new parameter, the radius of gyration (it) is introduced.
We notice that in all cases Moment of inertia can be expressed as I = Mk² form, where k has the dimension of length. ‘ k’ is a geometric property of the body and axis of rotation.
We know that if a point mass M is at a distance k from the axis of rotation, its moment of inertia, I = Mk². From this, we can define radius of gyration.
∴ k = \(\sqrt{\frac{I}{M}}\)
∴ I = Mk²
Radius Of Gyration Definition: If the whole mass of a body is assumed to be concentrated at a point such that the moment of inertia of the whole body equals the moment of inertia of that point, then the radial distance of the point from the axis of rotation is called the radius of gyration.
Radius Of Gyration Example: Moment of inertia of a solid sphere about its diameter is, I = \(\frac{2}{5}\) Mr². So, its radius of gyration with respect to its diameter,
k = \(\sqrt{\frac{\frac{2}{5} M r^2}{M}}=\sqrt{\frac{2}{5}} r\)
WBCHSE Class 11 Physics Rotation Of Rigid Bodies
Rotational Kinetic Energy
Let PQR be a rigid body, revolving about the axis AB with angular velocity co. Due to this motion, the body possesses some kinetic energy. This kinetic energy is called rotational kinetic energy.
The rigid body can be assumed as an aggregate of a number of particles. Let the masses of the particles be m1, m2, m3,….., etc., at distances r1, r2, r3,….., etc., respectively from the axis of rotation AB. Since the body is rigid, the angular velocity of the constituent particles is the same, i.e., ω. However, due to the difference in their distances from the axis of rotation, the linear velocities of different particles are different.
Let the linear velocity of the particle of mass m1 be v1
So, v1 = ωr1
System of particles motion
So, the kinetic energy of the particle of mass \(=\frac{1}{2} m_1 v_1^2=\frac{1}{2} m_1 \omega^2 r_1^2\)
Similarly, the kinetic energy of the particle of mass = \(\frac{1}{2} m_2 v_2^2=\frac{1}{2} m_2 \omega^2 r_2^2\) and so on.
In this way, adding the kinetic energies of all particles, the kinetic energy of the entire rigid body is obtained.
So, the rotational kinetic energy of the body
= \(\frac{1}{2} m_1 \omega^2 r_1^2+\frac{1}{2} m_2 \omega^2 r_2^2+\frac{1}{2} m_3 \omega^2 r_3^2+\cdot\)
= \(\frac{1}{2} \omega^2\left(m_1 r_1^2+m_2 r_2^2+m_3 r_3{ }^2+\cdots\right)\)
= \(\frac{1}{2} \omega^2 \sum_i m_i r_i^2\) = \(\frac{1}{2} \omega^2 I\) (where, \(I=\sum_i m_i r_i^2\))
[ri = the perpendicular distance of i-th particle from the axis of rotation, mi = mass of the i -th particle of the rigid body] = \(\frac{1}{2}\) Iω²
Here, I = \(\sum_i m_i r_i^2\) = moment of inertia of the body about the axis of rotation.
Comparing torque with force, it is seen that the moment of inertia in rotational motion plays the same role as that played by the mass in translational motion. Now, it is also seen that the same conclusion can be drawn by comparing the translational kinetic energy \(\frac{1}{2}\)mv² with the rotational kinetic energy \(\frac{1}{2}\) Iω².
WBCHSE Class 11 Physics Rotation Of Rigid Bodies
Relation Between Rotational Kinetic Energy With Work Done: Let a force F on a body of mass m cause a change in its kinetic energy (Δk) only. If the body under this force is displaced linearly from the initial position x1 to the final position x2, then work done by the force,
W = \(\int_{x_1}^{x_2} F d x\)
According to the work-kinetic energy theorem, Δk = W
i.e., \(\frac{1}{2} m v_2^2-\frac{1}{2} m v_1^2=\int_{x_1}^{x_2} F d x\)….(1)
[where v1 = initial speed, v2 = final speed]
Now consider a torque z on a body of moment of inertia I (about a certain axis of rotation) causes a change in its rotational kinetic energy (Δkr) only. If the body under this torque is displaced from the initial angular position θ1 to the final angular position θ2 by the torque,
W = \(\int_{\theta_1}^{\theta_2} \tau d \theta\)
Just like above equation (1) now we can relate work and rotational kinetic energy as below: Δkr = W
i.e., \(\frac{1}{2} I \omega_1^2-\frac{1}{2} I \omega_2^2=\int_{\theta_1}^{\theta_2} \tau d \theta\)….(2)
[where ω1 = initial angular speed, ω2 = final angular speed] when τ is constant, W = τ(θ2 – θ1)
Therefore, power, i.e., the rate at which the work is done, P = \(\frac{W}{dt}\) = τω
WBCHSE Class 11 Physics Rotation Of Rigid Bodies
Angular Momentum
The rotational analogues of the mass (m) of a body and its linear velocity (v) are moment of inertia (I) and angular velocity (ω), respectively. Hence, the rotational analogue of the linear momentum (mv) of the body is Iω. This physical quantity is called the angular momentum (L) of the body.
Angular Momentum Definition: The dynamical property generated in a body under rotational motion, due to the moment of inertia about an axis and angular velocity, is called the angular momentum of the body about that axis.
Angular momentum is measured by the product of moment of inertia and angular velocity, i.e., L = Iω.
Since I is a scalar and ω is an axial vector, angular momentum L is also an axial vector whose direction is along the axis of rotation, and in the direction of ω.
Unit And Dimension Of Angular Momentum:
CGS System: g · cm² · s-1
SI: kg · m² · s-1
Dimension of L = dimension of I x dimension of ω = ML² x T-1 = ML²T-1
Relation Between Linear Momentum And Angular Momentum: Suppose a body is revolving with an angular velocity ω about an axis. If m1, m2, m3,…. are the constituent particles of that body and they are at distances r1, r2, r3,…. respectively from the axis of rotation, then the moment of inertia of the body,
I = \(m_1 r_1^2+m_2 r_2^2+m_3 r_3^2+\cdots=\sum_i m_i r_i^2\)
In the case of pure rotation, the angular velocity of each particle becomes equal to the angular velocity of the body.
So, the angular momentum of the body,
L = \(I \omega=\sum_i m_i r_i^2 \cdot \omega=\sum_i m_i r_i v_i\) (because \(v_i=\omega r\))
= \(\sum_i r_i \times m_i v_i=\sum_i r_i \times p_i\)
[pi = mivi = linear momentum of i-th particle]
For the particles, the quantities r1 x m1v1, r2 x m2v2,…… etc., can be called the moments of linear momentum, or in brief, moments of momentum (in analogy with the moment of force).
So, the angular momentum of a body about an axis is the algebraic sum of the moments of linear momentum about the same axis, of all particles constituting the body.
Thus, for a particle rotating about a circle of radius r and having a linear momentum p, the angular momentum will be L = rp.
Vector Representation: The vector representation for the relation between linear and angular momentum is \(\vec{L} = \vec{r} \times \vec{p}\). This is often referred to as the defining equation of \(\vec{L}\).
WBCHSE Class 11 Physics Rotation Of Rigid Bodies
We know the vector representation for the relation between linear velocity and angular velocity is \(\vec{v}=\vec{\omega} \times \vec{r}\).
If \(\vec{v}\) and \(\vec{\omega}\) are replaced by \(\vec{p}\) and \(\vec{L}\), respectively, the geometric form for the relation of \(\vec{L}\), \(\vec{p}\) and \(\vec{r}\) is obtained.
Relation Between Angular Momentum And Torque: In case of rotational motion, when a torque is applied to a body, an angular acceleration is produced in it. If the initial angular velocity of the body is ω1 and its angular velocity after time t is ω2, then the angular acceleration of the body,
α = \(\frac{\omega_2-\omega_1}{t}\)
Again, torque = moment of inertia x angular acceleration
or, \(\tau=I \alpha=I \times \frac{\omega_2-\omega_1}{t}=\frac{I \omega_2-I \omega_1}{t}\)
or, \(\tau t=I \omega_2-I \omega_1\)
Hence, torque x time = change in angular momentum of the body during that interval
This is the relation between torque and angular acceleration. From this relation, it is evident that a change in angular momentum takes place about the axis along which the torque acts on the body.
We know that in the case of translational motion, Ft = mv – mu and the rotational analogue of this equation is τt = Iω2 – Iω1. The quantity Ft is known as the impulse of force. Similarly, the quantity τt is known as the angular impulse or the impulse of torque.
Law Of Conservation Of Angular Momentum: Suppose the moment of inertia of a body changes from I1 to I2 in time t. In this case, the equation τt = Iω2 – Iω1 changes to τt = I2ω2 – I1ω1 Now, if no external torque acts on the body, i.e., if τ = 0, then from the equation, τt = I2ω2 – I1ω1 we get, I2ω2 – I1ω1 = 0, or, τt = I2ω2 = I1ω1
It means that the final angular momentum of the body is equal to its initial angular momentum, i.e., the angular momentum is conserved.
Law: if the net external torque on a body is zero, the angular momentum of the body rotating about an axis always remains conserved.
So, this law is nothing but the rotational analogue of the law of conservation of linear momentum.
Again we know, \(\frac{dL}{dT}\) = τext
From this, it is clear that, if total external torque acts on a body is zero; its angular velocity decreases with the increase of its moment of inertia and vice versa i.e., angular momentum remains constant.
WBCHSE Class 11 Physics Rotation Of Rigid Bodies
Related Experiments And Practical Examples:
1. A man is sitting on a turntable holding a pair of dumbbells of equal mass, one in each hand with his arms out-stretched while the turntable rotates with a definite angular velocity, If the man suddenly draws the dumbbells towards his chest, the speed of rotation of the turntable is found to increase.
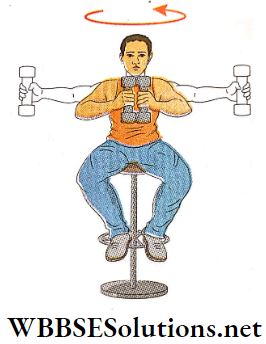
System of particles motion
- This is due to the fact that when the man draws the dumbbells towards his chest, the moment of inertia of the man about the axis of rotation decreases and his angular velocity increases due to conservation of angular momentum.
- If the man again stretches his arms, his angular velocity decreases due to an increase in moment of inertia, and the turntable consequently rotates slowly.
2. In a diving event, when a competitor dives from a high platform or springboard into water, he keeps his legs and arms outstretched and starts descending with less angular velocity, After that he curls his body by rolling the legs and arms inwards, his moment of inertia decreases.
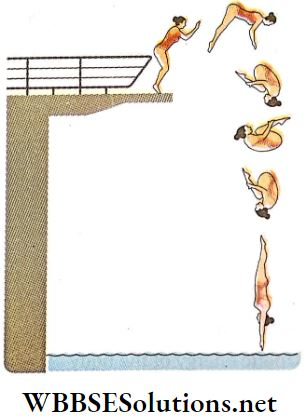
- As angular momentum is conserved, his angular velocity goes on increasing rapidly. As a result, his body begins to spin rapidly and before reaching the surface of the water, he can perform a good number of somersaults.
- In the case of skating on the surface of ice or during the performance of acrobatics, the principle of conservation of angular momentum can be applied in a similar way.
WBCHSE Class 11 Physics Rotation Of Rigid Bodies
Angular Momentum Numerical Examples
Example 1. If the radius of the earth decreases by \(\frac{1}{2}\)%, then what will be the change in the length of a day? Assume that the earth is a uniform sphere and its moment of inertia, I = \(\frac{2}{5}\)MR², where M and R are the mass and the radius of the earth.
Solution:
If the mass of a solid sphere remains unaltered, then its moment of inertia ∝ (radius)².
Here, the changed radius \(=\frac{100-\frac{1}{2}}{100} R=\frac{199}{200} R\).
So, if the moment of inertia of the earth for its present radius R is I and the moment of inertia for its changed radius is I’, then
⇒ \(\frac{I}{I^{\prime}}=\frac{R^2}{\left(\frac{199 R}{200}\right)^2}=\left(\frac{200}{199}\right)^2\)
If the present angular velocity of the earth is ω and its changed angular velocity is ω’, then according to the principle of conservation of angular momentum,
⇒ \(I \omega=I^{\prime} \omega^{\prime}\)
or, \(\omega^{\prime}=\frac{I \omega}{I^{\prime}}\)
or, \(\frac{2 \pi}{T^{\prime}}=\frac{I}{I^{\prime}} \times \frac{2 \pi}{T}\)
or, \(T^{\prime}=\frac{I^{\prime}}{I} \cdot T=\left(\frac{199}{200}\right)^2 \times 24=23.76 \mathrm{~h}\)
∴ The length of the day will decrease by (24-23.76) = 0.24 h = 14 min 24 s
System of particles motion
Example 2. A solid sphere of mass 1 kg and of radius 10 cm is rotating about one of its diameters with an angular; velocity of π rad · s-1. Calculate the kinetic energy of the sphere by using the relevant formula.
Solution:
Let the moment of inertia of the sphere about its diameter I = \(\frac{2}{5}\)MR², M = mass of the sphere and R = radius of the sphere.
The kinetic energy of the body = rotational kinetic energy of the body
= \(\frac{1}{2} I \omega^2=\frac{1}{2} \times \frac{2}{5} M R^2 \cdot \omega^2\)
= \(\frac{1}{5} \times 1000 \times(10)^2 \times \pi^2\)
= \(197392.09 \mathrm{erg} .\)
Example 3. A thin rod of length l and mass m per unit length is rotating about an axis passing through the midpoint of its length and perpendicular to it. Prove that its kinetic energy \(\frac{1}{24}\) mω2l3 = ω = angular velocity of the rod.
Solution:
Kinetic energy of the rod = \(\frac{1}{2}\) mω2
According to the problem,
I = \(\frac{1}{12}\)Ml² [M = mass of the rod = ml]
= \(\frac{1}{12}\) x ml x l² = \(\frac{m l^3}{12}\)
∴ Kinetic energy of the rod = \(\frac{1}{2} \times \frac{m l^3}{12} \times \omega^2=\frac{1}{24} m \omega^2 l^3 .\)
WBCHSE Class 11 Physics Rotation Of Rigid Bodies
Example 4. Calculate the moment of inertia of a solid cylinder of I length 10 cm and of radius 20 cm about its own axis. The density of the material of the cylinder = 9 g · cm-3.
Solution:
L= length of the cylinder, R = radius of the cylinder and M = mass of the cylinder
= volume of the cylinder x density
= πR²L X density
= π x (20)² x 10 x 9 g
Moment of inertia of a solid cylinder about its own axis,
I = \(\frac{1}{2} M R^2\)
I = \(\frac{1}{2} \times \pi \times(20)^2 \times 10 \times 9 \times(20)^2\)
= \(22.6 \times 10^6 \mathrm{~g} \cdot \mathrm{cm}^2\)
Example 5. A solid sphere of diameter 2 cm and of mass 20 g is rolling with a velocity of 3 cm · s-1. What is the total kinetic energy of the sphere?
Solution:
Let M = mass of the sphere, R = radius of the sphere, V = linear velocity of the sphere, I = \(\frac{2}{5}\)MR² (moment of inertia of the sphere about its diameter), ω = \(\frac{V}{R}\)
Total kinetic energy of the sphere = translational kinetic energy + rotational kinetic
= \(\frac{1}{2} M V^2+\frac{1}{2} I \omega^2=\frac{1}{2} M V^2+\frac{1}{2} \times \frac{2}{5} M R^2\left(\frac{V}{R}\right)^2\)
= \(\frac{1}{2} M V^2+\frac{1}{5} M V^2=\frac{7}{10} M V^2=\frac{7}{10} \times 20 \times(3)^2\)
= \(126 \mathrm{erg}\)
System of particles motion
Example 6. A stone of mass m tied with a thread Is rotating along a horizontal circular path (force of gravity is neglected). The length of the thread decreases gradually in such a manner that the angular momentum of the stone remains constant with respect to the centre of the circle. If the tension in the thread Is T = Arn, where A = constant, r = instantaneous radius of the circle, then find the value of n.
Solution:
If the instantaneous angular velocity of the stone is w, then angular momentum,
L = Iω = mr²ω = constant (according to the problem)
or, ω = \(\frac{L}{m r^2}\)
Here the tension in the thread provides the necessary centripetal force for rotation.
So, T = \(A r^n=m \omega^2 r=m \cdot \frac{L^2}{m^2 r^4} r=\frac{L^2}{m} r^{-3}\)
= \(A r^{-3} \quad\left(A=\frac{L^2}{m}=\text { constant }\right)\)
∴ n=-3 .
WBCHSE Class 11 Physics Rotation Of Rigid Bodies
Example 7. Two ends of a uniform rod weighing W, are placed on supports so that the rod remains horizontal. If a support at one end is suddenly removed, what will be the force exerted on the horizontal rod by the support at the other end?
Solution:
Let the length of the rod = l cm, its weight = W = Mg, where M is the mass of the rod. When the support at one end is removed suddenly, the centre of gravity of the rod falls downwards with an acceleration a. Let R = reaction force at the end with the support. Hence, if the C.G. now falls with an acceleration a, the rod will turn about the point P.
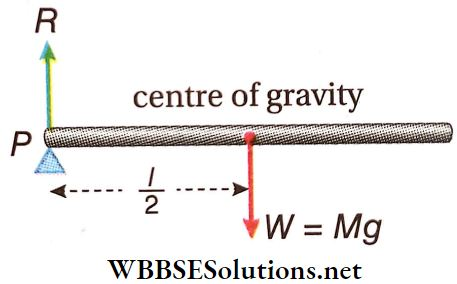
The torque on the rod = Mg · \(\frac{l}{2}\)
Also, Mg – R = Ma or, \(a=\frac{M g-R}{M}\)
Here moment of inertia, I = \(\frac{1}{3}\)Ml² = moment of inertia of the rod about the perpendicular axis passing through the end of the rod and the angular acceleration, α = \(\frac{a}{V / 2}=\frac{2 a}{l}\)
∴ \(\frac{1}{3} M l^2 \alpha=M g \frac{l}{2}\) (because \(\tau=I \alpha\))
or, \(\frac{1}{3} M R^2 \cdot \frac{2 a}{l}=M g \frac{l}{2} \text { or, } \frac{2}{3} a=\frac{g}{2} \text { or, } \frac{2}{3}\left(\frac{M g-R}{M}\right)=\frac{g}{2}\)
R = \(\frac{M g}{4}=\frac{W}{4}\)
Therefore, when one support is removed, the support at the other end will exert a reaction force of \(\frac{W}{4}\)
System of particles motion
Example 8. A rod of length L and M is attached with a hinge on a wall at point O. After releasing the rod from its vertical position OA, when it comes to position OA’, what is the reaction on point O of the rod by the hinge?
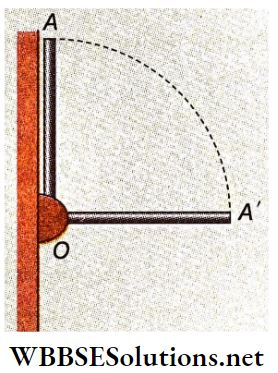
Solution:
Let, the angular velocity of the rod at the horizontal position OA’ is ω.
∴ At that instant its kinetic energy = \(\frac{1}{2} I \omega^2=\frac{1}{2} \cdot \frac{M L^2}{3} \cdot \omega^2=\frac{M L^2 \omega^2}{6}\)
The centre of mass of the rod shifts down by \(\frac{L}{2}\) from OA to OA’.
So, decrease in potential energy of the rod = Mg\(\frac{L}{2}\)
According to the kinetic energy conservation law, \(M g \frac{L}{2}=\frac{M L^2 \omega^2}{6} \quad \text { or, } \omega=\sqrt{\frac{3 g}{L}}\)…(1)
WBCHSE Class 11 Physics Rotation Of Rigid Bodies
Two forces act on the rod at position OA’
- Gravitational force (Mg) vertically downward direction and
- Reaction force (n) of the hinge
Let, the horizontal and the vertical n components of n are nx and ny respectively; the horizontal and the vertical components of the acceleration of the centre of mass of the rod area ax and ay respectively.
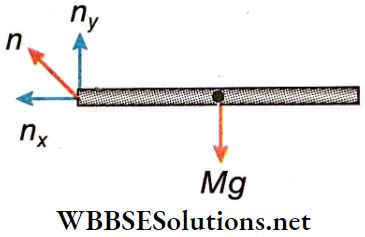
∴ According to \(M g-n_y=M a_y\)….(2)
and \(n_x=M a_x=M \omega^2 \cdot \frac{L}{2}\)
(because \(a_x=\) centripetal acceleration)
= \(M \cdot \frac{3 g}{L} \cdot \frac{L}{2}=\frac{3}{2} M g\)
[putting the value of ω from equation (1)]
The rod starts to rotate due to the action of torque created by ny and Mg.
If the angular acceleration of the rod is α, \(M g \cdot \frac{L}{2}=I \alpha=\frac{M L^2}{3} \alpha\)
∴ \(\alpha=\frac{3 g}{2 L}\)
The acceleration along the vertical direction, \(a_y=\frac{L}{2} \alpha=\frac{3 g}{4}\)
Putting the value of ay in equation (2) we get, \(M g-n_y=\frac{3 M g}{4} \text { or, } n_y=\frac{M g}{4}\)
∴ n = \(\sqrt{n_x^2+n_y^2}=\sqrt{\left(\frac{3}{2} M g\right)^2+\left(\frac{M g}{4}\right)^2}=\frac{\sqrt{37}}{4} M g\)
System of particles motion
WBCHSE Class 11 Physics Rotation Of Rigid Bodies
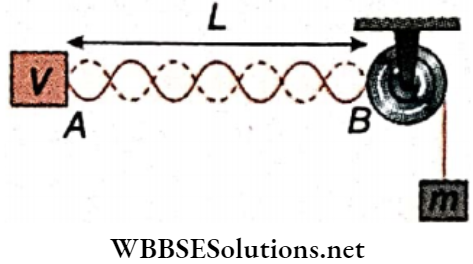
Motion of A Mass Suspended From A Rope Wrapped Around A Solid Cylinder
Let a solid cylinder of mass M and radius R is kept in such a way that it can rotate freely about its axis XX’. A mass m is suspended from a rope wrapped around the cylinder. It is then released from rest and for this, the cylinder begins to rotate about XX’.
Two forces act on the suspended mass m
- Its weight (vertically downward) and
- Tension of the rope T (vertically upward).
1. Acceleration Of The Attached Mass m: If the linear acceleration directed downward of mass m is a, then, mg – T = ma…..(1)

If the moment of inertia and angular acceleration about the axis of rotation is I and α respectively.
⇒ \(\tau=I \alpha=T R\)
∴ \(I \frac{a}{R}=T R \quad \text { or, } T=\frac{I a}{R^2}\)
From equation (1) we get, \(m a=m g-\frac{I a}{R^2}\)
or, \(m a+\frac{I a}{R^2}=m g\) or, \(a\left[m+\frac{l}{R^2}\right]=m g\)
or, \(a=\frac{g}{1+\frac{l}{m R^2}}\)….(2)
The moment of inertia about the axis of the cylinder.
I = \(\frac{1}{2} M R^2\)
∴ \(a=\frac{g}{1+\frac{1}{2} \frac{M R^2}{m R^2}}=\frac{g}{1+\frac{M}{2 m}}\)
2. Angular Acceleration Of The Cylinder: We know the angular acceleration
= \(\frac{\text { linear acceleration }}{\text { radius }}\)
Hence, \(\alpha=\frac{a}{R}\)
From equation (2) we get., \(\alpha=\frac{g}{1+\frac{1}{m R^2}}\)
3. Tension Of The Thread: From equation (1) we get,
T = \(m g-m a=m g-\frac{m g}{1+\frac{1}{m R^2}}\)
= \(m g\left[1-\frac{1}{1+\frac{1}{m R^2}}\right]=m g\left[\frac{\frac{l}{m R^2}}{1+\frac{l}{m R^2}}\right]=\frac{m g}{\frac{m R^2}{l}+1}\)
The moment of inertia about the axis of the cylinder,
I = \(\frac{1}{2} M R^2\)
∴ \(T=\frac{m g}{\frac{2 m R^2}{M R^2}+1}=\frac{m g}{1+\frac{2 m}{M}}\)
System of particles motion
WBCHSE Class 11 Physics Rotation Of Rigid Bodies
Mixed Motion
If a body undergoes translation and rotation simultaneously, then its motion is called a mixed motion. As for exam¬ ple, we will now discuss the rolling of a body without slipping.
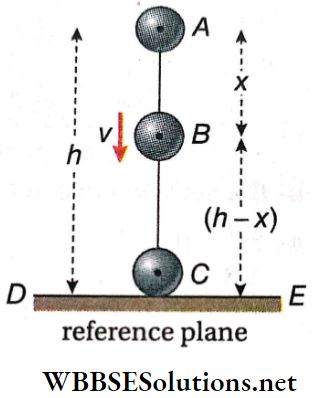
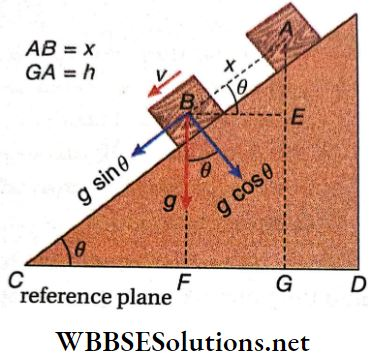
Downward Rolling Of A Body Without Slipping On An Inclined Plane: Let a body (say a cylinder or a sphere) of mass M and radius R roll down a plane without slipping inclined at an angle θ with the horizontal. We shall now find an expression for the linear acceleration (a) of the centre of mass of the body rolling down the plane. After that, the value of friction will also be calculated.
1. Linear Acceleration Of The Centre Of Mass Of The Body: Different forces with their components are shown.
Applying Newton’s 2nd law of motion along the inclined plane, we get, Mgsinθ – f = Ma…(1)
Here f is the static friction which generates torque and the angular acceleration. If the moment of inertia about an axis passing through the centre of mass are I and α respectively, then the torque acting on the body,

⇒ \(\tau=I \alpha=f R\)
∴ f = \(\frac{I \alpha}{R}=\frac{I a}{R^2}\)
(because \(\alpha=\frac{a}{R}\))
From the equation we get, \(M g \sin \theta-\frac{I a}{R^2}=M a\)
or, \(g \sin \theta=a+\frac{I a}{M R^2} or, a=\frac{g \sin \theta}{1+\frac{I}{M R^2}}\)
Special cases: In case of solid cylinder, I = \(\frac{1}{2} M R^2\)
∴ a = \(\frac{g \sin \theta}{1+\frac{\frac{1}{2} M R^2}{M R^2}}=\frac{2}{3} g \sin \theta\)
In case of solid sphere, I = \(\frac{2}{5} M R^2\)
∴ a = \(\frac{g \sin \theta}{1+\frac{\frac{2}{5} M R^2}{M R^2}}=\frac{5}{7} g \sin \theta\)
In the case of a hollow cylinder, I = MR²
∴ a = \(\frac{g \sin \theta}{1+\frac{M R^2}{M R^2}}=\frac{1}{2} g \sin \theta\)
In case of hollow sphere, \(I=\frac{2}{3} M R^2\)
∴ a = \(\frac{g \sin \theta}{1+\frac{\frac{2}{3} M R^2}{M R^2}}=\frac{3}{5} g \sin \theta\)
2. Friction acting on the body: From equation (2) we get, f = \(\frac{I a}{R^2}\)
Special cases: In the case of solid cylinders, \(f=\frac{1}{2} M R^2 \times \frac{1}{R^2} \times \frac{2}{3} g \sin \theta=\frac{1}{3} M g \sin \theta\)
WBCHSE Class 11 Physics Rotation Of Rigid Bodies
In the case of solid spheres,
∴ \(f=\frac{2}{5} M R^2 \times \frac{1}{R^2} \times \frac{1}{2} g \sin \theta=\frac{1}{2} M g \sin \theta\)
In the case of hollow cylinders,
∴ \(f=M R^2 \times \frac{1}{R^2} \times \frac{1}{2} g \sin \theta=\frac{1}{2} M g \sin \theta\)
In the case of hollow spheres,
∴ \(f=\frac{2}{3} M R^2 \times \frac{1}{R^2} \times \frac{3}{5} g \sin \theta=\frac{2}{5} M g \sin \theta\)
System of particles motion
WBCHSE Class 11 Physics Rotation Of Rigid Bodies
Comparison Between Linear And Rotational Motions
In the discussion of rotational motion, some physical quantities and numerical formulae, that we have already dealt with, are the rotational analogues of some physical quantities and numerical formulae of linear motion. These quantities and formulae are given below:

Unit 5 Motion Of System Of Particles And Rigid Body Chapter 2 Rotation Of Rigid Bodies
Linear And Rotational Motions Numerical Examples
Example 1. A particle of mass m is projected at an angle of 45° with the horizontal. At the highest point of its motion (h), what will be its angular momentum; concerning the point of projection?
Solution:
At any point, the horizontal component of the velocity of the particle = \(v_x=v \cos 45^{\circ}=\frac{v}{\sqrt{2}}\); the vertical velocity at the highest point = 0
If the time taken by the particle to reach the highest point is t, then 0 = vsin45°- gt
or, t = \(\frac{\nu}{\sqrt{2} g}\)[initial vertical velocity = vsin45°]…(1)
If the maximum height attained is h, then
h = \(\nu \sin 45^{\circ} \cdot t-\frac{1}{2} g t^2=\frac{v}{\sqrt{2}} \cdot \frac{\nu}{\sqrt{2} g}-\frac{1}{2} g \cdot \frac{v^2}{2 g^2}\)
= \(\frac{v^2}{2 g}-\frac{v^2}{4 g}=\frac{v^2}{4 g}\)….(2)
∴ Angular momentum of the particle with respect to the point of projection
= \(m v_x \times h=\frac{m \nu}{\sqrt{2}} \cdot \frac{v^2}{4 g}=\frac{m v^3}{4 \sqrt{2} g}\)
From equation (2) we get, \(v^2=4 g h \text { or, } v=2 \sqrt{g h}\)
∴ The angular momentum of the particle about the point of projection.
= \(\frac{m}{4 \sqrt{2} g} \cdot 8 \cdot(g h)^{3 / 2}=m h \sqrt{2 g h} .\)
System of particles motion
Example 2. Initially, a sphere of radius r is rotating with an angular velocity ω about its own horizontal axis. When the sphere falls on a surface (coefficient of friction μ), it begins to skid first and then starts rotating without skidding.
- What will be the final linear velocity of its centre of mass?
- How much distance will the sphere cover before reaching this velocity?
Answer:
1. Let the mass of the sphere be m. Then its moment of inertia about the axis of rotation, I = \(\frac{2}{5} m r^2\)

The moment of frictional force (μmg) resists the rotational motion of the sphere. If the angular retardation is α, then \(\mu m g r=I \alpha=\frac{2}{5} m r^2 \alpha\)
or, \(\alpha=\frac{5 \mu g}{2 r}\)…(1)
Due to this, if the angular velocity of the sphere becomes ω’ in time t, then \(\omega^{\prime}=\omega-\alpha t=\omega-\frac{5 \mu g t}{2 r}\)
The speed of a rotating point on the upper surface of the sphere, \(v^{\prime}=\omega^{\prime} r=\left(\omega-\frac{5 \mu g t}{2 r}\right) r\)…..(2)
Again, due to frictional force μmg, if the sphere skids over the surface with an acceleration a, then μmg = ma or, a = μg
∴ The linear velocity of the centre of mass of the sphere in time t, v = 0 + at= μgt…..(3)
The condition of rotational motion of the sphere without skidding is, v = v’. If the values of these two velocities become the same in time t, the sphere will undergo pure rotation.
From equations (2) and (3) we get, \(\mu g t =\left(\omega-\frac{5 \mu g t}{2 r}\right) r=\omega r-\frac{5 \mu g t}{2}\)
or, \(\frac{7}{2} \mu g t =\omega r \quad \text { or, } \quad t=\frac{2 \omega r}{7 \mu g}\)
∴ From equation (3) we get, \(\nu=\mu g \times \frac{2 \omega r}{7 \mu g}=\frac{2}{7} \omega r.\)
2. Distance covered, x = \(\frac{1}{2} a t^2=\frac{1}{2} \mu g\left(\frac{2 \omega r}{7 \mu g}\right)^2=\frac{2}{49} \cdot \frac{r^2 \omega^2}{\mu g} .\)
System of particles motion
Example 3. A small sphere of radius r at rest begins to slide down the surface of a hemispherical bowl from the brim of the bowl. When the sphere reaches the bottom of the bowl, what fraction of its total energy will be converted into translational kinetic energy and what fraction into rotational kinetic energy?
Solution:
Let the initial position of the small sphere be A
The velocity of the sphere, when it reaches the point B = V
∴ At the point B, translational kinetic energy of the sphere = \(K_t=\frac{1}{2} m V^2\) and rotational kinetic energy of the sphere
⇒ \(K_r =\frac{1}{2} I \omega^2\)
= \(\frac{1}{2} \times \frac{2}{5} m r^2 \times \omega^2\)
= \(\frac{1}{5} m V^2\)

∴ Total energy of the sphere, \(K =K_t+K_r\)
= \(\frac{1}{2} m V^2+\frac{1}{5} m V^2=\frac{7}{10} m V^2\)
∴ The ratio of the translational kinetic energy to the total kinetic energy, \(\frac{K_t}{K}=\frac{\frac{1}{2} m V^2}{\frac{7}{10} m V^2}=\frac{5}{7} \).
Again, the ratio of the rotational kinetic energy to the total kinetic energy, \(\frac{K_r}{K}=\frac{\frac{1}{5} m V^2}{\frac{7}{10} m V^2}=\frac{2}{7}\)
So, \(\frac{5}{7}\) part of the total energy will be converted into translational kinetic energy and \(\frac{2}{7}\) part into rotational kinetic energy.
Unit 5 Motion Of System Of Particles And Rigid Body Chapter 2 Rotation Of Rigid Bodies Useful Relations For Solving Examples
Torque, \(\vec{tau}\) (\(\vec{r} \times \vec{F}\) = (\(\vec{F}\) = force applied on the body, \(\vec{r}\) = position vector of the point of application of the force with respect to the origin)
Torque (τ) = moment of inertia (I) x angular acceleration (α); here, I = \(\sum_i m_i r_i^2\) = moment of inertia of the body about its axis of rotation.
Work done by the couple, W = τ · θ = torque x angular displacement
For n complete revolutions, work done by a couple, W = 2πnx torque
If the mass of an extended body is M and its moment of inertia about any axis of rotation is I, the radius of gyration,
K = \(\sqrt{\frac{I}{M}} \quad \text { or, } \quad I=M k^2\)
Rotational kinetic energy of a body = \(\frac{1}{2}\)Iω²
Angular momentum (I) = moment of inertia (I) x angular velocity (ω)
System of particles motion
If a particle revolves along a circular path of instantaneous radius vector \(\vec{r}\) and if the linear momentum of the particle is \(\vec{p}\), then the angular momentum of the particle, \(\vec{L} = \vec{r} \times \vec{p}\)
Torque x time = change in angular momentum of the body during that time = angular impulse or impulse of torque
∴ \(\vec{\tau}_{\text {ext }}=\frac{d \vec{L}}{d t}\)
The rate of change of angular momentum of a body is equal to the external torque acting upon the body.
Power, P = τω = torque x angular velocity
The total kinetic energy of rolling = translational kinetic energy+rotational kinetic energy
= \(\frac{1}{2}\)mv² + \(\frac{1}{2}\)Iω²
Unit 5 Motion Of System Of Particles And Rigid Body Chapter 2 Rotation Of Rigid Bodies Very Short Answer Type Questions
Question 1. What is the unit of angular momentum?
Answer: g · cm2 · s-1
Question 2. State whether the length of a day will increase or decrease if the radius of the earth becomes half of its present value keeping its mass constant.
Answer: Decrease
Question 3. What is the dimension of angular momentum?
Answer: ML²T-1
Question 4. What is the vector relation of linear momentum and angular momentum?
Answer: \(\vec{L}=\vec{r} \times \vec{p}\)
Question 5. A girl is standing at the centre of a rotating horizontal platform with her hands drawn inwards. What will happen if she stretches her hands horizontally?
Answer: The platform will rotate slowly
Question 6. Write down the dimension of torque.
Answer: ML2T-2
Question 7. When we turn on a tap we apply a _____ on it with the help of our fingers.
Answer: Couple
Question 8. What is the CGS unit of moment of inertia?
Answer: g · cm²
Question 9. Write down the expression of the moment of inertia of a circular disc (mass = m, radius = r) about the perpendicular axis passing through its centre.
Answer: \(\frac{1}{2}\)mr²
Question 10. Two spheres have equal masses and their external radii are the same. One of them is solid and the other hollow. Which one will have a greater radius of gyration?
Answer: Hollow sphere
System of particles motion
Question 11. Is the radius of gyration a constant quantity?
Answer: No
Question 12. What is the moment of inertia of a solid sphere (radius = r, mass = m ) about an axis passing through any of its diameters?
Answer: \(\frac{2}{5}\)mr²
Question 13. What is the radius of gyration of a solid sphere with respect to its diameter?
Answer: \(\sqrt{\frac{2}{5}} R\)
Question 14. What is the kinetic energy of a rotating body about its axis of rotation?
Answer: \(\frac{1}{2}\)Iω²
Question 15. What is needed to produce pure rotation?
Answer: Torque
Question 16. Write down the vector equation relating torque and angular momentum.
Answer: \(\vec{\tau}=\frac{d \vec{L}}{d t}\)
Question 17. If the moment of inertia of a body rotating about an axis is increased, state whether its angular velocity increases or decreases when no external torque acts on the body.
Answer: Decrease
Question 18. If the ice at the polar regions were to melt completely, state whether the length of a day would increase or decrease.
Answer: Increase
Question 19. What is the rotational analogue of the impulse of a force?
Answer: Angular impulse or impulse of a torque
Question 20. Which physical quantity is represented by the product of the moment of inertia and angular velocity?
Answer: Angular momentum (L = Iω]
Question 21. What is the relation between torque and moment of inertia?
Answer: Torque = moment of inertia x angular acceleration
Question 22. Torque x time = change of ______ of the body in that time.
Answer: Angular momentum
Question 23. Rotational analogue of force is _________
Answer: Torque
Unit 5 Motion Of System Of Particles And Rigid Body Chapter 2 Rotation Of Rigid Bodies Assertion Reason Type Questions And Answers
Direction: These questions have statement 1 and statement 2. Of the four choices given below, choose the one that best describes the two statements.
- Statement 1 is true, statement 2 is true; statement 2 is a correct explanation for statement 1.
- Statement 1 is true, and statement 2 is true; statement 2 is not a correct explanation for statement 1.
- Statement 1 is true, statement 2 is false.
- Statement 1 is false, and statement 2 is true.
Question 1.
Statement 1: The moment of inertia of a circular ring about a given axis is more than the moment of inertia of a circular disc of the same mass and same size, about the same axis.
Statement 2: The circular ring is hollow; so its moment of inertia is more than circular disc which is solid.
Answer: 2. Statement 1 is true, statement 2 is true; statement 2 is not a correct explanation for statement 1.
Question 2.
Statement 1: If the earth shrinks (without change in mass) to half its present size, the length of the day would become 6 hours.
Statement 2: As the size of the earth changes, its moment of inertia changes.
Answer: 1. Statement 1 is true, statement 2 is true; statement 2 is a correct explanation for statement 1.
System of particles motion
Question 3.
Statement 1: Many great rivers flow towards the equator. The sediments that they carry increase the time of rotation of the earth about its own axis.
Statement 2: The angular momentum of the earth about its rotation axis is conserved.
Answer: 1. Statement 1 is true, statement 2 is true; statement 2 is a correct explanation for statement 1.
Question 4.
Statement 1: The mass of a body cannot be considered to be concentrated at the centre of mass of the body for the purpose of computing its moment of inertia.
Statement 2: For then the moment of inertia of every body about an axis passing through its centre of mass would be zero.
Answer: 1. Statement 1 is true, statement 2 is true; statement 2 is a correct explanation for statement 1.
Question 5.
Statement 1: The moment of inertia of a uniform disc and solid cylinder of equal mass and radius about an axis passing through the centre and perpendicular to the plane will be the same.
Statement 2: Moment of inertia depends upon the distribution of mass from the axis of rotation, i.e., the perpendicular distance from the axis.
Answer: 1. Statement 1 is true, statement 2 is true; statement 2 is a correct explanation for statement 1.
Question 6.
Statement 1: The angular velocity of a rigid body in motion is defined for the whole body.
Statement 2: All points on a rigid body performing pure rotational motion are having same angular velocity.
Answer: 2. Statement 1 is true, statement 2 is true; statement 2 is not a correct explanation for statement 1.
Question 7.
Statement 1: The moment of inertia about an axis passing through the centre of mass is minimum.
Statement 2: The Theorem of the parallel axis can be applied only to a two-dimensional body of negligible thickness.
Answer: 3. Statement 1 is true, statement 2 is false.
Question 8.
Statement 1: In the rotational plus translational motion of a rigid body different particles of the rigid body may have different velocities but they will have the same accelerations.
Statement 2: The translational motion of a particle is equivalent to the translational motion of the rigid body
Answer: 4. Statement 1 is false, statement 2 is true.
Unit 5 Motion Of System Of Particles And Rigid Body Chapter 2 Rotation Of Rigid Bodies Match The Columns
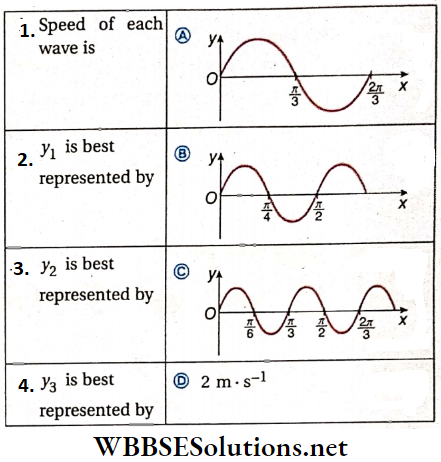
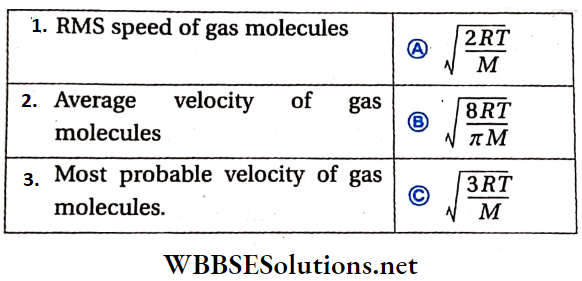
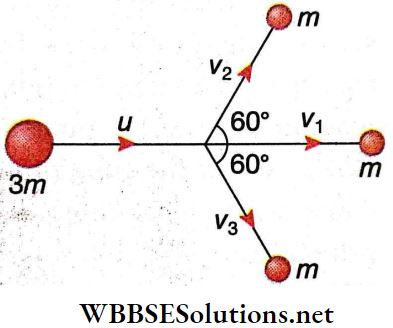
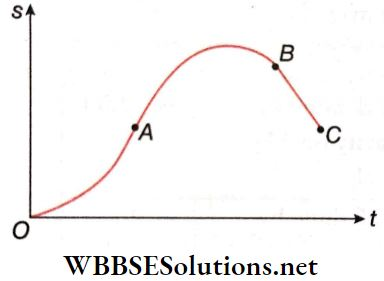
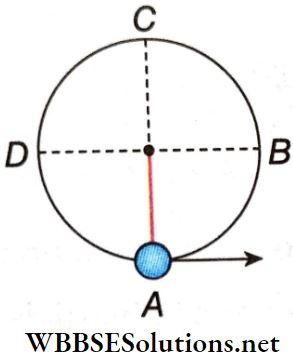
Question 1. If the radius of Earth is reduced to half without changing its mass, then match the following columns.

Answer: 1. C, 2. D, 3. B
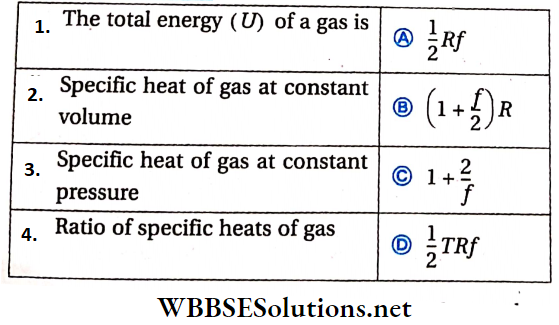
Question 2. From a uniform disc of mass M and radius R, a concentric disc of radius R/2 is cut out, For the remaining annular disc: I1 is the moment of inertia about axis ‘1’, I2 about ‘2’, I3 about ‘3′ and l4 about ‘4‘. Axes ‘1’ and ‘2’ are perpendicular to the disc and ‘3’ and ‘4’ are in the plane of the disc. Axes ‘2’, ‘3’ and ‘4’ intersect at a common point.


Answer: 1. C, 2. A, 3. A, 3. B
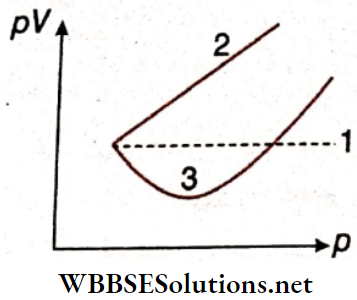
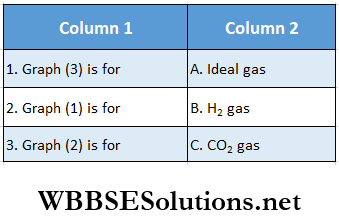
Question 3. A solid sphere is rotating about an axis as shown. An insect follows the dotted path on the circumference of the sphere


Answer: 1. B, 2. C, 3. A, 4. C
System of particles motion
Unit 5 Motion Of System Of Particles And Rigid Body Chapter 2 Rotation Of Rigid Bodies Comprehension Type Questions And Answers
Question 1. Two discs A and B are mounted co-axially on a vertical axle. The discs have moments of inertia I and 2I, respectively, about the common axis. Disc A is imparted an initial angular velocity 2ω using the entire potential energy of a spring compressed by a distance x1. Disc B is imparted an angular velocity ω by a spring having the same spring constant and compressed by a distance x2. Both the discs rotate in the clockwise direction
1. The ratio \(\frac{x_1}{x_2}\) is
- 2
- \(\frac{1}{2}\)
- √2
- \(\frac{1}{\sqrt 2}\)
Answer: 3. √2
2. When disc B is brought in contact with disc A, they acquire a common angular velocity in time t. The average frictional torque on one disc by the other during this period is
- \(\frac{2 I \omega}{3 t}\)
- \(\frac{9 I \omega}{2 t}\)
- \(\frac{9 I \omega}{4 t}\)
- \(\frac{3 I \omega}{2 t}\)
Answer: 1. \(\frac{2 I \omega}{3 t}\)
3. The loss of kinetic energy during the above process is
- \(\frac{I \omega^2}{2}\)
- \(\frac{I \omega^2}{3}\)
- \(\frac{I \omega^2}{4}\)
- \(\frac{I \omega^2}{6}\)
Answer: 2. \(\frac{I \omega^2}{3}\)
Question 2. A uniform solid sphere is released from the top of a fixed inclined plane of inclination 30° and height h. It rolls without sliding.
1. The acceleration of the centre of the sphere is
- \(\frac{3 g}{5}\)
- \(\frac{4 g}{5}\)
- \(\frac{4 g}{7}\)
- \(\frac{3 g}{7}\)
Answer: 4. \(\frac{3 g}{7}\)
2. The speed of the point of contact of the sphere with the inclined plane, when the sphere reaches the bottom of the incline, is
- \(\sqrt{2 g h}\)
- \(\sqrt{\frac{10 g h}{7}}\)
- \(zero\)
- \(2 \sqrt{2 g h}\)
Answer: 3. \(zero\)
3. The time taken by the sphere to reach the bottom is
- \(\sqrt{\frac{2 h}{g}}\)
- \(\sqrt{\frac{70 h}{9 g}}\)
- \(\sqrt{\frac{25 h}{18 g}}\)
- \(\sqrt{\frac{25 h}{6 g}}\)
Answer: 2. \(\sqrt{\frac{25 h}{18 g}}\)
Unit 5 Motion Of System Of Particles And Rigid Body Chapter 2 Rotation Of Rigid Bodies Integer Answer Type Questions
In this type, the answer to each of the questions Is a single-digit integer ranging from 0 to 9.
Question 1. A cube of mass 2 kg is held stationary against a rough wall by a force F = 40 N passing through centre C. Find the perpendicular distance of normal reaction between wall and cube from point C. Side of the cube is 20 cm. Take g = 10 m · s-2.
Answer: 5

Question 2. A wheel has an angular acceleration of 2.0 rad · s-2 and an initial angular speed of 1.0 rad · s-1 What will be the angular displacement (in radian) in 2 s?
Answer: 6
Question 3. A sphere of radius 2 m rolls on a plank. The accelerations of the sphere and the plank are indicated. What is the value of angular acceleration (in rad · s-2)?
Answer: 3

System of particles motion
Question 4. For two rings of radii R and nR are made up of the same material. The ratio of moments of inertia about axes passing through their centres is 1: 8. What should be the value of n?
Answer: 2