Dual Nature Of Matter And Radiation Long Questions And Answers
Question 1. The kinetic energy of a proton is the same as that of an α -particle. What is the ratio of their respective de Broglie wavelengths?
Answer:
Kinetic energy
E = ½mv²= \(\frac{1}{2} m v^2=\frac{(m v)^2}{2 m}\)
Or, mv = \(\sqrt{2 m E}\)
Hence, de Broglie wavelength,
λ = \(\frac{h^2}{m v}=\frac{h}{\sqrt{2 m E}}\)
Since the kinetic energy of a proton equals that of a-parade
⇒ \(\frac{\lambda_1}{\lambda_2}=\sqrt{\frac{m_2}{m_1}}=\sqrt{\frac{4}{1}}\)
= \(\frac{2}{1}\)
Question 2. What should be the percentage increase or decrease of kinetic energy of an electron so that its de Broglie wavelength is halved?
Answer:
Kinetic energy, E = \(\frac{p^2}{2 m}\)
[p = momentum]
∴ p = \(\sqrt{2 m E}\)
de Broglie wavelength = \(\frac{h}{p}=\frac{h}{\sqrt{2 m E}}\)
∴ \(\frac{\lambda_1}{\lambda_2}=\sqrt{\frac{E_2}{E_1}}\)
Here \(\lambda_2=\frac{\lambda_1}{2}\) Or, \(\frac{\lambda_1}{\lambda_2}\) = 2
Hence \(\sqrt{\frac{E_2}{E_1}}\) = 2 Or, E2 = 4E1
∴ Increase in kinetic energy
= \(\frac{E_2-E_1}{E_1} \times 100 \%\) × 100%
= \(\frac{4 E_1-E_1}{E_1} \times 100 \%\) × 100%
= 300%
Question 3. The kinetic energy of a free electron is doubled. By how many times, would its de Broglie wavelength increase
Answer:
Kinetic energy, E = \(\frac{p^2}{2 m}\) [p= momentum of electron]
∴ p = \(\sqrt{2 m E}\)
de Broglie wavelength
λ = \(\frac{h}{p}=\frac{h}{\sqrt{2 m E}}\)
i.e \(\frac{\lambda_1}{\lambda_2}=\sqrt{\frac{E_2}{E_1}} \text { or, } \lambda_2=\lambda_1 \sqrt{\frac{E_1}{E_2}}\)
= \(\lambda_1 \sqrt{\frac{1}{2}}=\frac{\lambda_1}{\sqrt{2}}\)
Hence, de Broglie wavelength will be \(\) times Original One.
Question 4. Energy of a photon = E; kinetic energy of a proton is also the same as energy of the photon. The corresponding wavelength of the photon and de Broglie wavelength of the proton are λ1 and λ2, respectively. What is the relation between E and the ratio of λ1 and λ2?
Answer:
In the case of photon, E = hf = hc/λ1
Or, λ1 = hc/E
In case of proton, E = \(\frac{p^2}{2 m}\) [p = momentum of proton]
Hence, p = \(\sqrt{2 m E}\) and, \(\frac{h}{p}=\frac{h}{\sqrt{2 m E}}\)
i.e \(\frac{\lambda_2}{\lambda_1}=\frac{h}{\sqrt{2 m E}} \cdot \frac{E}{h c}\)
= \(\frac{1}{c} \sqrt{\frac{E}{2 m}} \quad \text { or, } \frac{\lambda_2}{\lambda_1} \propto \sqrt{E}\)
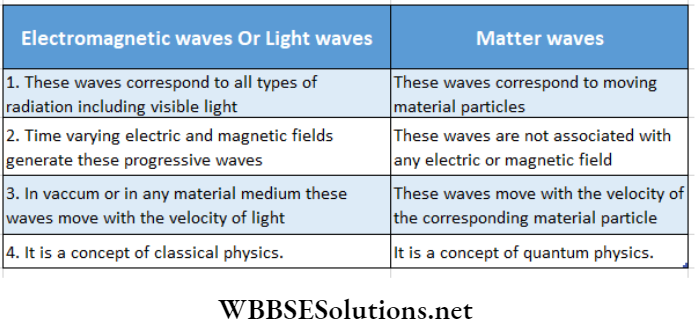
Question 5. Distinguish between matter wave and light wave
Answer:
Question 6. The maximum kinetic energies of photoelectrons emitted from a metal surface when illuminated by radiations of wavelengths λ1 and λ2 are K1 and K2, respectively. If λ1 = 3λ2, show that,\(K_1<\frac{K_2}{3}\)
Answer:
If the work function of the metal is W, from Einstein’s equation
K1 = \(K_1=\frac{h c}{\lambda_1}-W \text { and } K_2=\frac{h c}{\lambda_2}-W\) – W
Hence,K1 – \(K_1-\frac{K_2}{3}=h c\left(\frac{1}{\lambda_1}-\frac{1}{3 \lambda_2}\right)-W+\frac{W}{3}\)
= hc. 0 – \(\frac{2 W}{3}\)
Since λ1= 3λ2
= – \(\frac{2 W}{3}\)<0
K1 < \(\frac{K_2}{3}\)
Question 7. A proton and an electron have the same kinetic energy. Which one has a larger de Broglie wavelength?
Answer:
de Broglie wavelength, λ = \(\frac{h}{\sqrt{2 m K}}\)
Since h and K are constants, λ∝ \(\frac{1}{\sqrt{m}}\)
If I and mp are the masses of an electron and a proton respectively.
Then, \(\frac{\lambda_e}{\lambda_p}=\sqrt{\frac{m_p}{m_e}}>1\)
∴ λe > λp
So, the de Broglie wavelength of electrons is greater
Question 8. A proton and an electron have the same de Broglie wave¬ length. Which one has higher kinetic energy?
Answer:
de Broglie wavelength,
λ = \(\frac{h}{\sqrt{2 m K}}\) [ K= kinetic energy]
Or, mK = \(\frac{h^2}{2 \lambda^2}\)
For the same λ, mK = constant
i.e \(K \propto \frac{1}{m} \quad \text { or, } \frac{K_e}{K_p}=\frac{m_p}{m_e}>1\)
∴ Ke>Kp
∴ Electron has higher kinetic energy.
Question 9. A photon and an electron have the same de Broglie wavelength. Which one has greater total energy?
Answer:
The total energy of a photon of wavelength λ is given by
Ep = hf = hc/λ ……………………………………………… (1)
de Broglie wavelength J of an electron (same as bf photon) of mass m moving with velocity v is given by
λ = \(\frac{h}{m v} \text { or, } m=\frac{h}{\lambda v}\)
The total energy of electron of mass m
Ee = mc² (According to the theory of relativity)
= \(\frac{h c^2}{\lambda v}\) ………………………………… (2)
Dividing equation (2) by equation (1) we hive
⇒ \(\frac{E_e}{E_p}=\frac{\frac{h c^2}{\lambda v}}{\frac{h c}{\lambda}}=\frac{c}{v}\)
Since c>c.
∴ Ee >Ep
So the total energy of the electron is greater than that of a photon
Question 10. Find the exp-session of de Broglie wavelength of a particle moving with a velocity close to the velocity of light.
Answer:
When a particle moves with a velocity v close to the velocity of light c, then the effective mass of the particle
m = \(\frac{m_0}{\sqrt{1-\frac{v^2}{c^2}}}\)
Where mQ = rest mass of the particle
Hence, the de Broglie wavelength associated with the particle is
λ = \(\frac{h}{m v}=\frac{h \sqrt{1-\frac{v^2}{c^2}}}{m_0 v}\)
= \(\frac{h}{m_0 v} \sqrt{1-\frac{v^2}{c^2}}\)
Question 11. If another the speed particle of anis 3v, electron ratio iso f the v and de Brogliethe speed wavelength of the electron to that of the particle Is 104/8.13. Is the particle a proton or an α particle? Justify your answer
Answer:
de Broglie wavelength, λ = \(\frac{h}{m v}\)
[ h = Planck’s constant]
∴ For the electron and the other particle
⇒ \(\frac{\lambda_1}{\lambda_2}=\frac{m_2}{m_1} \cdot \frac{v_2}{v_1}\)
Or, = \(\frac{m_2}{m_1}=\frac{\lambda_1}{\lambda_2} \cdot \frac{v_1}{v_2}=\frac{10^4}{8.13} \times \frac{v}{3 v}\)
= \(\frac{410}{1}\)
∴ The other particle is 410 times heavier than an electron. It is neither a proton nor an α-particle
Question 12. Prove that the product of the slope of the V0-f graph and electronic charge gives the value of Planck’s constant.
Answer:
From Einstein’s photoelectric equation, we know that,
½mv² = hf – W0
But, ½mv²max = eV0
∴ eV0 = hf – W0
Or, \(\frac{h}{e} f-\frac{W_0}{e}\) ………………………………………….. (1)
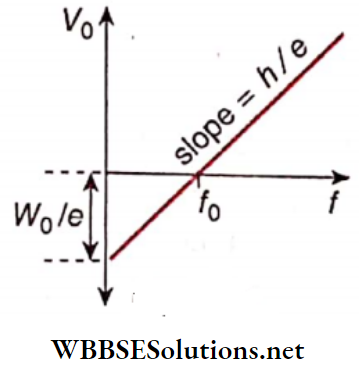
So the plot of stopping potential ( V0) versus frequency (f) will be a straight line
Comparing equation (1) with (the equation of a straight line y = mx + c, we get the slope of V0– f graph m = h/e
Planks constant h = me
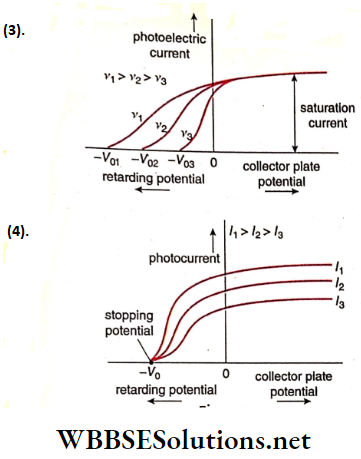
Question 13. The given graphs show the variation of photoelectric current (I) with the applied voltage ( V) when light of different Intensi¬ ties Is Incident on surfaces of different materials Identify the pairs of curves that correspond to different materials but the same intensity of incident radiations.
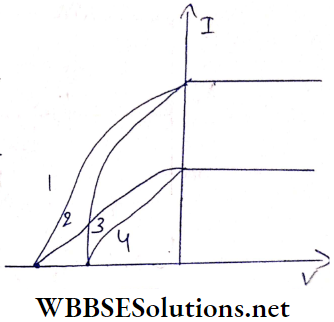
Answer:
Stopping potential depends on the material of the photocathode, but Is independent of the Intensity of the Incident radiation. So the pairs (1,3) and (2, 4) correspond to different materials but the same Intensity of incident radiation.
Question 14. The potential energy U of a moving particle of mass m varies with x as shown In the figure. The de Broglie wavelengths of the particle In the regions 0 <x< 1 and x > 1 are λ1 and λ2 respectively. If £ the total energy of the particle Is nE, find λ1/λ2
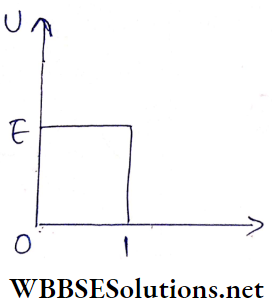
Answer:
Total energy =nE = potential energy + kinetic energy
In the region 0 < x < 1, potential energy, U1= E
∴ Kinetic energy Is K1 = nE- E = (n- 1 )E
∴ de Broglie wavelength,
λ1= \(\frac{h}{\sqrt{2 m K_1}}=\frac{h}{\sqrt{2 m(n-1) E}}\) ………………………………………….(1)
In the region x > 1 , potential energy, U2 = 0
Kinetic energy, K2 = nE- 0 = nE
de Broglie wavelength
λ2 = \(\frac{h}{\sqrt{2 m K_2}}=\frac{h}{\sqrt{2 m n E}}\)………………………………..(2)
From equations (1) and (2) we get
⇒ \(\frac{\lambda_1}{\lambda_2}=\frac{h}{\sqrt{2 m(n-1) E}} \times \frac{\sqrt{2 m n E}}{h}\)
= \(\sqrt{\frac{n}{n-1}}\)
Question 15. Monochromatic light of wavelength 632.8 nm Is produced by a helium-neon laser. The power emitted is 9.42 mW.
- Find the energy and momentum of each photon in the light beam.
- How many photons per second, on average, arrive at a target irradiated by this beam? (Assume the entire beam to be incident on a small area of the target)
- What should be the velocity of a hydrogen atom to have the same momentum ofthe photon?
Answer:
1. Wavelength, λ = 632.8 nm = 632.8 × 10 -9m
∴ The energy of each photon
E = \(\frac{h c}{\lambda}=\frac{6.626 \times 10^{-34} \times 3 \times 10^8}{632.8 \times 10^{-9}}\)
= 3.14 × 10-19 J and momentum
p = \(\frac{h}{\lambda}=\frac{6.626 \times 10^{-34}}{632.8 \times 10^{-9}}\)
= 1.05 × 10-27 kg. m. s-1
2. Emission power
P = 9.42 mW = 9.42 × 10 -19 W
Number of photons emitted per second
n = \(\frac{P}{E}=\frac{9.42 \times 10^{-3}}{3.14 \times 10^{-19}}\)
3. Mass of hydrogen atom
= Mass of proton = 1.67 × 10 -27 kg
∴ Velocity of the hydrogen atom
= \(\frac{\text { momentum of photon }}{\text { mass of hydrogen }}=\frac{1.05 \times 10^{-27}}{1.67 \times 10^{-27}}\)
= 0.63 m. s-1
Question 16. The energy flux of sunlight reaching the surface of the earth is 1.388 × 103 W .m-2. How many photons (nearly) per square meter are incident on the earth per second? Assume that the photons in the sunlight have an average wavelength of 550 nm.
Wavelength, μ = 550 nm = 550 × 10 -9 m
Energy flux, Φ= 1.388 × 10 3 W .m-2
The energy of each photon,
E = \(\frac{h c}{\lambda}=\frac{6.626 \times 10^{-34} \times 3 \times 10^8}{550 \times 10^{-9}}\)
= 3.61× 10 -19J
∴ Number of photons incident on earth per square meter per second,
n = \(\frac{\phi}{E}=\frac{1.388 \times 10^3}{3.61 \times 10^{-19}}\)
= 3.85 × 10 21
Question 17. The work function for a certain metal is 4.2 eV. Will this metal give photoelectric emission for incident radiation of wavelength 330 nm?
Answer:
Work function, W0 = 4.2 eV = 6.72 × 10-19 J
Threshold frequency, f0 = \(\frac{W_0}{h}\)
⇒ \(\frac{6.72 \times 10^{-19}}{6.606 \times 10^{-34}}\)
= 1.101 × 1015 Hz
∴ Threshold wavelength,
λ0 = \(=\frac{6.72 \times 10^{-19}}{6.626 \times 10^{-34}}\)
= \(\frac{c}{f_0}=\frac{3 \times 10^8}{1.01 \times 10^{15}}\)
= 297 nm
∴ λ0 < λ
Where λ = incident wavelength
i.e f0 >f
∴ Photoelectric emission is not possible in this case.
Question 18. Light of wavelength 488 nm is produced by an argon laser which is used in the photoelectric effect. When light from this spectral line is incident on the cathode, the stopping (cut-off) potential of photoelectrons Is 0.38 y and the work functional the material from which staff cathode Ht made
Frequency of Incidental light,
f = \(\frac{c}{\lambda}=\frac{3 \times 10^8}{488 \times 10^{-9}}\)
= 6.15 × 1014 Hz
∴ Work function,
W0 = hf-eV0
= 6.626 × 10-34 × 6.15 × 104 – 1.6 × 10-19 × 0.38
= 3.467 × 10-19 J = 2.167.eV
Question 19. The wavelength of light from the spectral emission line of sodium is 589 nm. Find the kinetic energy at which
- An electron and
- A neutron would have the same de Broglie wavelength
Answer:
de Broglie wavelength, A = \(\frac{h}{\sqrt{2 m E}}\)
Or, E = \(\text { or, } E=\frac{1}{2 m} \cdot \frac{h^2}{\lambda^2}\)
1. The kinetic energy of the electron
Ee= \(\frac{1}{2 \times 9.1 \times 10^{-31}} \times\left(\frac{6.626 \times 10^{-34}}{589 \times 10^{-9}}\right)^2\)
= 6.95 ×10-25 J
2. Kinetic energy of neutron
En= \(\frac{1}{2 \times 1.67 \times 10^{-27}} \times\left(\frac{6.626 \times 10^{-34}}{589 \times 10^{-9}}\right)^2\)
= 3.79 ×10-28J
Question 20. The wavelength of each photon and electron is 1.00 nm, Find their
- Momentum and
- Energy.
Answer:
1. Momentum, p = \(\frac{h}{\lambda}\)
Or, p = \(\frac{6.626 \times 10^{-34}}{1 \times 10^{-9}}\)
= 6.626 ×10-25J kg. m. s-1
2. Energy of photon
E = \(\frac{h c}{\lambda}=\frac{6.626 \times 10^{-34} \times 3 \times 10^8}{10^{-9}}\)
= 1.24 keV
The kinetic energy of the electron
½mv² = \(\frac{p^2}{2 m}\)
= \(\frac{\left(6.626 \times 10^{-25}\right)^2}{2 \times 9.1 \times 10^{-31}} \mathrm{~J}\)
= 1.51
Question 21. The average kinetic energy of a neutron at 300 K is \(\frac{3}{2}\) kT. Find Its do Broglie wavelength.
Answer:
de Broglie wavelength
λ = \(\frac{h}{\sqrt{2 m E}}=\frac{h}{\sqrt{3 m k T}}\)
Since E = \(\frac{3}{2}\) KT
= \(=\frac{6.626 \times 10^{-34}}{\left(3 \times 1.67 \times 10^{-27} \times 1.38 \times 10^{-23} \times 300\right)^{1 / 2}}\)
K = 1.38 × 10-23
= 1.456A°
Question 22. What is the dc Broglie wavelength of a nitrogen molecule at 300 K ? Assume the molecule is moving with its rms speed. (Atomic mass of nitrogen = 14.0076 u J)
Answer:
vrms = \(\sqrt{\frac{3 k T}{m}}\)
de Broglie wavelength of nitrogen molecule
λ = \(\frac{h}{m v_{\mathrm{rms}}}=\frac{h}{\sqrt{3 m k T}}\)
= \(\frac{6.626 \times 10^{-34}}{\left(3 \times 28.0152 \times 1.38 \times 10^{-23} \times 300\right)^{1 / 2}}\)
= 0.275 ×10-10 m
Question 23. In an accelerator experiment on high-energy collisions of electrons with positrons, a certain event Is interpreted as the annihilation of an electron-positron pair of total energy 10.2 GeV into two ϒ – rays of equal energy. What is the wavelength associated with each γ- ray ( lGeV = 109 eV )
Answer:
The energy of two γ- ray = 10.2 GeV
∴ A Energy of each γ- ray = 5.1 GeV = 5.1 ×109eV
λ = \(\frac{h c}{E}=\frac{6.626 \times 10^{-34} \times 3 \times 10^8}{5.1 \times 10^9 \times 1.6 \times 10^{-19}}\)
= 2.44 ×10-16 m
Question 24. Estimating the following two numbers should be interesting. The first number will tell you why radio engineers need not worry much about photons! The second number tells you why our eye can never ‘count photons! even In barely detectable light.
- The number of photons emitted per second by a medium wave transmitter of 10 kW power, emitting radiowaves of wave-length 500 m
- The number of photons entering the pupil of our eye per second corresponds to the minimum intensity of white light that we humans can perceive (-10-10W.m-2). Take the area ofthe pupil to be about 0.4 cm2 and the average frequency of white light to be about 6 ×1014 Hz.
Answer:
1. Number of photons emitted per second,
n = \(n=\frac{\text { power of transmitter }}{\text { energy of each photon }}\)
= \(\frac{P}{h c / \lambda}=\frac{10^4 \times 500}{6.626 \times 10^{-34} \times 3 \times 10^8}\)
= 2.52 ×1031
This number is so very large that radio engineers need not worry much about photons.
2. Minimum intensity, I = 10-10 W.m-2
Area of pupil = 0.4 cm2 = 0.4 ×10-4m2
Average frequency, f = 6 × 10-4 Hz
E = hf = 6.626 ×10-34 × 6 × 1014
= 3.98 ×10-19J
∴ Number of photons incident on the eye,
n = \(\frac{I A}{E}=\frac{10^{-10} \times 0.4 \times 10^{-4}}{3.98 \times 10^{-19}}\)
= 1.01 × 104
This is quite a small number, but still large enough to be counted
Question 25. Monochromatic radiation of wavelength 640.2 nm? from a neon lamp irradiates photosensitive material made of calcium or tungsten. The stopping voltage is measured to be 0.54 V. The source is replaced by an iron source and its 427.2 nm line irradiates the same photocell. Predict the new stopping voltage
Answer:
The work function of the photoelectric cell
W0 = \(\frac{h c}{\lambda}\)eV0
= \(\left(\frac{6.626 \times 10^{-34} \times 3 \times 10^8}{640.2 \times 10^{-9}}-1.6 \times 10^{-19} \times 0.54\right)\)
= 1.40 eV
In the second case, A = 427.2 nm
V0 = \(\frac{1}{e}\left(\frac{h c}{\lambda}-W_0\right)\)
= \(\frac{1}{1.6 \times 10^{-19}}\) \(\times\left(\frac{6.626 \times 10^{-34} \times 3 \times 10^8}{427.2 \times 10^{-9}}-2.24 \times 10^{-19}\right)\)
= 1.51 V
Question 26. An electron microscope uses electrons accelerated by a voltage of 50 kV. determine the die deBroglie wavelength associated with the electrons. If other factors (such as numerical aperture etc.) are taken to be roughly the same, how does the resolving power of an electron microscope compare with that of an optical microscope with yellow light?
Answer:
Wavelength of electron
λe = \(\frac{h}{\sqrt{2 m e V}}\)
= \(\frac{6.626 \times 10^{-34}}{\left(2 \times 9.1 \times 10^{-31} \times 1.6 \times 10^{-19} \times 50 \times 10^3\right)^{\frac{1}{2}}} \mathrm{~m}\)
= 0.0549 A°
∴ Resolving power of a microscope ∝ 1/ λ
The wavelength of yellow light, λy = 5900 A°
∴ \(\quad \frac{\text { resolving power of electron microscope }}{\text { resolving power of optical microscope }}\)
= \(\frac{\lambda_y}{\lambda_e}=\frac{5900}{0.0549}\)
= 1.07 × 105
Question 27. Find the typical de Broglie wavelength associated with a He atom in helium gas at room temperature (27 °C) and 1 atm pressure.
Answer:
Here, T = 27°C = 300 K,
P = 1 atm = 1.01 × 105 N . m-2
Mass of He atom,
mHe = \(\frac{4}{\text { Avogadro’s number }}=\frac{4}{6.023 \times 10^{23}}\)
= 6.64 × 105 N.m-2
∴ Typical de Broglie wavelength
λ = \(\frac{h}{\sqrt{3 m_{\mathrm{He}} k T}}\)
= \(\frac{6.626 \times 10^{-34}}{\left(3 \times 6.64 \times 10^{-27} \times 1.38 \times 10^{-23} \times 300\right)^{1 / 2}} \mathrm{~m}\)
= 0.73A°
Question 28. Einstein’s photoelectric equation shows how the cut-off voltage and threshold frequency for a given photosensitive material can be determined with the help of a suitable photograph.
Answer:
Einstein’s equation: eV0 = h(f-f0)
Where, V0 = cut-off voltage due to frequency/ of incident light,
f0 = threshold frequency
Now, V0= \(\frac{h f}{e}-\frac{h f_0}{e}\)
This is of the form y = mx + c. So the graph of V0 vs f will be a straight line AB of slope – v0,
The point of intersection of AB with the x-axis gives the value of f. For any incident frequency, corresponding to any point C on AB, the ordinate OD gives the value of the cut-off voltage V0.
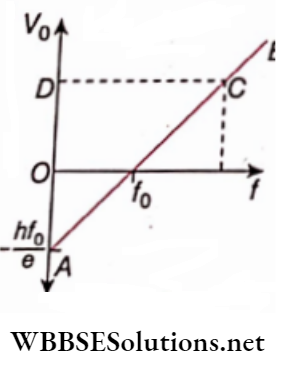
Question 29. An electron microscope uses electrons, accelerated by a voltage of 50 kV. Determine the de Broglie wavelength associated with the electrons. Taking other factors, such as numerical aperture, etc. to be the same, how does the resolving power of an electron microscope compare with that of an optical microscope that uses yellow light? C Kinetic energy of the electron
E = 50 keV = 50 × 1.6 ×10-16 J
∴ de Broglie wavelength
λ = \(\frac{h}{\sqrt{2 m E}}\)
= \(\frac{6.626 \times 10^{-34}}{\sqrt{2 \times\left(9.1 \times 10^{-31}\right) \times\left(50 \times 1.6 \times 10^{-16}\right)}} \times 10^{10} \mathrm{~A}\)
= 0.055 A°
λ<< λ’
The wavelength of yellow light,
λ’≈ 5900 A°
λ<<λ’
So, λ<<λ’
The least distance (d) between two points that a microscope can observe separately is proportional to the wavelength of light used, i.e, d ∝ λ
As λ<<λ’ we have d<<d’. So an electron microscope can resolve two points very much closer. Then its resolving power is much higher than that of an optical microscope
Question 30. The graph shows the variation of stopping potential with the frequency of incident radiation for two photosensitive metals A and B. Which one ofthe two has a higher value of work function? Justify your answer.
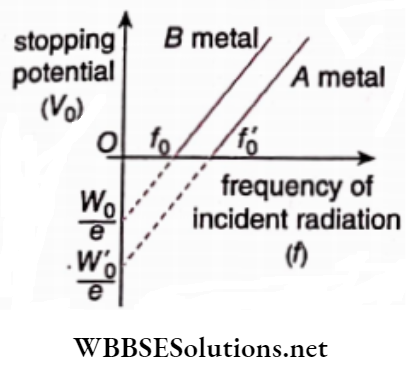
Answer:
For metal A, W’0 = hf’0; For metal B , W0 = hf0
As f’ >f0
∴ W’0 >W0
So, the function of metal A is greater than that of metal B
Question 31. Determine the value of the de Broglie wavelength associated with the electron orbiting in the ground state of the hydrogen atom (given E0 = -(13.6/n²) eV and Bohr radius r0 = 0.53 A ). How will the de Broglie wavelength change when it is in the first excited state?
Answer:
We know, the de Broglie wavelength
λ = \(\frac{h}{m v}=\frac{h r}{m v r}\)
= \(\frac{h r}{\frac{n h}{2 \pi}}=\frac{2 \pi r}{n}\)
For ground State, n= 1
∴ \(\lambda_1=\frac{2 \pi r_0}{1}\)
For ground state, n = 1.
∴ λ1 = 2π × 0.53 × 10-10
= 3.3 m × 10-10= 3.3 Å
Again , λn= \(\frac{2 \pi r_n}{n}=\frac{2 \pi n^2 r_0}{n}\)
= n2πr0
= n λ1
When n = 2 (for first excited state) = λ1 = 2λ1
Hence, de Broglie wavelength doubles in the first excited state
Question 32. Define the term ‘intensity of radiation’ in a photon picture of light Ultraviolet light of wavelength 2270 A° from 100 W mercury source irradiates a photocell made of a given metal. If the stopping potential is -1.3 V, estimate the work function of the metal. How would the photocell respond to a high intensity ( ~ 105 W. m-2 J red light of wavelength 6300 A° produced by a laser?
Answer:
The intensity of radiation can be defined as the amount of light energy incident per square meter per second.
V0 = 1.3 V; λ = 2270 × 10-10 m
We know that hf= hf0 + ±½ mv²max
Also, hf = W0+eV0
W0 = hf- ev0
W0 = hc/λ – eV0
W0 = \(\frac{6.62 \times 10^{-34} \times 3 \times 10^8}{2270 \times 10^{-10}}-1.3 \times 1.6 \times 10^{-19}\)
= \(6.669 \times 10^{-19} \mathrm{~J}=\frac{6.669 \times 10^{-19}}{1.6 \times 10^{-19}} \mathrm{eV}\)
= 4.168 eV
Again W0 = \(h f_0=\frac{h c}{\lambda_0}\)
Or, λ0 = \(\frac{h c}{W_0}=\frac{6.62 \times 10^{-34} \times 3 \times 10^8}{6.669 \times 10^{-19}}\) m
= 2.98 m = 2980 Å
The photocell would not respond to a high intensity ( ~ 105 W. m-2 ) red light of wavelength 6300 A° produced by a laser because this wavelength is greater than 2980 A°
Question 33. A proton and a particle are accelerated through the same potential difference. Which one of the two has (a) greater de Broglie wavelength, and (b) less kinetic energy? Justify your answer
Answer:
The de Broglie wavelength is related to the accelerating potential as
λ = \(\frac{h}{\sqrt{2 m e V}}\)
λp = \(\frac{h}{\sqrt{2 m_p e_p V}} \text { and, } \lambda_\alpha=\frac{h}{\sqrt{2 m_\alpha e_\alpha V}}\)
λα = And \(\)
∴ \(\frac{\lambda_p}{\lambda_\alpha}=\frac{h}{\sqrt{2 m_p e_p V}} \times \frac{\sqrt{2 m_\alpha e_\alpha V}}{h}=\frac{\sqrt{m_\alpha e_\alpha}}{\sqrt{m_p e_p}}\)
Now, the mass and charge of a -particle is greater than that of a proton
λp > λα for the same potential difference.
The kinetic energy is related to accelerating potential as
K = eV
∴ Kp = epV and Kα = eα V
∴ \(\frac{K_p}{K_\alpha}=\frac{e_p}{e_\alpha}\)
Since the charge on the proton is less than that on the alpha particle, Kp < Ka for the same potential difference
Question 34. In the study of a photoelectric effect the graph between the. stopping potential V and frequency u of the incident radiation on two different metals P and Q is shown in:
- Which one of the two metals has a higher threshold frequency?
- Determine the work function of the metal which has greater value.
- Find the maximum kinetic energy of electron emitted by light of frequency 8 × 10-33 Hz Hz for this metal.
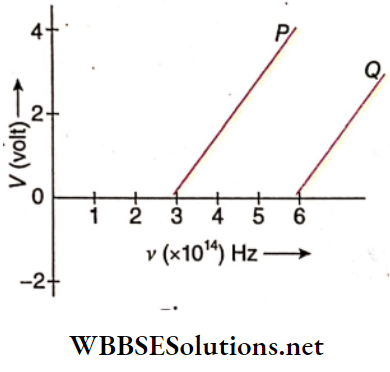
Answer:
1. Threshold frequency of P, νp = 3 × 1014 Hz
The threshold frequency of Q, νQ = 6 × 1014 Hz
So, metal Q has a higher threshold frequency.
2. Workfunction of metal Q,
W = hν0 .= 6.6 × 10-34 × 6 × 1014
= 39.6 × 10-20 J
= 2.47 eV
3. The maximum kinetic energy of electron emitted from metal Q by light of frequency 8 × 10-14 Hz is,
= 6.6 × 10-34 × (8 × 1014– 6 ×1014 )
=13.2 × 10-20 J
= 0.825 eV
Question 35. The following graph shows the variation of photocurrent for a photosensitive metal:
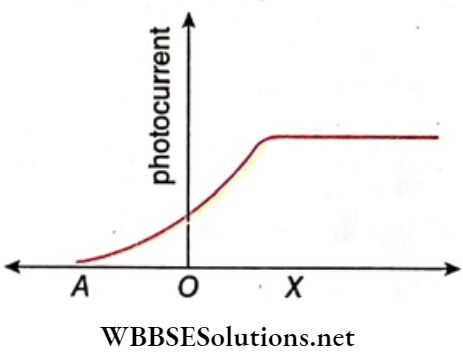
- Identify the variable X on the horizontal axis.
- What does point A on the horizontal axis represent?
- Draw this graph for three different values of frequencies of incident radiation, ν1, ν2, and ν3( ν1>ν2>ν3 )same intensity.
- Draw this graph for three different values of intensities of incident radiation I1 , I2 and I3(I1> I2> I3) having
Answer:
1. The variable X on the horizontal axis is the potential difference between the anode and the cathode.
2. The point A on the horizontal axis represents stopping potential
Question 36. The work functions of the following metals are given; Na = 2.75 eV, K = 2.3 eV, Mo = 4.17 eV, and Ni = 5.15 eV. Which of these metals will not cause photoelectric emission for radiation of wavelength 3300 A from a laser source placed 1 m away from these metals? What happens if the laser source is brought nearer and placed 50 cm away?
Answer:
Condition for photoelectric emission, hf> W0
Or, \(\frac{h c}{\lambda}>W_0\)
For λ = 3300 Å
⇒ \(\frac{h c}{\lambda}=\frac{6.6 \times 10^{-34} \times 3 \times 10^8}{3000 \times 10^{-10}} \mathrm{~J}\)
= \(\frac{6.03 \times 10^{-19}}{1.6 \times 10^{-19}} \mathrm{eV}\)
Mo and Ni will not cause photoelectric emission. If the laser source is brought nearer placed 50 cm away, then also there will be no photoelectric emission from Mo and Ni, since it depends upon the frequency of the source
Question 37. Radiation of frequency 1015 Hz Is Incident on two photosensitive surfaces P and Q. There is no photoemission from surface PhotoemUslon occurs from surface Q but photoelectrons have zero kinetic energy. Explain these observations and find the value of work function for surface
Answer:
There is no photoemission from surface I* because the frequency of incident radiation is less than its threshold frequency. Since photoemission occurs from surface Q and the photoelectrons have , zero kinetic energy, then the frequency of incident radiation is equal to the threshold frequency for surface Q .
Work function for surface Q, WQ = hf
= 6.6 × 10-34 × 1015
= 6.6 × 10-195
= 4.125 eV
Question 38. Find the energy required by an electron to have its de Broglie wavelength reduced from 10-10 m to 0.5 × 10-10 m.
Answer:
de Broglie wavelength
λ = \(\frac{h}{p}=\frac{h}{\sqrt{2 m E}}\)
Or, \(2 m E=\frac{h^2}{\lambda^2} \quad \text { or, } E=\frac{h^2}{2 m} \cdot \frac{1}{\lambda^2}\)
∴ The energy required by the electron
E1 – E2 = \(\frac{h^2}{2 m}\left(\frac{1}{\lambda_2^2}-\frac{1}{\lambda_1^2}\right)\)
= \(\frac{\left(6.62 \times 10^{-34}\right)^2}{2 \times 9.1 \times 10^{-31}} \cdot\left[\frac{1}{\left(0.5 \times 10^{-10}\right)^2}-\frac{1}{\left(10^{-10}\right)^2}\right]\)
= 7.2 × 10-17J
E1 – E2 = \(\frac{7.2 \times 10^{-17}}{1.6 \times 10^{-19}} \mathrm{eV}\)
= 450 eV
Question 39.
- Draw the curve showing the variation of de Broglie wavelength of a particle with its momentum. Find the momentum of a photon of wavelength 0.01A.
- Mention the inference of Davisson Garmer’s experiment.
Answer:
1. If momentum =p, then the de Broglie wavelength of a particle
λ = h/p
Or, λp = h = Planck’s constant Hence the λ-p graph will be a rectangular hyperbola,
Given, λ = 0.01 A° = 10-12 m
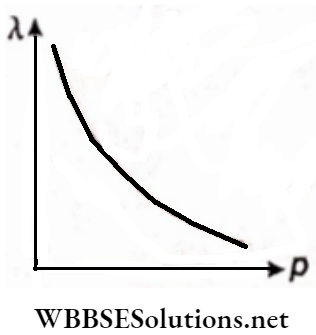
Momentum ofthe photon
p = \(\frac{E}{c}=\frac{h f}{c}=\frac{h}{\lambda}=\frac{6.63 \times 10^{-34}}{10^{-12}}\)
= 6.63 ×10-22 kg.m.s-1
2. The inference of this experiment is that an electron beam behaves as a matter wave. Hence the experiment proves de Broglie’s hypothesis.
Question 40. When light of wavelengths A and 2\ are incident on a metal surface, the stopping potentials are V0 and VQ/4 respectively. If c be the velocity of light in air, find the threshold frequency of photoelectric emission
Answer:
From Einstein’s photoelectric equation
eV0 = hf-W0 or , W0 = hf – eV0 = \(\frac{h c}{\lambda}\) – eV0
In first case W0= \(\frac{h c}{\lambda}-e V_0\) …………………….(1)
In the Second case
W0 = \(\frac{h c}{2 \lambda}-\frac{e V_0}{4}\)
Or, W0 = \(\frac{2 h c}{\lambda}-e V_0\) …………………………(2)
From equations (1) and (2) we get,
4 W0 – W0 = 3W0 = \(\frac{h c}{\lambda} \text { or, } w_0=\frac{h c}{3 \lambda}\)
Threshold frequency \(\frac{W_0}{h}=\frac{c}{3 \lambda}\) =
∴ Threshold wavelength = 3λ

