Class 12 Chemistry Unit 13 Organic Compounds Containing Nitrogen
The class of organic compounds having functional groups containing nitrogen is termed as ‘nitrogenous organic compounds.’ This chapter is devoted to the discussion of this special class of compounds, and their classifications with the corresponding functional groups in an elucidated way. Classification of important nitrogenous organic compounds and the related functional groups are as follows:

Alkyl Cyanide (R-CN)
Derivatives of hydrogen cyanide are called alkyl cyanide. These are obtained by replacing a hydrogen atom of a hydrogen cyanide molecule with an alkyl group.
![]()
In the molecules of alkyl cyanide, the alkyl group is directly attached to a carbon atom of the cyano group. Both the carbon and nitrogen atoms of the cyano group are sp- hybridised.
Nomenclature Of Alkyl Cyanides
Alkyl Cyanides General method (Common system): These are named by using the suffix ‘cyanide’ after the name of the alkyl group.

The compounds belonging to the class of cyanides are named by adding the suffix ‘nitrile’ in place of ‘ic-acid’ to the name of the corresponding acid produced by hydrolysis of the alkyl cyanide.
Alkyl Cyanides General method Example: Hydrolysis of methyl cyanide (CH3CN) gives acetic acid. So the other name of methyl cyanide is acetonitrile. Similarly, propionic acid is obtained by hydrolysis of ethyl cyanide (CH3CH2CN). Thus the other name of ethyl cyanide is propiononitrile.

IUPAC Method: In this system, cyanides are named alkane nitriles. The carbon atom of the — CN group is also counted as belonging to the parent chain.
- The positions of various substituents are indicated by numbering the carbon atoms in the longest parent chain starting from the carbon atom of the —CN group.
- These compounds are thus named by adding the suffix nitrile, to the names of the parent alkanes.

If the molecule of a compound contains two cyano groups ( —CN), ‘dinitrile’ is written after the name of the parent alkane.

IUPAC Method Example: \(\mathrm{N} \stackrel{1}{\mathrm{C}}-\stackrel{2}{\mathrm{C}} \mathrm{H}_2 \stackrel{3}{\mathrm{C}} \mathrm{H}_2 \stackrel{4}{\mathrm{C}} \mathrm{H}_2 \stackrel{5}{\mathrm{C}} \mathrm{H}_2-\stackrel{6}{\mathrm{C}} \mathrm{N} \text { (Hexanedinitrile) }\)
- When a molecule of a compound contains three or more cyano groups, the —CN group is treated as the substituent.
- The suffixes ‘tricarbonitrile’, ‘tetracarbonitrile’, etc. are used after the name of the alkane along with the positions of cyano groups in the chain of carbon atoms of that alkane.
- In this case, carbon atoms of cyano groups are not included in the chain of carbon atoms of the main alkane.
Example:
In the case of alicyclic, aromatic cyano compounds, the suffix ‘carbonitrile’ is added after the name of the parent hydrocarbon.
| Class 11 Physics | Class 12 Maths | Class 11 Chemistry |
| NEET Foundation | Class 12 Physics | NEET Physics |
Example:

Preparations Of Alkyl Cyanides
Alkyl Cynides From Alkyl Halides: Primary alkyl halides (preferably bromides and iodides) when heated with an alcoholic solution of NaCN or KCN, yield alkyl cyanides. In this reaction, alkyl isocyanide (RNC) is also obtained as a by-product.

Alkyl Cynides From Alkyl Halides Example:

Preparation Of Aryl Cyanide: In the reaction of diazonium salt with CuCN (extended Sandmeyer reaction), aryl cyanide is obtained.

Aryl Cyanide Reaction Mechanism: CN– ion, produced from KCN reacts both with 1° alkyl halides in aqueous solution via SN2 mechanism. Relatively larger and less electronegative carbon atom (electron cloud is less polarisable) of the ambident nucleophile ![]() attacks or -carbon atom of the alkyl halide, giving alkyl cyanide.
attacks or -carbon atom of the alkyl halide, giving alkyl cyanide.

Secondary alkyl halides (preferably bromides or iodides) also react with alcoholic solution of NaCN or KCN to give alkyl isocyanide as the major product.

The reaction proceeds through the SN2 mechanism.
From Acid Amides: When acid amide is heated in the presence of phosphorus pentoxide (P2O5) or thionyl chloride (SOCl2), alkyl cyanide is obtained. In this process, alkyl isocyanide is not obtained as a by-product.
Example:

From Ammonium Salts Of Carboxylic Acid: Ammonium salt of carboxylic acid when distilled in the presence of phosphorus pentoxide gives alkyl cyanide.
Carboxylic Acid Example:

Dehydration Of Aldoximes: Alkyl cyanide is obtained by heating a mixture of aldoxime and P205 or acetic anhydride.

Dehydration Of Aldoximes Example:

From Carboxylic Acids: Alkyl cyanide is produced on a large scale by passing a mixture of carboxylic acid vapours and ammonia over alumina (Al2O3) heated to 500°C.
![]()
Preparation of alkyl cyanide with the same number of carbon atoms from an aldehyde: Aldoxime, derived in the reaction between aldehyde and hydroxylamine, yields alkyl cyanide on dehydration.

Preparation of alkyl cyanide with the same number of carbon atoms from a carboxylic acid: Carboxylic acid is successively converted into acid chloride and acetamide and the latter is dehydrated by heating with P2O5.

From Grignard Reagent: Cyanogen chloride (ClCN) reacts with Grignard reagent to give alkyl cyanide. This method is highly suitable for the preparation of tertiary alkyl cyanide.
![]()
Grignard Reagent Example:

Properties And Uses Of Alkyl Cyanides
Physical Properties
- Lower members of alkyl cyanides are colourless liquids but higher members are crystalline solids.
- Generally, these alkyl cyanides are quite stable, have a sweet smell and are not poisonous like hydrogen cyanide (HCN).
- Molecules of alkyl cyanides have high dipole moments due to the presence of a polar cyano group. The boiling point of alkyl cyanides is higher than those of alkyl halides of comparable molecular mass. It is due to a stronger intermolecular force of attraction involving dipole-dipole attractive force.
CH3CN (Boilingpoint= 82°C); CH3CI (Boilingpoint= – 24°C)
Alkyl cyanides of lower molecular masses are soluble in water due to the formation of hydrogen bonds between the molecules of alkyl cyanides and water.

- With the increase in molecular mass, the size of the alkyl group present in an alkyl cyanide increases, i.e., the effect of the non-polar hydrocarbon part predominates. Consequently, solubility in water decreases.
- Alkyl cyanides are easily soluble in organic solvents such as alcohol, ether, benzene etc.
Chemical Properties
Chemical Properties Hydrolysis: Alkyl cyanides, when refluxed in the presence of dilute acid solution, undergo hydrolysis and produce carboxylic acids.
![]()
If an alkyl cyanide is refluxed with dilute alkali (for example., NaOH) it gets hydrolysed forming sodium salt of carboxylic acid (RCOONa). Acidification of the reaction mixture liberates free carboxylic acid.

Alkyl cyanides (RCN) undergo hydrolysis to produce carboxylic acids. The carbon atom of the carboxylic acid group is directly linked to the alkyl group R, so, the carbon atom of the —CN group in RCN is directly attached to the R group.
Partial hydrolysis: When alkyl cyanide is dissolved in concentrated H2SO4 and added to cold water or it is shaken with cold and cone. HCl, it suffers incomplete hydrolysis producing acid amide.
![]()
Formation Of Acid Amide: Alkyl cyanide reacts with an alkaline H2O4 solution to give the acid amide.

Acid Amide Reduction: Alkyl cyanides are reduced by hydrogen in the presence of Pt or Ni catalyst or (sodium+alcohol) or lithium aluminium hydride (LiAlH4), to give primary amines with the same number of carbon atoms.

Stephen Process: In this process, alkyl cyanides are reduced by stannous chloride and concentrated HCl in ether medium to form a complex which on hydrolysis gives aldehydes.

Formation Of Esters: Esters are formed when an alcoholic solution of alkyl cyanides is heated with concentrated H2SO4 or HCl.

Reaction With Grignard Reagent: Alkyl cyanide reacts with Grignard reagent to form a complex, which on hydrolysis with dilute acid gives a ketone.

Uses Of Alkyl Cyanides
- Some of the alkyl cyanides, particularly methyl cyanide (acetonitrile), is used as a solvent.
- Alkyl cyanides are used in the preparation of nitrile rubber and in the cotton industry.
- The process of conversion of a lower homologue into its higher homologue is accomplished through the formation of alkyl cyanide.
- In the synthesis of organic compounds such as amine, aldehyde, ketone, acid, ester, amide etc., alkyl cyanide is used as an intermediate.
Alkyl Isocyanide (R-NC)
Alkyl isocyanide is an isomer of alkyl cyanide. These compounds are also known as alkyl isonitrile or arylamine. The alkyl group present in a molecule of alkyl isocyanide is attached to a carbon atom through a nitrogen atom.

Nomenclature Of Alkyl Isocyanides
General method (Common system): In this system, compounds belonging to this class are named as isonitriles or isocyanides or carbylamines. The system of nomenclature for iso-nitrite is similar to that used for isomeric alkyl cyanide. In the case of names such as isocyanide or arylamine, the suffix ‘isocyanide’ or ‘arylamine’ is added to the alkyl group present in the molecules under consideration.
IUPAC Method: In this system, compounds belonging to this class are named iso carbonitride alkane or alkane isocarbonitrile.

Preparation Of Alkyl Isocyanides
Secondary alkyl halides (preferably bromides or iodides) react with an alcoholic solution of silver cyanide (AgCN) to give alkyl isocyanide as the major product. [If tertiary alkyl halide is used as the substrate, then an alkene is formed as the major product via elimination reaction.] In this reaction, alkyl cyanide is formed as a minor product.
![]()
Example:

Reacted Mechanism: In the presence of AgCN, silver halide is precipitated from alkyl halide producing a carbocation. Consequently, the reaction follows an SN1 path. Here cyanide ion is an ambident nucleophile![]() in which Natom is relatively smaller and more electronegative. Hence N-atom attacks the carbocation forming alkyl isocyanide as the main product.
in which Natom is relatively smaller and more electronegative. Hence N-atom attacks the carbocation forming alkyl isocyanide as the main product.

Primary alkyl halides (preferably bromides and iodides) also react with an alcoholic solution of silver cyanide to give alkyl cyanide as the major product.

The reaction occurs by the SN2 mechanism.
From Primary Amines: When primary amine is heated in the presence of chloroform and alcoholic potassium hydroxide (KOH) solution, alkyl isocyanide is obtained. This reaction is called arylamine reaction.

From N-alkyl Formamide: When IV-alkyl formamide is heated with POCl3 in the presence of pyridine, alkyl isocyanide is formed.

Properties And Uses Of Alkyl Isocyanides
- Alkyl isocyanides are poisonous liquids having obnoxious smell.
- The boiling point of an alkyl isocyanide is lower than that of the isomeric alkyl cyanide due to its lower polarity. CH3CN (boiling. = 82°C); CH3NC (boiling. = 49°C)
- Alkyl isocyanides are almost insoluble in water because of their inability to form hydrogen bonds with water molecules. But they are fairly soluble in organic solvents like ether, benzene etc.
Alkyl Isocyanides Chemical Properties
Alkyl Isocyanides Chemical Properties Hydrolysis: Alkyl isocyanides when shaken with dilute acids, are hydrolysed even in cold conditions producing primary amine and formic acid (different from alkyl cyanide).

In the hydrolysis of alkyl isocyanide, no acid other than formic acid is produced. Alkyl isocyanides are not hydrolysed by alkaline solutions.
In an isocyanate group ( — N+ =C–) both the N and C-atoms have filled octets of electrons. So, the e-nucleophile (O–H) can attack neither the C-atom nor the Natom of the isocyanide molecule.
Alkyl Isocyanides Chemical Properties Reduction: Catalytic hydrogenation of alkyl isocyanide or its reduction with sodium and alcohol, leads to the formation of a 2° amine (note the difference from alkyl cyanide). The 2° amine, produced contains a methyl group attached to the N-atom.

Alkyl Isocyanides Chemical Properties Example:

Isomerisation: On prolonged heating, alkyl isocyanide isomerises to form more stable isomeric alkyl cyanide.

Isomerisation Reducing Action: The carbon atom of the isocyanide group in alkyl isocyanide contains a lone pair of electrons. Due to the presence of a lone pair of electrons, alkyl isocyanides act as reducing agents.

Isomerisation Example: Methyl isocyanide reduces HgO to Hg, itself being oxidised to methyl Isocyanate.

Use Of Alkyl Isocyanide: It is used in the preparation of secondary amines with N-methyl group (R—NH—CH3).
Distinction Between Alkyl Cyanide And Alkyl Isocyanide:

Nitroparaffin Or Nitroalkane (R-NO2)
The organic compound obtained by the replacement of one hydrogen atom from a molecule of saturated hydrocarbon or alkane is called nitroalkane or nitroparaffin.
![]() Nitroalkanes are classified as primary, secondary or tertiary based on the nature of the carbon atom (i.e., 1°, 2° or 3°) to which the nitro group (—NO2) is attached.
Nitroalkanes are classified as primary, secondary or tertiary based on the nature of the carbon atom (i.e., 1°, 2° or 3°) to which the nitro group (—NO2) is attached.

Nomenclature Of Nitroalkane
In both the trivial and IUPAC systems of nomenclature, nitroalkanes are named as derivatives of alkanes. The position of the nitro group in the longest chain of carbon atoms is marked by the lowest possible number.
Example:

Preparation Of Nitroalkanes
From Alkyl Halides: Primary alkyl halides (preferably bromides or iodides i.e., RCH2Br and RCH2I) dissolved in dimethyl formamide (DMF) as solvent give nitroalkane on reaction with NaNO2 or KNO2. To increase the solubility of nitrite salts, some urea is added to the reaction mixture. If dimethyl sulphoxide (DMSO) is used as a solvent, the addition of urea is unnecessary.

Alkyl nitrite is formed in small amounts as a by-product.
Reaction Mechanism: In the solvent DMF or DMSO, the reaction proceeds through the SN2 path. In the given ambident nucleophile, the N-atom is relatively larger and less electronegative, having a more polarizable electron cloud O– — N=O↔O=N—O–. Hence N-atom of the nitrite ion attacks the carbon atom of the alkyl halide forming nitroalkane as the major product.

The reaction of ethanolic silver nitrate solution with a primary alkyl halide (preferably bromide or iodide) gives a satisfactory yield of primary nitroalkane.
![]()
Here also the reaction proceeds through the SN2 mechanism and the O–—N:=O (nitrite) ion acts as the nucleophile.

From Tertiary (3°) Alkyl Amines
Oxidation of a primary amine-containing tertiary alkyl group with potassium permanganate gives a 3° nitroalkane.
Example:

By Vapour Phase Nitration Of Alkanes: A mixture of gaseous alkane and HNO3 vapour when heated to 400-475°C, yields nitroalkanes.
Example:

Nitration of alkanes containing two or more carbon atoms at high temperatures brings about the cleavage of the C—C bond, consequently producing a mixture of nitroalkanes.
Example:

From α-Halogeno Acids: After heating sodium or potassium salt of α-halogen acid with a solution of sodium nitrite, the reaction mixture is acidified to produce α-nitro acid, which eliminates CO2 gas forming nitroalkanes.
Example:

Properties And Uses Of Nitroalkanes
Physical Properties
- In the pure state, nitroalkanes are colourless liquids with a pleasant smell.
- Nitroalkanes are sparingly soluble in water, lower nitroalkanes being relatively more soluble. In organic solvents, they dissolve easily.
- The dipole moments of nitroalkanes, (μ – 3-4D) are very high because the molecules remain strongly bound by dipole-dipole attractive forces. Therefore, the boiling point of nitroalkanes is much higher than that of alkanes with comparable molecular mass.

The polarity of nitroalkanes is much higher relative to isomeric alkyl nitrites. So the nitroalkanes have much higher boiling points than the isomeric alkyl nitrites. For example, the boiling points of nitroethane and ethyl nitrite are 115°C and -12°C respectively.
Chemical Properties
Reduction: Nitroalkanes are reduced involving the following stages—
 The nature of the product obtained by reduction depends on the nature of the reducing agent and the pH of the reaction medium.
The nature of the product obtained by reduction depends on the nature of the reducing agent and the pH of the reaction medium.
Reduction in acid medium: Nitroalkanes are reduced by tin and HCl or iron and HCl or zinc and HCl to give primary amines. This reaction proves that the nitrogen atom of the nitro group is directly bonded to the alkyl group.

Reduction in neutral medium: Nitroalkane, when heated with zinc dust and ammonium chloride solution in the presence of a few drops of alcohol yields alkyl-substituted hydroxylamine.

Identification Of Nitroalkanes By Muliiken-Barker Test: The nitro compound is boiled with zinc dust and aqueous NH4Cl solution in the presence of a few drops of ethyl alcohol.
The resulting solution is filtered into a freshly prepared Tollens reagent. The appearance of a grey precipitate indicates the presence of the —NO2 group.
Reduction By Hydrogen: Nitroalkanes are reduced by H2 in the presence of Ni, Pt or, Pd catalyst to give 1° amines.
![]()
Reduction by LiAlH4: Nitroalkanes are converted into primary amines by reduction with LiAlH4.
![]()
Hydrolysis: If primary nitroalkane is treated with boiling concentrated HCl or 85% concentrated H2SO4, it undergoes hydrolysis yielding carboxylic acid and hydroxylamine. This reaction is the basis of industrial production of hydroxylamine.

Secondary nitroalkanes are reduced by boiling with concentrated HCl to produce ketone and nitrous oxide.

Tertiary nitroalkanes are not hydrolysed by concentrated HCl or concentrated H2SO4.
Nitro-Aci-Nitro Tautomerism
Nitroalkanes containing ar-H atom, (i.e., primary and secondary nitroalkanes) display tautomerism.
Example: One H-atom from a -carbon atom of nitroethane detaches and gets itself linked to an O-atom of —NO2, giving rise to an isomeric compound with a completely different structure. The form of the compound is called nitro-form and the form is called an acid-nitro-form.

Out of the two tautomers of nitroethane (1 and 2), nitroform Is more stable than aci-nitro-form because of the resonance stabilisation of nitro-form
Hence, in the tautomeric mixture, the concentration of nitro-form is quite greater than that of aci-nitro-form 2.

Tertiary alkanes do not exhibit tautomerism (no α-H ).
Acidic Nature
- Nitroalkanes have no action on litmus paper as they are neutral compounds. But primary and secondary nitroalkanes which have ar-H atom, dissolve in alkali forming salts. From this, it is evident that primary and secondary nitroalkanes act as mild acids.
- Primary and secondary nitroalkanes behave as acids only in the presence of alkali, so these are called pseudo acids. These compounds are converted into aciform before they are reacted with alkalies.
- The nitro-form of nitroalkanes is called ‘pseudo acid’ and the aci-nitro-form is called ‘nitronic acid’. Aci-microforms are crystalline solids which dissolve in sodium hydroxide to give red solutions.

Owing to the absence of any α-H atom in tertiary nitroalkanes, they cannot exist in aci-nitro-form. As a result, tertiary nitroalkanes do not react with alkalies and hence, do not show any acidic properties.
Reaction With Nitrous Acid: Depending on their behaviour towards HNO2, primary, secondary and tertiary nitroalkanes can be differentiated by their reactions with nitrous acid as follows:
1° nitroalkanes react with nitrous acid, forming a crystalline compound, nitrolic acid, which dissolves in NaOH to give a red solution.

2° nitroalkanes react with HN02 forming a crystalline compound, pseudonitrole, which forms a blue solution with NaOH, indicating the presence of nitroso group.

3° nitroalkanes do not contain any ar-H atom. So they do not react with nitrous acid.
Halogenation: Primary and secondary nitroalkanes react with alkalies in the presence of halogen-producing halogen nitroalkanes. In this reaction, α-H atoms of nitroalkanes are successively replaced by halogen atoms.
Example:

When nitromethane is allowed to react with an excess chlorine, trichloro nitromethane or chloropicrin is formed. This Is also known as tear gas.

Uses Of Nitroalkanes
- Lower nitroalkanes are used as solvents for oil, fats, resin and paints.
- Nitroalkanes are used as intermediate compounds for the preparation of detergent, propellant, etc.
- Chloropicrin, a derivative of nitromethane, finds use as an insecticide and tear gas.
- In the manufacture of hydroxylamine, primary nitroalkane is used.
Alkyl Nitrite (R-ONA)
Alkyl nitrite is an ester of the inorganic acid, HNO2 (nitrous acid). Their general formula: is R—O—N=O. They are well-known as isomers of nitroalkanes. The alkyl group present in the molecule of alkyl nitrite is bonded to the nitrogen atom through an oxygen atom. Among the compounds belonging to this class, ethyl nitrite and isoamyl nitrite are important.

Preparations Of Alkyl Nitrites
From Alkyl Halides: In the reaction of 2° and 3° alkyl halides (preferably bromides and iodides) with silver nitrate solution, alkyl nitrites are obtained as the major product.
![]()
Reaction Mechanism: In the reaction between silver nitrite and alkyl halide, alkyl carbocation is produced.
- As a result, the reaction follows the SN1 path. Here nitrite ion (NO–2) is an ambident nucleophile in which O-atom is relatively smaller and more electronegative having higher electron density
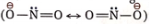 .
. - Hence O-atom attacks the carbon atom of the carbocation, resulting in the formation of alkyl nitrite as the major product.

If a secondary or tertiary alkyl halide (preferably bromide or iodide) is treated with an aqueous NaNO2 solution, then also alkyl nitrile is formed as the major product.

From Alcohols: On adding concentrated HCl or concentrated H2SO4 to a solution of C2H5OH and aqueous NaNO2, ethyl nitrite is obtained.

Isoamyl nitrite is produced when concentrated HCl is added to a solution of isopentyl alcohol and NaNO2.

Properties And Uses Of Alkyl Nitrites
Physical Properties: Ethyl nitrites and isoamyl nitrites are liquids with pleasant smell. Their boiling points are 17°C and 99°C, respectively.
Chemical Properties
Hydrolysis: Alkyl nitrite, when heated with aqueous solution of alkali undergoes hydrolysis to yield alcohol and nitrite salt.

Reduction: When alkyl nitrite is reduced by (Sn + HCl) or (Zn + HCl), the major products obtained are alcohol and hydroxylamine. This reaction proves that in alkyl nitrite, the alkyl group is attached to the N-atom through an O-atom.

Uses Of Alkyl Nitrites
- In the preparation of nitrous acid in an anhydrous medium, alkyl nitrites are used.
- A 4% alcoholic solution of ethyl nitrite is known as the sweet spirit of nitre. It is used as a heart stimulant and diuretic.
Distinction Between Nitroalkane And Alkyl Nitrite:

Aromatic Nitro Compounds
Aromatic nitro compounds are formed by the replacement of one or more hydrogen atoms in the benzene ring. Nitrobenzene is an ideal representative of aromatic nitro compounds.
Nomenclature Of Aromatic Nitro Compounds
Aromatic nitro compounds are named as nitroarenes.
Nitro compounds Example:

Preparation Of Aromatic Nitro Compounds
With the help of nitrating reagents Aromatic nitro compounds are prepared by the reaction of a suitable aromatic compound with any one of the following nitrating reagents—
- Mixed acid (concentrated HNO3 + concentrated H2SO4) or (fuming nitric acid + concentrated H2SO4) or (fuming nitric acid + fuming H2SO4),
- Concentrated HNO3 dissolved in glacial acetic acid or nitromethane,
- Acetyl nitrate (concentrated HNO3 dissolved in acetic anhydride),
- Nitronium salt dissolved in organic solvents [for example., nitronium perchlorate, (NO+2ClO–4), nitronium tetrafluoroborate (NO+2BF–4).
Preparation Of Aromatic Nitro Compound Using Mixed Acid
Nitrobenzene is prepared by heating benzene with a mixture of concentrated (HNO3 + H2SO4) at 50-60°C.

During the reaction, temperature is strictly controlled as at higher temperatures (>60°C), meta-dinitrobenzene is produced.
Nitrobenzene Reaction Mechanism: In the reaction between concentrated HNO3 and concentrated H2SO4 (nitrating reagent), the nitronium cation (NO2) acts as an electrophile.

It is known from experiments that the nitrations of C6H6 and C6D6 occur at the same rate. So the second step (in which the C—H or C—D bond cleaves), is not the rate-determining step. Hence, the first step which involves the formation of σ-complex between the electrophile and the substrate is the rate-determining step.

Nitration of toluene with mixed acids at ordinary temperature gives a mixture of o-and p-nitrotoluene.

Nitration of chlorobenzene with mixed acid at 100°C, gives a mixture o-and of p-chloronitro benzenes.

In the reaction of mixed acid with acetanilide, p-isomer is produced as a major product.

(—CH3, —Cl, —NHCOCH3 groups are o-/p-directing.)
When nitrobenzene is heated with a mixture of fuming nitric acid and concentrated H2SO4 in a boiling water bath, m-dinitrobenzene is obtained.

2,4-dinitrotoluene, obtained in the nitration of toluene, is subjected to further nitration at high temperature in the presence of fuming nitric acid and fuming H2SO4, when 2, 4, 6-trinitrotoluene (TNT) is obtained.

Oxidation of 2,4,6-trinitrotoluene (TNT), obtained in the nitration of toluene, gives rise to 2,4,6-trinitrobenzoic acid, which on decarboxylation yields 1,3,5-trinitrobenzene.

If fuming nitric acid is used during nitration, NO–2 ion is produced.
![]()
In the case of nitration with N2O5 dissolved in a polar solvent, the NO+2 ion is formed according to the following equilibrium.
![]()
Preparation of aromatic nitro compound using dil. HNO3
The presence of hydroxyl group ( —OH) in the benzene ring activates the ring to such an extent that treatment of phenol even with dilute HNO3 at ordinary temperature gives a mixture of o- and p- nitrophenols. A small amount of phenol is, however, oxidised by HNO3 where nitric acid is itself reduced to nitrous acid.

Preparation of aromatic nitro compound Reaction Mechanism: In the case of nitration with dilute HNO3, the reaction is initiated through the formation of nitrosonium ions. The nitroso compound so produced undergoes oxidation to form the corresponding nitro compound.

From Diazonium Salts: Fluoroboric acid reacts with arene diazonium chloride to form arene diazonium fluoroborate. To replace the —N–2BF+4 group with the nitro group, the salt so produced is decomposed in the presence of an aqueous solution of NaNO2 and Cu powder.
Diazonium Salts Example:


From Aromatic Amines: Aromatic amines are oxidised by trifluoroacetic acid to their corresponding nitro compounds.
Aromatic Amines Example:

Properties And Uses Of Aromatic Nitro Compounds
The physical and chemical properties of nitrobenzene as an ideal representative of aromatic compounds, are discussed below:
Nitro Compounds Physical Properties:
- Nitrobenzene is a light yellow oily liquid. Its commercial name is ‘oil of mirabane’. It has a characteristic smell of bitter almonds. It is immiscible in water.
- The boiling point of nitrobenzene is 211°C. It is solidified by cooling on ice. Solid nitrobenzene melts at 5.8°C.
- Nitrobenzene is heavier than water (sp. gravity: 1.204). It is steam volatile and has poisonous vapours.
- Nitro group present in nitrobenzene withdraws electrons from the ring through -I and -R effects. Consequently, the ring acquires a partial positive charge while the —NO2 group acquires a partial negative charge, which makes the molecule sufficiently polar (dipole moment: 3.95D).
Such a high dipole moment is responsible for the dipole-dipole interactions among the molecules, making nitrobenzene a high boiling liquid (b.p. 211°C ).
Chemical Properties: Nitrobenzene is a stable compound, generally not attacked by acids, alkalis or oxidising agents. So, it is used as an effective solvent In various oxidation reactions. The reactions of nitrobenzene may be classified under two heads—
- Reactions of nitro group (—NO2) (where the benzene ring remains unaffected) and
- Substitution reactions in the benzene ring (where the nitro group remains unaffected).
Reactions Of Nitro Group: The most important reaction of the nitro group in nitrobenzene is its reduction. As the nitro group is easily reduced, it is frequently used as an oxidising agent. The reduction of nitrobenzene takes place through the following steps:

Depending on the nature of the reducing agent and the concentration of hydrogen ions in the reduction medium, the products of reduction are found to be different.
Reduction in acid medium: Nitrobenzene, when reduced by tin, zinc or iron and concentrated HCl or zinc and acetic acid, gives aniline. All aromatic nitro compounds can be similarly reduced to give primary amines.

Reduction by metal in a strong acidic medium forms an intermediate compound, phenylhydroxylamine (C6H5NHOH) which undergoes rearrangement producing p-aminophenol.

Reduction In Neutral Medium: When nitrobenzene dissolved in 50% alcohol is warmed with zinc dust and an aqueous solution of ammonium chloride, it is reduced to phenylhydroxylamine.

Electrolytic reduction of nitrobenzene in acetic acid and aqueous solution of sodium acetate produces phenylhydroxylamine.

Reduction In Alkaline Medium: Depending on the nature of reducing agents, different products such as azoxybenzene, azobenzene and hydrazobenzene are formed in an alkaline medium.
when nitrobenzene is reduced by zinc dust and a methanolic solution of NaOH, at first azoxybenzene and then azobenzene is formed.

Nitrobenzene is reduced by Zn-dust an aqueous solution of NaOH to form hydrazobenzene.

Reduction by LiAIH4: In the reduction of nitrobenzene by lithium aluminium hydride, azobenzene is produced.

It is interesting to note that the reduction of aliphatic nitro compounds with lithium aluminium hydride gives the corresponding amines.
Electrolytic Reduction In Acidic Medium: Electrolytic reduction of nitrobenzene in a mild acidic medium gives aniline. On the other hand, electrolytic reduction of nitrobenzene in the strong acidic medium at first produces phenylhydroxylamine, which on rearrangement gives p-aminophenol.

Selective Reduction: m-dinitrobenzene on partial reduction by NH4HS gives m-nitroaniline.

m-nitroaniline is also obtained by controlled reduction of nitrobenzene using Na2S or (NH4)2S.
Hydrogenation: Nitrobenzene on catalytic hydrogenation (in the presence of Raney nickel, Pd or, Pt-C ) under 30 atm pressure gives aniline.

Substitution Reaction In Benzene Ring
Electrophilic substitution reaction: As the nitro group (—NO2) is meta-orienting in the electrophilic substitution reactions, the incoming substituent mainly enters the meta-position.
In the presence of electron attracting nitro group, the electron density of the benzene ring decreases and consequently, the rate of electrophilic substitution in nitrobenzene is much slower than that in benzene. Different types of electrophilic substitution reactions of nitrobenzene are given below:

Nitration: Nitrobenzene when heated with fuming nitric acid and fuming sulphuric acid in a boiling water bath, gives a deep yellow liquid, meta-dinitrobenzene.

If nitrobenzene is refluxed with fuming nitric acid and fuming sulphuric acid, 1,3,5-trinitrobenzene (TNB) is produced. The reaction takes five days, as it is extremely difficult to introduce the third nitro group. TNB is a highly explosive substance.

Chlorination: When chlorine gas is passed into hot nitrobenzene in the presence of iron powder or aluminium chloride, meta-chloronltrobenzene is formed.

Similarly, bromine reacts with nitrobenzene in the presence of iron powder to give meta-bromonitrobenzene.
Sulphonation: On heating with fuming sulphuric acid, nitrobenzene gives meta-nitrobenzene sulphonic acid.

Friedel-Crafts reaction: The electrophilic species involved in Friedel-Crafts reaction are very weak. Nitrobenzene fails to undergo substitution reactions with such electrophiles because the ring system of this molecule is highly electron deficient. This can be attributed to the -I and -R effects of the —NO2 group. Hence, nitrobenzene does not participate in the Friedel-Crafts reaction.
Nucleophilic Substitution Reaction: Nitro group (—NO2) present in nitrobenzene decreases the electron density of ortho- and para-positions to a greater extent, relative to meta-position. Hence, the meta-position becomes comparatively electron-rich, while the ortho- and parapositions are reduced to electron-deficient sites. Therefore, ortho- and para-positions of the molecule of nitrobenzene are easily attacked by nucleophiles.

Nucleophilic Substitution Example: When nitrobenzene is fused with caustic potash in the presence of air, it mainly gives orthonitrophenol as its potassium salt, which on subsequent acidification produces o-nitrophenol.

Uses Of Nitrobenzene
- Nitrobenzene is used
- As a high-boiling solvent,
- As a mild oxidising agent in organic synthesis
- In the preparation of aniline, benzidine and some azo-dyes,
- In boot polish,
- In the polishing of the floor using wax,
- In lowgrade scented soaps and
- In the preparation of explosives such as TNT, TNB, etc.
Tests For Nitro Group: Identification Of Nitrobenzene
Identification Nitrobenzene Reduction Test: Nitrobenzene when heated with Sn and concentrated HCl is reduced to aniline.
- The resulting solution is cooled (0-5°C) and treated with dilute HCI and dilute NaNO2 solution, producing benzenediazonium chloride.
- The addition of a few drops of this solution to a cold alkaline p-naphthol solution gives a bright, scarlet red azo dye.

- It is a test for the identification of the aromatic primary amino (—NH2) group. As the —NO2 group is reduced to the —NH2 group, it may be regarded as an indirect test for the detection of the —NO2 group in the benzene ring.
- This test can be applied to identify the —NO2 group in the absence of the —NH2 group in the benzene ring.
Mulliken-Barker Test: Nitrobenzene is boiled with zinc dust and an aqueous solution of NH4Cl in 50% C2H5OH, producing phenylhydroxylamine.
- The resulting solution is filtered into a freshly prepared Tollens’ reagent (ammoniacal silver nitrate solution).
- The appearance of a black or grey precipitate of silver indicates the presence of the —NO2 group.
- This test can be applied to identify the —NO2 group even in the presence of the —NH2 group in the benzene ring.
- Hence, this reaction Is a confirmatory test for the detection of the —NO2 group, when the —NH2 group is also present in the benzene ring.

Nitro (—NO2) group present in any organic compound (aliphatic or aromatic) is identified with the help of this test.
Limitations of Mulliken-Barker test: This test for the detection of the —NO2 group is not applicable when an organic compound already contains any other reducible functional group.
- For example, if an aldehyde (—CHO) group or α-hydroxyketo [—CH(OH)CO—] group is present in any organic compound, then, the Mulliken-Barker test for the identification of the —NO2 group cannot be used.
- Because aldehydes and α-hydroxyketones reduce Tollens’ reagent to give a precipitate of metallic silver (Ag).

Amines Introduction
Amines are considered as an important class of organic compounds. Amines are derived by the replacement of one or more H -atoms of ammonia molecules by alkyl or aryl groups.
- These are commonly found in nature as proteins, vitamins, hormones, alkaloids, etc.
- A large number of artificially prepared amino compounds are used as polymers, dyes and drugs. Adrenaline (a hormone) and ephedrine (a drug) in which a secondary amino group is present are used to increase blood pressure.
- Novocaine, an artificially prepared amino compound is used in dental treatment as an anaesthetic agent. Antihistamine drug viz. benadryl contains a tertiary amino group. Quaternary ammonium salts are widely used as surface active agents.
- Diazonium salts find extensive application as intermediates in the preparation of aromatic compounds and dyes.
Classification And Structure Of Amines
Classification Of Amines
Primary, Secondary And Tertiary Amines: Aliphatic amines are regarded as derivatives of ammonia. Amines are divided into three classes—primary (1°), secondary (2°) and tertiary (3°). Replacement of one, two or three H -atoms of ammonia molecule by alkyl or aryl groups produces primary (1°), secondary (2°) and tertiary (3°) amines, respectively.

R = alkyl or aryl group ( —CH3, —C2H5, —C6H5, etc.)
Functional groups present in 1°, 2° and 3° amines are:-

Aliphatic And Aromatic Amines
Aliphatic Amines: Aliphatic amines are derived by the replacement of one or more H -atoms of ammonia molecules by alkyl groups.
Aliphatic Amines Example:

Aromatic amines: In aromatic amines, at least one aryl group is attached to the amino nitrogen atom.
Aromatic amines Example:

The amines in which the N-atom is linked to the side chain of the aromatic ring are called aryl-substituted aliphatic amines.
Aromatic amines Example:

Simple And Mixed Amines
Simple Amines: If the alkyl or aryl groups attached to the N-atom are identical, then such amines are known as simple amines.
Simple Amines Example:

Mixed Amines: If the alkyl or aryl groups bonded to the N-atom are different, then such amines are known as mixed amines.
Mixed Amines Example:

Quaternary Ammonium Salts: Besides these three types of amines, there is another class of nitrogenous compounds containing quaternary N-atom.
- These compounds are known as tetraalkyl ammonium salts or quaternary ammonium salts.
- These compounds are produced by the substitution of 4 H -atoms of the ammonium salts by the alkyl group.

Quaternary Ammonium Salts Example: [(CH3)4N]+Cl– (tetramethylammonium chloride)
[(C2H5)2N(CH3)2]+OH– (diethyl dimethylammonium
Structure Of Amines: Like ammonia molecules, the structure of amines is pyramidal. The central N-atom is sp3-hybridised.
- The sp3-hybrid orbitals of N-atom form three σ-bonds with H-atom or an alkyl group and the fourth sp3-hybrid orbital contains a lone pair of electrons.
- Since Ip-bp repulsion is more than bp-bp repulsion, the angle between any two H-atoms or alkyl groups is less than the expected value (109°28′)- The bond angle is generally 107-108°.

- Despite having structural chirality, 3° amines with formula R1R2R3N: do not display optical activity.
- This is because, with the exchange of a small amount of energy (~25kJ-mol-1), rapid interconversion between a pair of enantiomers occurs and hence, they exist as ± or dl -mixture.

- Rapid interconversion between a pair of enantiomers
- However, ammonium salts with the formula R1R2R3R4N+X– exhibit optical activity due to their chiral structure.

Nomenclature Of Amino Compounds
Nomenclature Of Aliphatic Amines
General Method (Common System): According to this system, the amines are named using the suffix ‘amine’ after the name of the alkyl group(s) present in the amine.
General Method Example:

In the case of simple secondary and tertiary amines, the prefixes di- and tri-respectively are added before the name of the alkyl group.
General Method Example:

In the case of mixed secondary and tertiary amines, the names of the alkyl groups attached to the N-atom are arranged in alphabetical order.
General Method Example:

IUPAC Method: According to this system, the amines are named by replacing ‘e’ from the name of the parent alkane with the suffix ‘amine’ i.e., primary amines are regarded as alkanamine. They are named by replacing ‘and’ from the name of the alkane derived based on the number of carbon atoms in the longest carbon chain containing the amino group ( —NH2) with ‘anamine’.
IUPAC Method Example:

Secondary or tertiary amines are considered N-substituted derivatives of primary amines. The longest carbon chain attached to the nitrogen atom is taken as the alkyl group of primary amine. The other alkyl
groups are written before the name of the parent primary amine (1°) with the prefix ‘N’.
IUPAC Method Example:

Nomenclature Of Aromatic Amines: According to the conventional system of nomenclature, aromatic amines are called arylamines. The simplest aromatic amine is called aniline.
- Generally, substituted aromatic amines are considered derivatives of aniline. In some cases, special names are also used.
Aromatic Amines Example: o-/m-/p- methyl anilines are called o-/m-/ptoluidines while o-/m-/p- methoxy anilines are known as anisidines.
- According to the IUPAC system, the suffix ‘e’ of the arene is replaced by ‘amine’.
Aromatic Amines Example: Aminobenzene is named as benzenamine. The name Aniline is, however, accepted by IUPAC.


Isomerism In Amino Compounds
Amino Compounds Chain Isomerism: This type of isomerism arises due to the difference in the carbon chain attached to the amino group.
Amino Compounds Chain Isomerism Example:

Amino Compounds Position Isomerism: This type of isomerism occurs due to the difference in the position of the —NH2 group i.e., a functional group in the carbon chain.
Amino Compounds Position Isomerism Example:

Functional Group Isomerism: In compounds having the same molecular formula, the presence of different classes of amino groups (1°, 2° or 3°) gives rise to this type of isomerism.
Example: The functional group isomers of C3H9N are:

Metamerism: In the compounds having the same molecular formula and belonging to the same class of amino compounds, the presence of different alkyl groups bonded to N-atom gives rise to this type of isomerism. Hence, secondary and tertiary amines exhibit this type of isomerism.
Example:

Methods Of Preparation Of Mixture Of Amines
By Ammonolysis: Hofmann’s Method
An alcoholic solution of NH3, when heated with an alkyl halide in a closed glass tube at 100°C, produces a mixture of primary, secondary and tertiary amines along with quaternary ammonium salts. This reaction is known as ammonolysis.
Hofmann’s Method Example:
⇒ \(\mathrm{C}_2 \mathrm{H}_5 \mathrm{I}+\mathrm{NH}_3 \longrightarrow \mathrm{C}_2 \mathrm{H}_5 \mathrm{NH}_2+\mathrm{HI}\)
⇒ \(\mathrm{C}_2 \mathrm{H}_5 \mathrm{NH}_2+\mathrm{C}_2 \mathrm{H}_5 \mathrm{I} \longrightarrow\left(\mathrm{C}_2 \mathrm{H}_5\right)_2 \mathrm{NH}+\mathrm{HI}\)
⇒ \(\left(\mathrm{C}_2 \mathrm{H}_5\right)_2 \mathrm{NH}+\mathrm{C}_2 \mathrm{H}_5 \mathrm{I} \longrightarrow\left(\mathrm{C}_2 \mathrm{H}_5\right)_3 \mathrm{~N}+\mathrm{HI}\)
⇒ \(\left(\mathrm{C}_2 \mathrm{H}_5\right)_3 \mathrm{~N}+\mathrm{C}_2 \mathrm{H}_5 \mathrm{I} \longrightarrow\left[\left(\mathrm{C}_2 \mathrm{H}_5\right)_4 \mathrm{~N}\right]^{+} \mathrm{I}^{-}\)
- In this reaction, amine (base) and HI (acid) combine C2H5NH2 + HI → C2H5NH+3I– ). If the acid present in the reaction mixture is neutralised with the addition of excess base and then the resulting solution is distilled, a mixture of primary, secondary and tertiary amines is obtained in the receiving flask.
- Primary, secondary and tertiary amines are separated from their mixture by fractional distillation or Hinsberg’s method.
- In ammonolysis, the composition of the final mixture is determined by the initial mole ratio of the reactants—alkyl halide and ammonia. If excess ammonia is used, primary amine is obtained as the major product. If an excess of alkyl halide is used, tertiary amine is obtained as the major product.
- In ammonolysis reaction, the order of reactivity of alkyl halides: R—I > R—Br > R—Cl
- Ammonolysis is not effective in preparing arylamine due to the low reactivity of aryl halides towards nucleophilic substituents.
From Alcohols: Aliphatic amines of low molecular mass may be prepared industrially by passing a mixture of alcohol and ammonia in the vapour phase at high pressure over heated alumina or copper chromite as catalyst at 300° – 400°C to give a mixture of primary, secondary and tertiary amines.
From Alcohols Example: CH3OH + NH3 → CH3NH2 + H2O
CH3NH2 + CH3OH → (CH3)2NH + H2O
(CH3)2NH + CH3OH → (CH3)3N + H2O
In this process, quaternary ammonium salt is not produced. If ammonia is used in excess, primary amine is obtained as the major product.
General Methods Of Preparation Of Amines
Preparation Of Primary Amines
Preparation Of Primary Amines By Reduction Of Nitro Compounds: Reduction of nitro compounds by Sn/HCl, Zn/HCl, Fe/HCl, H2/Ni or LiAlH4 gives primary amines.

Preparation Of Primary Amines Nitro Compounds Example:

Preparation Of Primary Amines By Reduction Of Alkyl Cyanides: Alkyl cyanides on being reduced by H2/Ni, LiAlH4 or (Na + C2H5OH) yield primary amines.
![]()
Preparation Of Primary Amines Alkyl Cyanides Example:

Preparation Of Primary Amines By Reduction Of Acid Amides: Acid amides are reduced by sodium and ethanol or LiAlH4 to give primary amines.
![]()
Preparation Of Primary Amines Example Acid Amides:

Preparation Of Primary Amines By Reduction Of Aldoximes Or Ketoximes: When aldoximes or Ketoximes are reduced by (Na + C2H5OH) or LiAlH4, primary amines are produced.

Preparation Of Primary Amines By Reductive Amination Of Aldehydes And Ketones: Aldehydes or ketones react with a mixture of excess NH3 and H2 at 140°-150°C under high pressure, in the presence of Raney Ni, to form primary amines.
- The reaction occurs in two, steps. In the first step, amine is produced which is reduced by H2 to yield primary amine.
- This process of converting a carbonyl compound into imine by treatment with ammonia and its subsequent reduction is called reductive amination.

Preparation Of Primary Amines By Hofmann Degradation Or Hofmann Bromamide Reaction: Acid amides (RCONH2) react with Br2 in the presence of alkali (NaOH, ArCONH2 or KOH) at about 70°C to give primary amines. The amine formed has one C-atom less than the parent acid amide. The reaction also occurs in the presence of sodium or potassium hypobromite (NaOBr or KOBr).

Hofmann Bromamide Reaction Example:

This reaction is used to prepare lower members of different homologous series.
From Alkyl Halides: Gabriel Phthalimide Synthesis:
- This is a method of converting an alkyl halide to a 1° amine free from 2° and 3° amines. In this process, phthalimide is first converted into potassium phthalimide by reacting with ethanolic KOH.
- Potassium phthalimide on treatment with alkyl halide yields IV-alkyl phthalimide, which is hydrolysed by boiling with NaOH or KOH to give pure primary amines.

N-alkyl phthalimide can also be hydrolysed by HCl under heat and pressure to produce primary amine. Primary amines are also prepared by hydrazinolysis (cleavage by hydrazine) of N-alkylphthalimide. This method is more effective and efficient than acidic or alkaline hydrolysis.

It Is important to note that in Gabriel phthalimide synthesis, aromatic amines cannot be prepared using aryl halide instead of alkyl halide because aryl halide does not participate in nucleophilic substitution reaction.
Gabriel Phthalimide Synthesis Example: This method can be used to prepare α-amino acids.

From Grignard Reagent: Grignard reagent reacts with chloramine to form primary amine.
R—MgBr + Cl—NH2 R —NH2 + MgBrCI
It is an effective method for preparing primary amine in which the —NH2 group is attached to a tertiary carbon atom.
From Grignard Reagent Example:

By Schmidt Reaction: Carboxylic acid reacts with hydrazoic acid (HN3) in the presence of concentrated H2SO4 to give a primary amine, which contains one carbon atom less than the carboxylic acid.
![]()
By Schmidt Reaction Example:

By Hydrolysis Of Alkyl Isocyanides: At ordinary temperature, hydrolysis of alky! isocyanide by dilute HCl gives primary amine.

By Curtius Rearrangement: Acyl azide, on heating in an inert solvent (benzene, chloroform, etc.) gives alkyl isocyanate. The latter on hydrolysis yields lcamine. This reaction is called the Curtius reaction.

By Curtius Rearrangement Reaction Mechanism

By Lossen rearrangement: When heated with concentrated HCl or XaOH, hydroxamic acids undergo Lossen rearrangement forming a primary amine which involves the formation of an intermediate, alkyl isocyanate.

By Lossen rearrangement Example:

Hydroxamic acids exhibit tautomerism; keto form 1 is called hydroxamic form and enol form 2, a hydroximic form.

Preparation Of Secondary Amines
Secondary Amines From Alkyl Isocyanides: Alkyl isocyanides are reduced by H2/Ni or (Na + C2H5OH) to form secondary amines.

From Primary Amines: Secondary amines are prepared by heating primary amines with the requisite amount of alkyl halides (preferably alkyl iodides).

From Primary Amines Example:

From Alkyl Halides: Alkyl halides when heated with aniline form dialkyl aniline. Dialkyl aniline on treatment with nitrous acid gives p nitroso-N, N-dialkyl aniline which on alkaline hydrolysis yields secondary amine.
From Alkyl Halides Example:

Using this process, secondary amines, free from primary and tertiary amines are produced.
Preparation Of Diethylamine From Ethyl Iodide:

Preparation Of Tertiary Amines
From Alkyl Halides: Tertiary amines are prepared by heating an excess amount of alkyl halide with an alcoholic solution of ammonia. In this case, the quantity of alkyl halide to be used should be more than the stoichiometric amount.

From Quaternary Ammonium Hydroxide: Quaternary ammonium hydroxide on heating gives tertiary amines.
Quaternary Ammonium Hydroxide Example:

Separation Of Primary, Secondary And Tertiary Amines By Hinsberg’s Method
This method is also used to distinguish between primary, secondary and tertiary amines. The mixture of amines when reacted with benzene sulphonyl chloride (Hinsberg’s reagent), primary and secondary amines form N-alkylbenzene sulphonamide and N, N-dialkyl benzene sulphonamide, respectively but tertiary amines do not react.

- The resulting mixture is made alkaline by adding a KOH solution. Consequently, N-alkylbenzene sulphonamide forms potassium salt which remains dissolved in the reaction mixture.
- N, N-dialkylbenzene sulphonamide does not react with KOH but remains in the mixture as an insoluble compound.
C6H5—SO2—NHR + KOH → C6H5 —SO2 —NKR + H2O (soluble potassium salt)
C6H5—SO2—NR2 + KOH→ No reaction
- The alkaline mixture on distillation gives tertiary amine which separates as the distillate.
- The residual mixture left in the distillation flask is filtered and lV,iV-dialkylbenzene sulphonamide is obtained as residue. The filtrate on subsequent acidification gives Nalkylbenzenesulphonamide.
C6H5—SO2—NKR + HCl → C6H5—SO2—NHR + KCl
- N-alkylbenzene sulphonamide and N, A-dialkylbenzene sulphonamide are separately hydrolysed by 20% HCl or 70% H2SO4, to give primary and secondary amines, respectively.

At present, in the separation of an amine mixture, para toluenesulphonyl chloride![]() is used, instead of benzenesulphonyl chloride.
is used, instead of benzenesulphonyl chloride.

Physical Properties Of Amine
Odour And Nature
Among aliphatic amines, lower members (for example., methylamine, ethylamine, dimethylamine) are gases having an ammoniacal smell but the higher members are volatile liquids with a fishy odour.
Boiling Point: Due to the presence of polar N—H bonds, all amines, except tertiary amines, are capable of forming H -bonds.
- The electron density of the N-atom in the secondary amine is more than that of the N-atom in the primary amine.
- This is due to the presence of two electron-repelling (+1 effect) alkyl groups attached to the N -atom in 2° amine.
- As a result greater polarity of N—H bond in primary amines is observed. Consequently, primary amines form stronger intermolecular H-bonds and have higher boiling points than secondary amines.
- The isomeric tertiary amines have the lowest boiling points as they cannot participate in intermolecular H-bond formation.

Intermolecular Primary amines (consequently effective molecular mass increases) Due to the presence of polar N—H bonds in their molecules, 1° and 2° amines (except 3° amines) can form H -bonds.

- The N—H bond is less polar than the O—H bond. So, intermolecular H -bonds in amines are weaker than those in alcohols and carboxylic acids.
- Therefore, the boiling points of amines are comparatively lower than alcohols and carboxylic acids of comparable molecular mass. But their boiling points are higher than those of the alkanes and ethers of comparable molecular mass.

Solubility: Amines of lower molecular masses are water-soluble because their molecules can form H-bonds with water molecules.
- With the Increase In molecular mass, the size of the hydrocarbon part of the amines becomes larger. Consequently, their solubility in water decreases.
- When several carbon atoms in an amine exceed 6, then the amine becomes insoluble In water. AmlncN of higher molecular masses is, however, soluble In organic solvents (e.g., alcohol, ether, benzene, etc.).

Basic Character Of Amines
Amines are regarded as organic bases. N-atoms present in their molecules contain lone pairs of electrons and hence, can accept protons. In reactions with water, they produce OH– ions. In fact, they are as stronger bases than water.
⇒ \(\mathrm{R} \ddot{\mathrm{N}} \mathrm{H}_2+\mathrm{H}^{\oplus} \rightleftharpoons \mathrm{R}-\stackrel{\oplus}{\mathrm{N}} \mathrm{H}_3\)
⇒ \(\mathrm{RNH}_2+\mathrm{H}-\mathrm{OH} \rightleftharpoons \mathrm{R} \stackrel{\oplus}{\mathrm{N}} \mathrm{H}_3+\mathrm{OH}^{\ominus}\)
Strength Of Bases In Terms Of Kb and pKb: In an aqueous solution, any base (B:) can establish the following equilibrium.
⇒ \(\mathrm{B}:+\mathrm{H}-\mathrm{OH} \rightleftharpoons \mathrm{BH}^{\oplus}+\mathrm{OH}^{\ominus}\).
∴ Equilibrium constant,
⇒ \(K=\frac{\left[\mathrm{BH}^{\oplus}\right]\left[\mathrm{OH}^{\ominus}\right]}{[\mathrm{B} ;][\mathrm{HOH}]}\)
⇒ \(K \times\left[\mathrm{H}_2 \mathrm{O}\right]=\frac{\left[\mathrm{BH}^{\oplus}\right]\left[\mathrm{OH}^{\ominus}\right]}{[\mathrm{B}:]} \text { or, } K_b=\frac{\left[\mathrm{BH}^{\oplus}\right]\left[\mathrm{OH}^{\ominus}\right]}{[\mathrm{B}:]}\)…1
[H2O is present in large quantities in solution and it does not suffer any change quantitatively. So [H2O] can be treated as a constant. In that case AT[H2O] = constant (Kb) .]
The constant (Kb) in equation (1) is called the basicity constant. Taking negative logarithms on both sides of the equation,

⇒ \(p K_b=\log \frac{[\mathrm{B} ;]}{\left[\mathrm{BH}^{\oplus}\right]\left[\mathrm{OH}^{\Theta}\right]} \quad \cdots(2)\)
⇒ \(\left[p K_b=-\log K_b\right]\)
From equation (1), we see that the higher the value of Kb, the greater the concentration of OH– ions and the stronger the base.
Similarly, the lower the value of Kb, the weaker the base. From equation (2) It is clear that the higher the value of pKb , the weaker the base and vice-versa.
If the acidity constant of the conjugate acid (BH+) of any base (B:) is Ka, then it can be shown that with a decrease in the value of pKa, the strength of the corresponding base decreases and vice-versa.
pkb And pka Values Of Amines In Aqueous Solutions:

Basic Character Of Aliphatic Amines
Basic Of Amines In Aprotic Solvents: In nitrogenous bases, the higher the electron density on the N-atom, the more easily the nitrogen atom donates its lone pair of electrons to the proton. So, with an increase in electron density, the basicity of amine increases.
As the number of electron-repelling alkyl (methyl) groups attached to the N -atom in methylamine, dimethylamine and trimethylamine increases, basicity also increases, i.e., the increasing order of basicity is—

The basicity of the amines dissolved in aprotic solvents (for example., chlorobenzene) also increases with an increase in the number of alkyl groups attached to the N-atom. However, in an aqueous solution or any other hydroxylic or protein solvent, this trend is not observed.
Basicity Of Amines In Aqueous Solution: In this case, basicity depends on two factors. Firstly, the higher the electron density of the N-atom in the amine molecule, the greater the basicity of the amine.
- In other words, an increase in the number of electron-repelling alkyl groups linked to the N-atom results in an increase in the basicity of the amines.
- Hence, based on relative electron density, the basicity of ammonia and primary, secondary and tertiary amines is in the following order:

Secondly, the basicity of amines depends on the relative stability of the conjugate acids (cations) formed by the combination of the amines with protons. The greater the stability of the conjugate acid, the greater the basicity of the amine.

The conjugate acid produced from the primary amine attains maximum stability through intermolecular H-bond formation with water molecules while the cation formed by tertiary amine has the least stability.

- So, the order of stability of the conjugate acid (cation) in an aqueous solution is— RN+H3 > R2N+H2 > R3N+H. Thus, based on the stability of the cation, the basicity of the amines follows the order RNH2> R2NH>R3N.
- The difference in basicity among primary, secondary and tertian’ amines can be explained based on the two opposing factors mentioned above, viz. electron density on N-atom and stability of the conjugate acid (cation).
- Considering these two factors, it has been observed that in aqueous solution the secondary amines are always stronger bases than both primary and tertiary amines.
- The difference between the basicities of primary and tertiary amines is relatively small. In some cases, the basicity of primary amines is more than that of tertiary amines while in some cases, the reverse order is observed.
For example, in an aqueous solution, the respective order of basicity of the methylamine and ethylamine series including ammonia, is as follows:
(CH3)2NH > CH3NH2 > (CH3)3N > NH3(C2H5)2NH > (C2H5)3N > C2H5NH2 > NH3
Basic Character Of Aromatic Amines: Aromatic amines (for example., aniline, pKb = 9.38 ) are much weaker bases than ammonia (pKb = 4.75) and aliphatic amines (for example., ethylamine, pKb = 3.33).
The decreased basicity of aromatic amines may be explained in the following way—
The Hybridisation Of The C-atom Attached To The Amino Group: The N-atom of an aromatic amine is bonded to an sp2-hybridised carbon atom of the aromatic ring.
- But in the case of aliphatic amines, the N-atom is linked to an sp3-hybridised carbon atom.
- It should be noted that the order of electron-attracting property and electronegativity of carbon atoms based on hybridisation is— C(sp)>C(sp2)>C(sp3).
Effect Of Resonance: The lone pair of electrons N-atom of aniline (the simplest member of aromatic amines) takes part in resonance or delocalisation with the electrons of a benzene ring. Consequently, the electron pair on ammo nitrogen becomes available to a lesser extent to combine with a proton. This reduces the basic character to a large extent.

Decrease In The Stability Of The Conjugate Acid Relative To The Aromatic Amine: As the electron pair on N-atom in conjugate acid formed by protonation of aniline is not available, it cannot participate in delocalisation with the π-electrons of the benzene ring. So, aniline exhibits the least tendency to combine with a proton.

Due to the absence of the above effects in aliphatic amines, their basicity is found to be much higher than aromatic amines. It is interesting to note that cyclohexyl amine —NH2 having no aromatic ring displays strong basic properties (pKb = 3.32) like aliphatic amines.
Comparison Of Basicity Of Different Amino Compounds
Diphenyl And Triphenyl Amines: With the increase in the number of aromatic rings attached to the N-atom of an amino group, electron density on the N-atom decreases with a consequent gradual decrease in the basicity of the amines. This is because the lone pair on N-atom participates in delocalisation with π-electrons associated with a large number of aromatic rings.

Benzylamine And Methylamine: In benzylamine, the amino group is connected to the benzene ring through the —CH2 group. For this reason, it behaves as a strong base like aliphatic amines. Due to the -I effect of the phenyl group, it is a weaker base than methylamine.

N-methyl Aniline And N, N-dimethylaniline: As the number of electron-releasing methyl groups attached to the N-atom of aniline increases, electron density also increases, leading to an increase in basicity.

The Basic Strength Of o-, m- and p-substituted Aromatic Amines: The presence of electron-donating groups [for example., —CH3, —NH2, —OCH3, —OH) in the aromatic ring increases the basic strength of the corresponding aromatic amines. This is because, these groups, with a few exceptions, increase the electron density on the N-atom or any nearest atom.

On the other hand, electron-attracting groups [for example., —NO2, —CN, —X(halo) ] present in the aromatic ring decreases the basicity of the aromatic amines. This is because the electron-attracting groups diminish the electron density of the N-atom in the amino group.

Ortho-, meta- and para-toluidine (or methyl aniline): Due to ortho-effect, the basicity of ortho-toluidine is less than aniline. On the other hand, due to the +1 effect of the —CH3 group, its meta-isomer i.e.„ meta-toluidine is slightly more basic than aniline.
Owing to the +1 effect coupled with the hyperconjugation effect of a methyl group, para-toluidine becomes more basic than the meta-isomer i.e., m-toluidine

ortho-, meta- and para-anisidine (or methoxy aniline): Due to the ortho-effect orthomethoxyaiuline is less basic than aniline.
- Again +R effect of the — OCH3 group cannot influence the basic strength of meta-methoxy aniline because it causes no increase in electron density on the ring carbon attached to the —NH2 group.
- However, only the -I effect of the —OCH3 group is active in the case of meta-isomer. The net outcome is the least basicity of meta-isomer.
- In para-isomer, due to greater distance, the -I effect of the —OCH3 group is not perceptible to an appreciable extent. However, due to the +R effect, it is found to be the most basic.

Ortho-, meta- and para-nitroaniline: Given the -I and -R effect of the —NO2 group, nitroanilines are always found to be less basic than aniline.
- In the case of the o-isomer, due to the shorter distance, the -I effect of the NO2 group is most effective.
- Besides, because of the electron-attracting -R effect and H bond formation, as depicted below, the o-isomer displays the least basicity.

- Due to the combined -I (more effective at a shorter distance) and -R effects of the —NO2 group, the o-isomer is less basic than the p-isomer.
- In the case of the m-isomer, the -R effect of the —NO2 group does not cause any reduction of basic character but due to the -I effect, basicity decreases significantly.
- Despite this, it exhibits more basic character than the p-isomer.

Organic Nitrogen Compounds
Chemical Properties Of Amines
Chemical Properties Of Primary Amines
Chemical Properties Reaction With Mineral Acids: Mineral acids react with primary amines to form salts.
Chemical Properties Reaction With Mineral Acids Example:

Chemical Properties Reaction With Alkyl Halide: Primary amines when treated with excess alkyl halides give successively secondary and tertiary amines. In the presence of a large excess of alkyl halide, quaternary ammonium salts are produced. This reaction is known as the alkylation of amine.

Organic Nitrogen Compounds
Chemical Properties Reaction With Alkyl Halide Example:

Reaction With Acetyl Chloride Gr Acetic Anhydride: Primary amines on treatment with acetyl chloride (CH3COCI) or acetic anhydride [(CH3CO)2O] give acetyl derivatives. In this reaction, one H-atom of the amino group is replaced by the acetyl group. So, it is called acetylation reaction.
Acetic Anhydride Example:

Organic Nitrogen Compounds
Reaction With Benzoyl Chloride (Benzoylation): In an alkaline medium, primary amines react with benzoyl chloride where one H-atom of the —NH2 group is replaced by a benzoyl (—COC6H5) group.
Reaction With Benzoyl Chloride Example:

Reaction With Benzenesulphonyl Chloride And P-Toluenesulphonyl Chloride: In the reaction of benzenesulphonyl chloride or paratoluenesulphonyl chloride with primary amines, N-alkyl sulphonamide is formed. These sulphonamides dissolve in KOH or NaOH forming soluble sodium or potassium salts.
Reaction With Benzenesulphonyl Chloride Example:

Organic Nitrogen Compounds

Reaction With Nitrous Acid: Nitrous acid (HNO2) is an unstable acid, produced in situ in the reaction medium by the action of sodium nitrite and dilute HCl. Aliphatic primary amine in reaction with nitrous acid gives alcohol and N2 gas.
Nitrous Acid Example:

Aromatic primary amines react with nitrous acid at low temperatures to form diazonium salts.
Nitrous Acid Example:

Reaction With Carbon Disulphide (CS2): In the reaction of carbon disulphide with primary amines, dithiocarbamic acid is produced.
- This decomposition with mercuric chloride (HgCl2) yields alkyl isothiocyanate (RNCS).
- Alkyl isothiocyanate has a pungent smell like mustard oil and hence, this reaction is known as the Hofmann mustard oil reaction.
- This reaction is used as an identification test of primary amines.

Organic Nitrogen Compounds
Carbylamine Reaction: Primary amine on heating with chloroform and alcoholic KOH solution yields alkyl isocyanide (RNC) or arylamine. This reaction is called arylamine reaction. Alkyl isocyanides have an extremely unpleasant smell. So, a primary amine can be easily detected by this reaction.
![]()
Carbylamine Reaction Example:

Reaction With Grignard Reagent: The two H-atoms attached to the N-atom of primary amines are highly reactive concerning the Grignard reagent. So, each molecule of primary amine reacts with two molecules of Grignard reagent to form two molecules of alkane.
RNH2 + 2CH3MgI → 2CH4 (Methane) + RN(MgI)2
Reaction With Aldehydes: The reaction of primary amines with aldehydes produces imines. The imine thus formed is called Schiff’s base.

Catalytic hydrogenation (reduction in the presence of Ni catalyst) of Schiff’s base gives secondary amines.

Methylation Of Primary Amines
Eschweiler-Clarke Reaction: When a 1° amine is heated with a mixture of formaldehyde and formic acid at 100°C, one H -atom of the amino group is replaced by a methyl group. Here, a 2° amine is obtained where a methyl group is attached to the N -atom.

This reaction is known as Eschweiler-Clarke methylation.
Oxidation: Primary amines are oxidised by KMnO4 to form aldimine ketimine or nitroalkane, depending upon their structures.

Organic Nitrogen Compounds
Aldimines or ketimines on hydrolysis with dilute acid regenerate aldehydes and ketones.


Reaction With Transition Metal Ion: Primary and secondary amines react with transition metal ions to produce soluble coordination compounds.
Transition Metal Ion Example: AgCl dissolves in methylamine to form a complex

The electrophilic substitution reactions of aromatic 1° amine have been discussed later.
Chemical Properties Of Secondary Amines
Reaction With Mineral Acids: Like primary amines, secondary amines also react with mineral acids to form salts.
Reaction With Mineral Acids Example:

Reaction With Alkyl Halides: The reaction of secondary amines, with alkyl halides produces tertiary amines In the presence of an excess of alkyl halides, quaternary ammonium salts are formed.
Reaction With Alkyl Halides Example:

Organic Nitrogen Compounds
Reaction With Acetyl Chloride And Acetic Anhydride: Like primary amines, 2° amines also react with acetyl chloride or acetic anhydride to produce acetyl derivatives.
Acetyl Chloride And Acetic Anhydride Example:
![]()

Reaction With Benzenesulphonyl Chloride And Para-Toluenesulphonyl Chloride: Secondary amines react with benzene sulphonyl chloride or para-toluene sulphonyl chloride to form, N, N-dialkyl sulphonamide. These sulphonamides are insoluble in alkali because there is no H-atom attached to their N-atom.
Reaction With Benzenesulphonyl Chloride Example:

Reaction With Nitrous Acid (HNO2): Secondary amines react with nitrous acid to form a yellow oily compound, N-nitrosamine. In this reaction, nitrogen gas does not evolve.
R2N H + HO —N=O R2N —N=O(N-nitrosoamine)+ H2O
Reaction With Nitrous Acid Example:

N-nitrosamine on heating with dilute HCl decomposes to reproduce the secondary amine.

Reaction With Carbon Disulphide: Secondary amines react with carbon disulphide (CS2) to form dithiocarbamic acid but unlike primary amines, it is not decomposed by mercuric chloride(HgCl2).

Carbylamine Reaction: Secondary amines do not participate in this reaction.
Reaction with Grignard reagent: The H-atom attached to the N-atom of secondary amines is highly reactive with respect to the Grignard reagent. So, one molecule of Grignard reagent reacts with one molecule of secondary amine to liberate one molecule of alkane.

Organic Nitrogen Compounds
Reaction with aldehyde and ketone: Aldehyde and ketone having α-H react with secondary amines to produce enamine.

Methylation Of Secondary Amine
Eschweiler-Clarke Reaction: 2° amines are methylated on heating with a mixture of formaldehyde and formic acid at 100°C. One H-atom of the amino group is replaced by a methyl group. As a result, a tertiary amine is obtained where a methyl group is attached to the N-atom.

Oxidation: Secondary amines on oxidation by potassium permanganate give tetra alkylhydrazine.

2° amines when oxidised by Caro’s acid (H2SO5) give N, N-dialkylhydroxylamine.

Chemical Properties Of Tertiary Amines:
Reaction With Mineral Acids: Like primary and secondary amines, tertiary amines also react with mineral acids to form salts.
⇒ \(\mathrm{R}_3 \mathrm{~N}+\mathrm{HCl} \longrightarrow \mathrm{R}_3 \stackrel{\oplus}{\mathrm{N}} \mathrm{H} \stackrel{\ominus}{\mathrm{C}}\)
Reaction With Mineral Acids Example:

Reaction With Alkyl Halides: In the reaction of tertiary amines with alkyl halides, quaternary ammonium salts are formed.
Reaction With Alkyl Halides Example:

Reaction With Nitrous Acid (HNO2): Tertiary amines dissolve in cold nitrous acid producing nitrite salts. Nitrogen gas is not evolved in this reaction.

Oxidation: Tertiary amines are not oxidised by KMnO4 but are oxidised by Caro’s acid (H2SO5) to form amine oxide.

Due to the absence of any H-atom attached to N-atom, tertiary amines do not react with the following reagents:
- Acetyl chloride and acetic anhydride,
- Benzenesulphonyl chloride and para-toluene sulphonyl chloride,
- Carbon disulphide,
- Chloroform in the presence of alcoholic KOH (Carbylamine reaction)
- Grignard reagent
- A mixture of formaldehyde and formic acid (Eschweiler-Clarke reaction)
- Aldehyde.
Exhaustive Methylation: When primary, secondary and tertiary amines are reacted with excess methyl iodide, quaternary ammonium salt is obtained as the end product. This process is known as exhaustive methylation.

Quaternary ammonium iodide reacts with moist silver oxide (AgOH) to give quaternary ammonium hydroxide which on heating decomposes to give an alkene and a tertiary amine. By identifying products, the initial amine can be determined.
Identification Of Different Types Of Amines
Different Types Of Amines Hinsberg’s Test: In this test, Hinsberg’s reagent, i.e., benzene sulphonyl chloride (C6H5SO2CI) is added to the sample of amine. If a precipitate appears, then the reaction mixture is made alkaline with a KOH solution. Consequently, primary, secondary and tertiary amines display different chemical reactions.
Primary Amines react with the Hinsberg reagent to give a precipitate (N-alkyl benzene sulphonamide) which dissolves in the KOH solution.

Organic Nitrogen Compounds
With C6H5SO2Cl, secondary amines give a precipitate (N, N-dialkyl sulphonamide), which remains insoluble in the KOH solution.

Tertiary amines do not react with C6H5SO2Cl. Hence, no precipitate is formed.
Identification Of Primary Amines
Carbylamine Test: This test is employed to identify both aliphatic and aromatic primary amines. In this test, a sample of primary amine is warmed with chloroform and alcoholic KOH solution when alkyl isocyanide or arylamine (RNC) having an extremely unpleasant smell is produced.
![]()
Hofmann Mustard Oil Reaction: When a 1° amine is warmed with alcoholic CS2 solution, followed by heating with HgCl2, an oily liquid (alkyl isothiocyanate) having the pungent smell of mustard oil is formed.

Identification Of Secondary Amines
Liebermann’s Nitroso Test
- In cold conditions, dilute HCl and NaNO2 are added to a sample of secondary amine when a yellow oily liquid, Nnitrosoamine, is formed.
- The oily substance is separated and heated with a small amount of phenol and a few drops of concentrated H2SO4. The mixture turns green.
- The solution on dilution with water becomes red. The solution when made alkaline, with an aqueous solution of sodium hydroxide, turns deep blue.
R2N — H + HO — N = O → R2N — N=O + H2O
Comparison Among Primary, Secondary And Tertiary Amines:

Aromatic Amine: Aniline (C6H5NH2)
When —NH2, —NHR or —NR2 group (where R = alkyl or aryl group) is directly attached to the aromatic ring, then the compounds formed are known as primary, secondary and tertiary aromatic amines, respectively.
- Aniline (C6H5NH2) is considered the simplest member among all aromatic primary amines. In 1826, Unverdorben first prepared aniline by destructive distillation of a mixture of indigo and lime.
- The Portuguese name of indigo is anil and hence, the compound was named aniline in 1841.
Preparation Of Aniline
Laboratory Preparation Of Aniline
Principle: In the laboratory, aniline is prepared by reducing nitrobenzene with tin and concentrated HCl.

2C6H5NO2 + 3Sn + 12HCl→ 2C6H5NH2 + 3SnCl4 + 4H2O
In the presence of excess acid, aniline exists as its hydrochloride salt [C6H5NH2.HCl]. To get free aniline, the reaction mixture is made alkaline with an excess of sodium hydroxide.
![]()
Industrial Preparation Of Aniline
From Chlorobenzene: Aniline is commercially produced by heating a mixture of chlorobenzene and excess aqueous solution of ammonia to 250°-350°C in the presence of cuprous oxide as a catalyst, under high pressure (about 60 atm pressure). This is known as the Dow process. The ammonolysis reaction involves nucleophilic substitution via the formation of a ‘benzyne’ intermediate.
![]()
From Nitrobenzene: Aniline is also produced industrially by the
- Reduction of nitrobenzene with iron, 30% HCl solution and
- Catalytic (Raney nickel) hydrogenation of nitrobenzene.
Example:

Organic Nitrogen Compounds
Other Methods Of Preparation Of Aniline
From Phenol: Ammonolysis of phenol in a closed vessel in the presence of anhy. zinc chloride catalyst, at high temperature yields aniline.

From Benzamide: Benzamide on heating with bromine and NaOH (or KOH ) solution gives aniline. This is known as ‘Hofmann rearrangement’ or ‘Hofmann degradation.

Properties And Uses Of Aniune
Physical Properties Of Aniline
- Freshly distilled aniline is a colourless oily liquid with an unpleasant smell. It has a boiling point of 184°C and is poisonous.
- Aniline is almost insoluble in water but dissolves in organic solvents like alcohol, ether, benzene, etc.
- Aniline cannot turn moist red litmus paper blue. So, the litmus experiment suggests that aniline is a neutral liquid.
Freshly distilled aniline is oxidised in the presence of light and air to form different coloured compounds and slowly assumes a brown colour.
- The boiling points of these compounds are much higher than aniline. When impure aniline is subjected to distillation, colourless pure aniline is obtained, leaving behind the coloured compounds in the distillation flask.
Comparison Of Solubility Of Aniline And Aliphatic Primary Amine In Water: A lone pair of electrons on the N-atom of the amino group conjugates with π-electrons of benzene ring through resonance, i.e., delocalisation of electron pair occurs.
- Consequently, the —NH2 group becomes partially positively charged and the ring acquires partial negative charge. So aniline is a polar molecule.
- Its dipole moment is 1.70D. Owing to this delocalisation, the availability of lone electron pair on N-atom decreases. So aniline is incapable of forming an effective H-bond with water. Hence, aniline is almost insoluble in water.

- On the other hand, in any aliphatic amine for example., methylamine (CH3NH2), the lone electron pair on the nitrogen atom does not participate in delocalisation.
- Consequently, methylamine can form strong H -bonds with water through this lone pair of electrons.
- This causes high solubility of methylamine in water. (But electronegativity of nitrogen atom being high, relatively weaker moment due to -I effect acts in the direction of —NH2 group.
- This moment neutralises to some extent the strong moment, caused by the +R effect which acts in the direction of the group towards the ring.)
Organic Nitrogen Compounds
Chemical Properties Of Aniline: Due to resonance, aniline displays the following chemical properties—O As a result of the +R effect in the amino ( —NH2) group, the electron density at ortho and para positions of the ring, relative to meta-position increases.
- Hence, electrophilic substitution takes place mainly at ortho- and para-positions relative to the —NH2 group and this reaction occurs more easily than that in benzene.
- The benzene ring is electron-rich due to resonance and hence, aniline is easily oxidised, producing varieties of coloured organic compounds. As a result of resonance, aniline is converted into a weaker base than aliphatic 1° amine (for example., methylamine, ethylamine, etc.).
- Any electron-releasing group such as —CH3, —NH2 present in the ring increases the basicity of aromatic amines while the presence of any electron-attracting group, e.g., —NO2, —CHO decreases the basicity of aromatic amines.
- The electron-releasing groups push the electrons towards nitrogen and hence, the lone pair of electrons on the N-atom, necessary for bond formation with proton (H+) is more easily available than benzene.
- So an aromatic amine having an electron-releasing group is found to be more basic than aniline.
- Conversely, the electron-attracting group tends to shift the lone pair of electrons from N-atom.
- Consequently, the lone pair of electrons on the N-atom required for bond formation with proton is not so easily available as in the case of aniline.
- So, the basicity of an aromatic amine-containing electron-attracting group is less than that of aniline.
The chemical reactions of aniline may be divided into two classes, viz.,
- Reactions of the amino group and
- Reactions of the benzene ring. But as the benzene ring influences the reactions of the —NH2 group, the reactions of the benzene ring are also influenced by the —NH2 group.
For example, the presence of a benzene ring enables aniline to form diazonium salt. —NH2 group being present in the ring, increases the stability and in substitution reactions, H-atoms of ortho-/para-positions are substituted leading to ortfto-and para- compounds.
Reactions Of Amino (-NH2) Group
Reaction Of Amino Salt Formation: Aniline being a weak base reacts with strong mineral acids to produce salts.
Salt Formation Example: In the reaction of aniline with cone. HCl and cone. H2SO4, crystalline solid aniline hydrochloride and aniline sulphate salts are respectively formed.

- Aniline does not dissolve in water, but these salts obtained from aniline are soluble in water. So, aniline dissolves in a dilute aqueous solution of HCl or H2SO4 with the formation of aniline salt.
- As these salts are produced in the reaction between weak base and strong acid, they undergo hydrolysis in aq. the solution, consequently producing an acidic solution.
- Aniline is liberated when an excess sodium hydroxide solution is added to these salts in cold conditions.

N-alkylation: When a mixture of aniline and methyl chloride is heated in a closed reaction vessel, hydrogen atoms of the —NH2 group are replaced by methyl groups successively.
- This results in the consecutive formation of N-methylaniline (secondary amine), N, N-dimethylaniline (tertiary amine) and finally N, N, N-trimethylanilinium chloride (quaternary ammonium salt).
- This process is called N-methylation. Other alkyl halides also react similarly with aniline.

This quaternary salt on heating at 300°C undergoes rearrangement (Hofmann-Martius reaction) to form 2,4,6- trimethylanilinium chloride.

N-arylation: Aniline on being heated to 250°C with aniline hydrochloride in a closed vessel yields diphenylamine.

Acetylation: At ordinary temperature, aniline reacts with acetyl chloride when one H-atom of the —NH2 group is replaced by an acetyl group (—COCH3) forming acetanilide, a white crystalline solid with a melting point of 114°C. Acetanilide may also be produced when aniline is heated with acetic anhydride or glacial acetic acid. This reaction is called the acetylation reaction.

Benzoylation: When aniline is shaken with benzoyl chloride and excess sodium hydroxide solution, one H-atom of the —NH2 group is replaced by a benzoyl (—COC6H5) group to form white crystalline benzanilide. This reaction is known as benzoylation. It is also known as the Schotten-Baumann reaction.

Organic Nitrogen Compounds
Formation Of Schiff Base: Aniline participates in the condensation reaction with aromatic aldehydes to form a class of compounds, called anils or Schiff bases.
Schiff Base Example: Heating a mixture of aniline and benzaldehyde to 125°C gives benzylidene aniline (a Schiff base).

Schiff bases are easily hydrolysed. So, in certain reactions, (for example., nitration), the —NH2 group may be protected by forming Schiffbase.
Reaction With Phosgene: Aniline reacts with phosgene to give phenyl isocyanate.

Reaction With Carbon Disulphide: When aniline is heated with caustic potash powder and an ethanolic solution of carbon disulphide, N, N’-diphenyl thiourea (thiocarbamide) is produced.

Carbylamine Reaction: When aniline is heated with a mixture of chloroform and alcoholic KOH solution, phenyl isocyanide or phenyl carbylamine having nauseating and unpleasant smell is formed. This reaction is called arylamine reaction. Aniline may be detected with the help of this reaction.
![]()
Diazotisation: When a cold aqueous solution of sodium nitrite is added to a cold acidified solution of any primary aromatic amine, benzene diazonium salt is produced.
Diazotisation Example: When aniline dissolved in dilute hydrochloric acid is cooled to 0-5°C and sodium nitrite solution is added to it slowly with constant stirring, a pale yellow-coloured solution of benzenediazonium chloride is formed. This reaction is called diazotisation. Nitrous acid produced in the reaction between NaNO2 and HCl reacts with aniline.

In the reaction of nitrous acid with aliphatic primary amine (RNH2), alcohol is formed with the liberation of nitrogen gas.
Oxidation: As the benzene ring of aniline is electron-rich, it is easily oxidised by various oxidising agents. The nature of the products obtained on oxidation depends on the oxidising agents.
Oxidation Example: Aniline when oxidised by chromic acid or MnO2/ H2SO4 yields p-benzoquinone. Trifluoroperacetic acid oxidises aniline to nitrobenzene while personal sulphuric acid (Caro’s acid) oxidises it to nitrosobenzene.

Electrophilic Substitution Reaction Of Benzene Ring
Halogenation Amino (—NH2) group sufficiently increases electron density at ortho- and para-positions of the ring through the +R effect. So, the ring becomes so active towards electrophilic substitution reaction that all the H-atoms of ortho- and para-positions can be substituted by halogens without any halogen carrier.
Chlorination: At ordinary temperature, chlorine water reacts with aniline to give a white precipitate of 2,4,6- trichloroaniline.

Organic Nitrogen Compounds
Bromination: At ordinary temperature, bromine water reacts with aniline to give a white precipitate of 2,4,6-trihydroaniline.

lodination: Iodine reacts with aniline in the presence of aq. sodium bicarbonate solution to form p-iodoaniline.

Substitution in aniline ring in a particular position (Selective substitution): For preferential substitution at a particular position of aniline ring, the high reactivity of the ring must be reduced. For this purpose, the —NH2 group is converted into —NHCOCH3 by acetylation.
- The lone pair of electrons on the nitrogen atom of the —NHCOCH3 group is attracted by the adjacent carbonyl (C=O) group through resonance.
- This consequently interferes with its participation in resonance with π-electrons in the ring, thereby reducing its reactivity.

Substitution in aniline ring Example: Preparation of p-bromoaniline:

Preparation Of o-bromoaniline:

Preparation Of m-bromoaniline:

Organic Nitrogen Compounds
Nitration: The benzene ring in aniline is highly activated due to the presence of the — NH2 group. Hence, direct nitration of aniline with a mixture of cone. HNO3 and cone. H2SO4 gives rise to a complex mixture of mono-, di- and trinitro compounds.
- Moreover, the electron-rich aniline ring being highly susceptible to oxidation, gets oxidised by a strong oxidising agent like HNO3, to yield different products. So, direct nitration of aniline is not carried out.
- To prevent oxidation by deactivating the benzene ring, aniline is first converted to acetanilide by acetylation.
- Nitration of acetanilide with mixed acid (concentrated HNO3 and concentrated H2SO4) results in the formation of ortho- and para-nitroacetanilide.
- Due to the large size of —NHCOCH3 (steric hindrance), p-nitro acetanilide (90%) Is obtained as the major product.
- The two isomers thus obtained are separated by crystallisation from ethanol. The para-isomer on being hydrolysed by acid or alkali produces p-nitroaniline.

Thus amino groups should be protected by acetylation before carrying out nitration of aniline.
Preparation Of O-nitroaniline: When the p-position of acetanilide is blocked by sulphonation and nitration, the product formed is hydrolysed and desulphonated by heating with dilute H2SO4, to obtain o-nitroaniline.

Preparation Of M-nitroaniline: m-nitroaniline is produced by selective reduction of one nitro group of dinitrobenzene using an ethanolic solution of ammonium hydrogen sulphide, m-nitroaniline may also be prepared by nitration of aniline in presence of 98% H2SO4.
In the presence of conc.H2SO4, the —NH2 group acts as an electron-attracting (-I effect) & mete-directing —N+H3 group, consequently producing m-nitroaniline as the chief product.

Organic Nitrogen Compounds
Sulphonation: When aniline is heated with fuming sulphuric acid (sulphuric acid containing 10% SO3) at 180°C or with concentrated H2SO4 at 200°C, p-amino benzene sulphonic acid or sulphanilic acid is produced as the major product.
In this reaction, anilinium hydrogen sulphate salt is first formed, which eliminates a molecule of water to form phenylsulphamic acid, which rearranges to give sulphanilic acid.

Uses Of Aniline
- Aniline is widely used in the preparation of various azo dyes and as an antioxidant and vulcanisation accelerator in the rubber industry.
- It is also extensively used in the synthesis of a wide range of important organic compounds like hydroquinone, acetanilide, sulphanilic acid, nitroaniline, etc.
- It is also used in the pharmaceutical and plastic industries.
Tests For Aromatic Amino Group: Identification Of Aniline
Azo-Dye Test: Aniline dissolved in dilute HCl solution is cooled to 0-5°C, to which, aqueous sodium nitrite solution is added. A few drops of this solution are then slowly added to a cold solution of β-naphthol dissolved in sodium hydroxide. As a result, a scarlet red azo-dye is formed,
Azo-Dye Test Reaction Mechanism: It is an electrophilic substitution reaction, where benzene diazonium ion, C6H5N+2 (produced in the reaction of aniline with NaNO2/HCl; i.e., HNO2) acts as an electrophile.

Distinguishing Test For Aliphatic And Aromatic 1° Amine:

Organic Nitrogen Compounds
Carbyiamine Or Isocyanide Test: A few drops of aniline, mixed with a few drops of chloroform and alcoholic caustic potash solution is heated in a test tube. Phenyl isocyanide or phenyl carbamide having an obnoxious smell, is produced. (This test is also applicable for the aliphatic —NH2 group.)

Bromine Water Test: A few drops of aniline are taken in a test tube to which excess bromine water is added. The mixture on shaking gives a white precipitate of 2,4,6-tribromoaniline.

Diazonium Salts (ArN2X)
A primary aromatic amine reacts with nitrous acid (NaNO2 and HCl) in cold conditions (0-5°C) to form an unstable compound known as diazonium salt.
- The general formula of this class of compounds is ArN+2X–, where Ar = aryl group for example., phenyl (C6H5—), p -nitrophenyl (p-NO2C6H4—) etc., and X– = acid anion, for example., Cl–, HSO–4, BF–4, Br–, etc.
- The functional group of this class of compounds is diazonium ion (—N+=N). Since the molecules of these compounds contain two nitrogen atoms and their nature is very much similar to that of ammonium salts, these compounds are known as ‘diazonium salts’.

Diazonium Salts Example:

Organic Nitrogen Compounds
Preparations Of Diazonium Salts
Laboratory preparation of C6H5N2CI
Principle: Aniline, dissolved in dilute hydrochloric acid is cooled to 0-5°C to which, a cold aqueous solution of sodium nitrite is added. A reaction occurs to form benzene diazonium chloride.
- The reaction involves two steps. In the first step, nitrous acid is produced in the reaction between sodium nitrite and HCl.
- In the second step, aniline reacts with nitrous acid and HCl to yield benzene diazonium chloride.
Laboratory preparation Of Principle Reaction Mechanism

Since diazonium salts slowly decompose even at low temperatures (0-5°C), they are used immediately after preparation.
Diazotisation: The process which involves the reaction of primary aromatic amines with dilute mineral acids and sodium nitrite in ice-cold conditions (0-5°C) to form diazonium salts is called diazotisation. In 1858, Peter Griess discovered this reaction in Hofmann’s laboratory in London.
Causes Of Stability Of Aromatic Diazonium Salts: Diazonium salts, both aliphatic and aromatic, are unstable because of the extra stability of nitrogen gas (N2) produced when they are decomposed.
- But all the aromatic diazonium salts including the benzenediazonium chloride, are much more stable than the aliphatic diazonium salts.
- So, in cold conditions, aromatic diazonium salts can be prepared but not aliphatic diazonium salts.
- The dispersal of the positive charge of diazonium cation into the benzene ring brings stability to the diazonium salts, i.e., these salts are stabilised by resonance.
The resonating structures of benzene diazonium cation may be represented as follows:
Organic Nitrogen Compounds

Aliphatic Diazonium Salts Cannot Be Stabilised Through Resonance. So, they are extremely unstable and easily decompose with the evolution of N2 gas-forming carbocation. In the reaction of this carbocation with water, alcohol is produced.
Aliphatic Diazonium Salts Example: Ethylamine reacts with NaNO2/HCl to give ethanol.

If aliphatic amines contain electron-withdrawing groups like —CN, —COR, and — COOR they can be converted into aliphatic diazo compounds. Thus, ethyldiazoacetate (CHN2COOC2H5) may be readily obtained by treating a cold solution of the hydrochloride of ethylglycine ester with a cold NaNO2 solution.
C–lH3N+CH2COOC2H5 + NaNO2 → CHN2COOC2H5 + NaCl + 2H2O
Organic Nitrogen Compounds
Properties Of Diazonium Salts
Physical Properties Of Diazonium Salts
- Benzene diazonium salts are generally colourless crystalline solids.
- Most of the diazonium salts, especially nitrates, are explosives in the solid state. They decompose with explosions when heated or at the slightest impact. So, these salts, except aryldiazonium fluoroborate (ArN+2 B–F4), cannot be separated in the dry state. Hence, all important reactions of diazonium salts are carried out in aqueous medium.
- They are highly soluble in water and dissociate into Ions in aqueous solution.
Chemical Properties Of Diazonium Salts: Diazonium salts are chemically very reactive. Like Grignard reagent, it is a very important synthetic reagent, being the starting material for the preparation of various aromatic compounds, dyes and drugs. The reactions of these salts are of three types. They are discussed in the following table—

Substitution Reaction
Substitution By Hydroxyl (—OH) Group: When diazonium salt solution is slowly added to boiling water acidified with dilute sulphuric acid or when highly acidified diazonium salt solution is heated, the salt is hydrolysed leading to replacement of the diazo group (—N+2) by a hydroxyl group (—OH). For example, in this reaction, benzene diazonium chloride is converted into phenol.
Substitution By Hydroxyl Group Example:

Substitution By Hydroxyl Group Reaction Mechanism: It is an aromatic SN1 reaction. At higher temperatures, benzene diazonium cation decomposes to liberate N2 gas and thereby produces phenyl cation which in turn combines with water to form phenol.

Organic Nitrogen Compounds
Substitution By Hydrogen Atom: When heated in the presence of hypophosphorus acid (H3P02), die diazonium salt is reduced and the diazo group (—N2) is replaced by hydrogen. For example, benzene diazonium chloride is converted into benzene in this reaction.
Hydrogen Atom Example:

The Diazo group may also be replaced by hydrogen by heating diazonium salts with ethanol.
Hydrogen Atom Example:

Deamination: The removal of the —NH2 group from the benzene ring by diazotisation of primary aromatic amine, followed by reduction (substitution of diazonium group by H-atom) is called deamination. This process is extensively used in the synthesis of different organic compounds.
Deamination Example: Synthesis of 1,3,5-tribromobenzene from aniline.

Organic Nitrogen Compounds
Substitution By Halogen: Sandmeyer Reaction
Substitution By Chlorine Or Bromine: On treating aromatic diazonium salt solution with a solution of cuprous chloride dissolved in hydrochloric acid or a solution of cuprous bromide dissolved in hydrobromic acid, diazo group (—N+2) is replaced by chlorine or bromine and N2 gas is liberated.
- In this case, cuprous salt acts as the catalyst.
- This Reaction Is Called The Sandmeyer Reaction.
Chlorine Or Bromine Example: When benzene diazonium chloride solution is added to cuprous chloride dissolved in HCl, chlorobenzene is obtained as the product. Similarly, benzene diazonium chloride when added to cuprous bromide dissolved in HBr, bromobenzene is obtained as the product.

Gattermann reaction Diazonium salt when heated with MCI or HBr in the presence of copper powder gives chlorobenzene or bromobenzene. This reaction is called the Gattermann reaction which is a simplified form of the Sandmeyer reaction.

- Substitution by fluorine: Arenediazonium fluoroborate on gentle heating yields aryl fluoride. This is the best method for preparing aryl fluoride. This reaction is known as the Schiemann reaction.
- When fluoroboric acid is added to a diazonium salt solution, insoluble fluoroborate is precipitated.
- It is separated, dried and then heated slowly when aryl fluoride is obtained.
Chlorine Or Bromine Example: Benzenediazonium fluoroborate obtained from benzenediazonium chloride is heated gently to form fluorobenzene.

Organic Nitrogen Compounds
Substitution By Iodine: Benzene diazonium salt solution when heated with KI solution, produces aryl iodide. This is the best method for introducing iodine atoms Into the benzene ring.
Substitution By Iodine Example: Benzene diazonium chloride solution when heated with KI solution, produces iodobenzene.

Substitution By Cyano (—CN) Group: In the reaction of diazonium salt solution with cuprous cyanide dissolved in an aqueous solution of KCN or with an aqueous solution of KCN in the presence of copper powder, the diazo group is replaced by cyano group and aryl cyanide is produced.
- This substitution reaction by the cyano group is, in fact, a special form of Sandmeyer and Gattermann reactions.
- Cyanobenzene (benzonitrile) may be prepared from benzene diazonium chloride or sulphate with the help of this reaction.
Substitution By Cyano Group Example:

Preparation Of Benzoic Acid From Aniline:

Substitution By Nitro (—NO2) Group: Diazotisation of primary aromatic amine with fluoroboric acid and sodium nitrite gives rise to arene diazonium fluoroborate salt. This salt decomposes In the presence of an aqueous solution of sodium nitrite and copper powder, thus replacing the —N2BF4 group with the nitro group.
Substitution By Nitro Group Example: This reaction is used to prepare o and p-dinitro benzene which cannot be produced easily by general methods.

Synthesis Of P-dinltrobenzene From Aniline:

Substitution By Aryl (— Ar) Group: This type of substitution may be carried out by the addition of an aromatic compound to the alkaline solution of diazonium salt or ethanol and copper to diazonium hydrogen sulphate solution. The first method is known as the Gomberg reaction.
Substitution By Aryl Group Example:

Organic Nitrogen Compounds
Schematic Diagram Of Different Substitution Reactions Of Diazonium Salts

Reduction Reactions
Reduction By SnCl2 In Acidic Medium: Diazonium salt when reduced by SnCl2/HCl or sodium sulphite (Na2SO3) gives aryl hydrazine hydrochloride.
Acidic Medium Example: Benzene diazonium chloride in this reaction produces phenylhydrazine hydrochloride.

Organic Nitrogen Compounds
Reduction By Zn/Concentrated HCl: Reduction of the N—with N present agent diazoniums like zinc/conc.salt with HCl the formation of aromatic amine and NH3.
Reduction By Zn/Concentrated HCl Example: Benzene diazonium chloride on reduction gives aniline and NH3

Coupling Reactions: Diazonium salts readily combine with phenols, naphthols and aromatic amines to form brightly coloured (orange, red or yellow) azo compounds.
- In azo compounds, two aromatic rings are linked together by the diazo group (—N=N—). These reactions are called coupling reactions.
- Phenols couple readily in weakly alkaline solution (the rate of coupling increases with a change in pH from 5 to 8).
- Coupling reactions of aromatic amines are carried out in weakly acidic solutions, but not in strongly acidic solutions (the rate of coupling decreases as pH changes from 6 to 2).
Coupling Reaction With Phenol: When a cold solution of benzene diazonium chloride is added to a cold alkaline solution of phenol, a coupling reaction occurs and an orange azo compound called p-hydroxy azobenzene is produced.

Due to steric hindrance, coupling preferentially occurs in the p-position of the hydroxyl group. But if this position is blocked, then o-coupling occurs. For example, o-cresol gives an o-azo compound. If both the positions are blocked, then coupling reactions cannot occur.

Coupling Reaction With β-Naphthol: When a cold solution of benzenediazonium chloride is added to a cold alkaline solution of β-naphthol a brilliant red azo-dye is produced.
Coupling occurs at a or 1-position of α-naphthol. In an alkaline solution, this azo dye exists as sodium salt. Primary aromatic amines can be easily identified with the help of this reaction.

Organic Nitrogen Compounds
Reaction Mechanism: The coupling reaction is an electrophilic substitution reaction where the diazonium cation acts as an electrophile.

Coupling Reactions With Amines: Coupling with tertiary amine: In neutral or weakly acidic solution, N, N -dimethylaniline undergoes a coupling reaction with benzene diazonium chloride.
- The coupling occurs at the carbon atom (C-coupling) of the ring to form p-( N, N -dimethylamino) azobenzene.
- As the nitrogen atom of N, N-dimethylaniline does not contain any hydrogen atom, N-coupling does not occur.

Coupling With Secondary Amine: Coupling with secondary amine generally occurs at the nitrogen atom of the amine i.e., N-coupling occurs. Some C-coupling also occurs.
Coupling With Secondary Amine Example: N-methyldiazoaminobenzene is produced in the reaction between N-methylene and benzene diazonium chloride. At the same time, due to the C-coupling in the ring, some amount of aminoazobenzene is also produced.

Organic Nitrogen Compounds
It should be noted that o-phenylenediamine (1,2-diaminobenzene) reacts with NaNO2/HCl at low temperature when one —NH2 group is diazotised, which undergoes N-coupling with the other —NH2 group leading to the formation of cyclic diazoamino compounds.

Coupling With Primary Amine: In the case of 1° amines, coupling occurs mainly at N-atom (AT-coupling).
Coupling With Primary Amine Example: In the reaction of benzene diazonium chloride with aniline, diazoaminobenzene is produced.

- During the diazotization reaction, if the acidity of the solution is low, then auto-coupling occurs and yellow diazoaminobenzene Is precipitated.
- Diazoaminobenzene when hydrochloride or with HCl, undergoes rearrangement producing p-aminoazobenzene (C -coupling).

Coupling With Primary Amine Reaction Mechanism: In the case of tertiary amine, C-coupling is an electrophilic substitution reaction.

Organic Nitrogen Compounds
For primary and secondary amines, N-coupling occurs in the following way:

Transformations

Organic Nitrogen Compounds


Organic Nitrogen Compounds


Organic Nitrogen Compounds
Distinctive Chemical Tests
Methyl Cyanide And Methyl Isocyanide:
Organic Nitrogen Compounds

Methyl Cyanide And Acetamide:

Ethylamine And Dimethylamine:

Methylamine And Aniline:
Organic Nitrogen Compounds

Dimethylamine And Trimethylamine:

Nitromethane And Methylamine:

Organic Nitrogen Compounds
Aniline Hydrochloride And P-chloroaniline:

Benzamide And Acetanilide:

Acetamide And Benzamide:

Organic Nitrogen Compounds
Cyanobenzene And Aniline:

Aniline And Benzylamine:

Nitrobenzene And Aniline:

Class 12 Chemistry Unit 13 Organic Nitrogen Compounds Very Short Questions And Answers
Question 1. Write the structural formula of vinyl cyanide and its IUPAC name.
Answer:
Structural formula: CH2=CH—CN,
Organic Nitrogen Compounds
IUPAC name: prop-2-enenitrile
Question 2. Can 3° amine participate in the acetylation reaction?
Answer:
The tertiary or 3°amine (R3N) molecule does not contain any replaceable hydrogen atom attached to the N-atom. Hence, 3° amine does not take part in acetylation reaction.
Question 3. Why does the preparation of nitrobenzene necessitate strict control of temperature?
Answer:
In the preparation of nitrobenzene, it is essential to control the temperature because if the temperature exceeds 60°C, m -dinitrobenzene, instead of nitrobenzene is obtained.
Question 4. How will you convert p-H2NC6H4NH2 into p-O2NC6H4NO2 in a single step?
Answer:
p-H2NC6H4NH2 can be converted into p-O2NC6H4NO2 In a single step by oxidation with peroxy trifluoroacetic acid
Question 5. Name the experiment through which aliphatic and aromatic amines can be distinguished.
Answer:
By the azo-dye test, aliphatic and aromatic amines can be distinguished from each other.
Question 6. Write the IUPAC name of an alkyl cyanide containing 5 carbon atoms and only primary hydrogens.
Answer: 2,2-dimethylpropanenitrile.
Question 7. Why tetramethylammonium iodide is not basic?
Answer: There is no lone pair of electrons on N -atom
Question 8. What is enamine?
Answer: Compound formed by the reaction between secondary amine and aldehyde having α-H atom
Question 9. Which class of amine is formed by Gabriel phthalimide synthesis?
Answer: Primary amine
Question 10. Which class of amine is obtained by reducing alkyl isocyanide?
Answer: Secondary amine
Question 11. Ethyl nitrite belongs to which class of compounds?
Answer: Ester (involving mineral acid)
Organic Nitrogen Compounds
Question 12. Give examples of 1° amine containing 2° and 3° alkyl groups
Answer: (CH3)2CH —NH2,(CH3)3C—NH2
Question 13. Why does tertiary nitroalkane not exhibit acidic properties?
Answer: Due to the absence of α-H, they do not exist as acids
Question 14. Give an example of a compound where the Mulliken-Barker test can not be applied for the detection of the nitro group.
Answer: p-nitrobenzaldehyde (p-O2NC6H4CHO)
Question 15. Diazonium salts are not generally separated in a solid state—why?
Answer: Solid diazonium salts are explosive
Question 16. How aliphatic and aromatic 1° amines are distinguished?
Answer: Azo-dye test
Question 17. How will you convert isocyanide into its isomeric cyanide?
Answer: Prolonged heating (rearrangement)
Question 18. What happens when quaternary ammonium hydroxide is heated?
Answer: Tertiary amine
Question 19. What is Hinsberg reagent?
Answer: Benzenesulphonyl chloride (C6H5SO2Cl);
Question 20. Why is benzene diazonium chloride not stored and used immediately after its preparation?
Answer: Benzene-diazonium chloride is unstable. So it is not stored but used immediately after its preparation.
Question 21. Why does acetylation of the — NH2 group of aniline reduce its activating effect?
Answer:
On acetylation, aniline forms acetanilide (C6H5NHCOCH3) in which the lone pair on N is involved in delocalization not only with the benzene ring but also with the adjacent carbonyl group. Thus, acetylation of the —NH2 group of aniline reduces its activating effect.

Question 22. Explain why MeNH2 is a stronger base than MeOH.
Answer:
Nitrogen is less electronegative than oxygen. So, a lone pair of electrons on N is more easily available for donation to a proton. Hence, MeNH2 is more basic than MeOH.
Question 23. What is the role of pyridine in the acylation reaction of amines?
Answer: Pyridine acts as a basic catalyst and removes HCl.
![]()
Question 24. What is the structure and IUPAC name of the compound, allyl amine?
Answer:![]()
Question 25. Write down the IUPAC name
Organic Nitrogen Compounds
Answer: N,N -dimethylbenzenamine.
Question 26. A compound Z with molecular formula C3H9N reacts with C6H5SO2Cl to give a solid, insoluble alkali. Identify Z.
Answer:
- The compound Z (C3H9N) reacts with C6H5SO2Cl to form a solid (i.e., sulphonamide) which is insoluble in alkali.
- This shows that there is no H-atom on the N-atom of the sulphonamide. Thus ‘Z’ is a 2° amine with the structure CH3CH2NHCH3 (N-methylethanamine).
Question 27. A primary amine, RNH2 can be reacted with CH2X to get a secondary amine, RNHCH3 but the only disadvantage is that 3° amine and quaternary ammonium salts are also obtained as side products. Can you suggest a method where RNH2 forms only 2° amine?
Answer:

Question 28. Complete the following reaction,
Answer:

Question 29. Why is aniline soluble in aqueous HCl?
Answer: Aniline (base) reacts with HCl to form the salt, anilinium chloride, which is water soluble

Question 30. Suggest a route by which the following conversion can be accomplished.
Answer:

Organic Nitrogen Compounds
Question 31. Identify A and B in the reaction.
Answer:

Organic Nitrogen Compounds Short Questions And Answers
Question 1. An organic compound with formula C3H9N reacts with Hinsberg’s reagent to give a product, which is insoluble in alkali but dissolves in ether. Identify the compound.
Answer:
A secondary amine reacts with Hinsberg’s reagent to form N, N-dialkyl sulphonamide which is insoluble in alkali but soluble in ether. Hence, the compound with the formula C3H9N is CH3CH2NHCH3 (Ethylmethylamine).

Organic Nitrogen Compounds
Question 2. How will you prepare ethylamine, free-form diethyl and triethyl amine from ethyl iodide?
Answer:
By Gabriel-phthalimide synthesis, ethylamine, not contaminated by diethyl and triethyl amine, is prepared.

Question 3. Why is methylamine a stronger base than ammonia?
Answer:
- Molecules of both ammonia and methylamine contain a lone pair of electrons on the N-atom. So both act as Lewis bases.
- But due to the presence of an electron-repelling methyl group, attached to the N-atom in methylamine, the electron density on the N-atom increases, i.e., the availability of electrons on the N-atom increases.
- Hence, CH3NH2 can form a bond with the proton by donating its lone pair to N-atom with greater ease than NH3. So CH3NH2 behaves as a stronger base than NH3

Organic Nitrogen Compounds
Question 4. Silver chloride is insoluble in water but soluble in ethylamine—explain.
Answer:
Silver chloride is insoluble in water but when it is heated with ethylamine, it forms a complex salt which is soluble in water.
Question 5. Write the names and structural formulae of the isomeric amines having molecular formula C4H11N.
Answers:

Question 6. Starting from n -butyric acid how will you prepare n-propylamine?
Answer:

Organic Nitrogen Compounds
Question 7. How will you transform methylamine into dimethyl amine, free from trimethyl amine?
Answer:

Question 8. Between methyl cyanide and methyl isocyanate which one is more soluble in water and why?
Answer:
N-atom in the molecule of methyl cyanide is partially negatively charged. So, it can easily participate in the hydrogen bond formation with the water molecules. Hence, methyl cyanide shows sufficient solubility in water.

On the contrary, the N-atom in the molecule of methyl isocyanide becomes partially positively charged and hence, it fails to form an H-bond with water molecules. So it is almost insoluble in water.

Organic Nitrogen Compounds
Question 9. Can acetonitrile exhibit acidic properties? If so, explain with an example.
Answer:
Acetonitrile reacts with a strong base like sodamide, thus removing a proton from α-carbon. In the presence of a strong base, acetonitrile (methyl cyanide) displays its mild acidic character. The mild acidic character of acetonitrile is due to the stability of its conjugate base through resonance.

Question 10. Why does an aqueous solution of methylamine give a precipitate of ferric hydroxide with ferric chloride?
Answer:
In aqueous solution, methylamine establishes the following equilibrium:
![]()
OH– ion present in the solution reacts with Fe3+ ion, produced by the dissociation of FeCl3, to give a brown precipitate of ferric hydroxide.
![]()
Question 11. Why is it difficult to prepare pure primary amine by ammonolysis of alkyl halide?
Answer:
Ammonolysis of alkyl halides produces a mixture of primary, secondary, tertiary and quaternary ammonium salts. Isolation of primary amine from the mixture is very difficult. Hence, the preparation of pure primary amine by the ammonolysis of alkyl halide is quite difficult.

Organic Nitrogen Compounds
Question 12. Give a suitable method for the preparation of tertiary butylamine from tertiary butyl bromide.
Answer:
Tertiary butyl bromide is first converted into Grignard reagent which on subsequent treatment with chloramine produces tertiary butylamine.

Question 13. Out of methyl cyanide and methylamine, which one is more basic and why?
Answer:
- Nitrogen atoms of methyl cyanide and methylamine are respectively sp- and sp3-hybridised. The electronegativity of sp-hybridized N -atom is greater than that of sp3-hybridised N -atom.
- So, the electron density on the N-atom of methylamine (CH3NH2) is more than that on the N-atom of methyl cyanide (CH3—C=N:).
- Moreover, due to the attachment of an electron-repelling methyl group directly to the N-atom in methylamine, the electron density of the N-atom is further enhanced.
- This concerted effect eventually imparts a more basic character to methylamine than methyl cyanide.

Organic Nitrogen Compounds
Organic Nitrogen Compounds
Question 14. What happens when the product obtained in the reaction of tetramethyl ammonium iodide with moist silver oxide is heated?
Answer:
When tetramethyl ammonium iodide reacts with moist silver oxide, tetramethyl ammonium hydroxide is obtained. The latter when heated in dry conditions, produces trimethylamine and methyl alcohol.

Question 15. Although aniline is a colorless liquid, it is generally found as a brown-colored liquid—why? How is pure colorless aniline obtained from this impure variety of aniline?
Answer:
- Any organic compound having an electron-rich center is prone to oxidation by aerial oxygen. The nitrogen atom of aniline contains a lone pair of electrons and the —NH2 group enhances the electron density of the ring through resonance.
- So both —the NH2 group and the ring are susceptible to oxidation. Therefore, aniline gets oxidized easily, producing different colored oxidized products.
- The colored aniline when distilled using an air condenser gives pure, colorless aniline as a distillate.
Question 16. What happens when a cupric chloride or copper sulfate solution is mixed with methylamine?
Answer:
When cupric chloride or copper sulfate solution is mixed with methylamine, a deep blue complex salt is formed and the solution turns blue.
- CuCl2 + 4CH3NH2 → [CU(CH3NH2)4]Cl2 (deep blue)
- CuSO4 + 4CH3NH2 → [Cu(CH3NH2)4]SO4 (deep blue)
Organic Nitrogen Compounds
Question 17. The observed boiling point of nitrobenzene is much higher than that expected based on its molecular mass—why?
Answer:
- Nitrobenzene is sufficiently polar (dipole moment μ= 3.95D ). So, its molecules experience strong dipole-dipole attraction.
- Consequently, separation of the molecules requires higher thermal energy, and hence, the actual boiling point of nitrobenzene is much higher than that expected based on its molecular mass.
Question 18. Which is more basic out of benzylamine (C6H5CH2NH2) and isomeric p -toluidine (p-CH3C6H4NH3)? Explain.
Answer:
- As the — NH2 group in p -toluidine is directly attached to the benzene ring, the lone pair of electrons of the N-atom undergoes delocalization with n -electrons of the aromatic ring. This results in a decrease in electron density on N-atom.
- On the other hand, the — NH2 group in benzylamine is not directly linked to the benzene ring and so the lone pair of electrons on N-atom do not participate in resonance.
- Hence, the electron density on N-atom is not reduced. Therefore, benzylamine is more basic than p -toluidine.
Question 19. Why is nitrobenzene not easily oxidized? Mention j 1 one practical application based on this property.
Answer:
- Due to the strong electron-attracting property of the — NO2 group, the electron density in the benzene ring is drastically reduced i.e., the ring becomes highly deactivated.
- So it is not oxidized even by a strong oxidant. Its boiling point is sufficiently high. Hence, nitrobenzene is used as an ideal solvent for the oxidation reactions occurring at high temperatures.
Question 20. How will you prepare isopropyl amine directly from acetone? Which class does it belong to?
Answer:
When acetone vapor mixed with excess ammonia and H2 gas is passed over Raney nickel (catalyst) under high pressure at 140°-150°C, isopropylamine is produced. Isopropylamine belongs to primary amines containing a secondary alkyl group.

Organic Nitrogen Compounds
Organic Nitrogen Compounds
Question 21. Nitration of toluene occurs very easily and at a much faster rate relative to benzene. But nitration of nitrobenzene is very difficult and takes place at a much slower rate concerning benzene. Explain.
Answer:
- An increase in electron density in the benzene ring increases the reactivity of the compound while a decrease in electron density reduces its reactivity. The —CH3 group present in the toluene molecule is electron-repelling.
- By its +1 effect and hyperconjugation, the electron density in the ring is significantly enhanced. On the other hand, in the nitrobenzene molecule, the —NO2 group present is a strong electron-attracting group.
- It considerably decreases the electron density in the ring with the help of its -I effect as well as the -R effect.
- Given the reasons mentioned above, nitration of toluene (an electrophilic substitution reaction) can be accomplished very easily and with greater ease than benzene. But nitration of nitrobenzene becomes very difficult and proceeds at a much slower rate than benzene.

Question 22. Arrange in the order of increasing basicity (with reasons): aniline, p-nitroaniline, p-toluidine, N, N,4- trimethylamine
Answer:
- Each of the given compounds is an aromatic amine. An increase in the electron density of the amino-nitrogen increases the basicity of the corresponding compound.
- So if the electron-donating alkyl group is attached to the amino N -atom, the basicity of the amine increases.
- Moreover, if the electron-attracting nitro group (having -I and -R effect) is present in the ring, the basicity of the amine decreases.
- Based on the above information, the given compounds can be arranged in the following order of their increasing basicity:

Organic Nitrogen Compounds
Question 23. Why does the nitration of chlorobenzene occur at a slower rate compared to benzene?
Answer:
- Cl -atom present in chlorobenzene increases the electron density of the ring through the +R effect but it draws the electron from the ring through its -I effect.
- In this case, as the -I effect is stronger, it outweighs the +R effect. So, the electron density of the ring eventually decreases. Hence, nitration of chlorobenzene occurs slowly concerning benzene.
Question 24. What happens when: one. HCl cone. H2SO4 are added to aniline? In each case, what happens if the addition of acid is followed by the addition of water?
Answer:
- When cone. HCl is added to aniline, and crystalline solid aniline hydrochloride is precipitated.
- The addition of cone H2SO4 to aniline gives crystalline solid aniline sulfate. The salts are ionic. So when water is added, these salts dissolve giving a colourless solution.

Question 25. How will you prove that aniline is basic?
Answer:
Aniline is insoluble in water but soluble in HCl as, aniline reacts with the acid (HCl) to form a salt, aniline hydrochloride which is soluble in water. This suggests that aniline is basic.

Question 26. Why is Gabriel phthalimide synthesis unsuitable for the preparation of aniline?
Answer:
- In the preparation of aniline by Gabriel-phthalimide synthesis, the first step requires a nucleophilic substitution reaction of a halobenzene using potassiophthalimide as the nucleophile.
- However, aryl halides (for example., C6HgCl ) generally do not participate in nucleophilic substitution reactions. So, it is not possible to prepare aniline by Gabriel’s phthalimide synthesis.

Organic Nitrogen Compounds
Question 27. How will you prepare paracetamol, a widely used antipyretic drug from nitrobenzene?
Answer:
At first, nitrobenzene is reduced to phenylhydroxylamine, which in the presence of dil. HCl rearranges to p-aminophenol. Then it reacts with acetic anhydride to form paracetamol.

Organic Nitrogen Compounds
Question 28. How is the —NO2 group in an aromatic compound identified in the presence of the —NH2 group? Give example.
Answer:
- Nitro group in an aromatic compound in the presence of the —NH2 group can be detected by the Mulliken-Barker test.
- In this test, the aromatic compound is heated with Zn-dust, NH4Cl, and 50% aqueous alcohol and the resulting mixture is filteredin Tollens’ reagent.
- The appearance of a grey or black precipitate indicates the presence of a nitro group in the compound.

Organic Nitrogen Compounds
Question 29. Why is acetamide more acidic than ethylamine?
Answer:
- If one proton is lost from the N-atom of the acetamide molecule, the anion produced i.e., conjugate base acquires stability through resonance.
- But in ethylamine, if one proton is lost from the N-atom conjugate base cannot be stabilized through resonance.
- So acetamide has a greater tendency to lose proton than ethylamine and thus, behaves as a stronger acid than ethylamine.

Question 30. Why is aniline less basic than methylamine?
Answer:
- The basicity of a nitrogenous base depends on the ease with which the N-atom present in the molecule of the base can donate its lone pair of electrons to a proton, i.e, the effective electron density on the N-atom present in the molecule of the base decides its basicity.
- In an aniline molecule, the lone pair of electrons present on the N-atom of the —NH, the group participates in resonance with the n electrons of the benzene ring, causing delocalization of the lone pair of electrons.
- Consequently, electron density on the N-atom diminishes. So, the N-atom cannot donate its electron pair to the proton and thus, aniline behaves as a weak base.

Organic Nitrogen Compounds
Organic Nitrogen Compounds
- On the other hand, in methylamine, the lone pair of electrons on the N-atom can not be involved in delocalization.
- Hence, the lone pair resides entirely on the N atom itself Besides this, due to the effect of — CH, group the electron j density on the N-atom increases.
- So, the N-atom can donate Us lone pair of electrons to a proton. Therefore, methylamine is a stronger base than aniline.
Question 31. How can CH3CN be used for the synthesis of CH3NH2 and CH3CH2NH2?
Answer:

Question 32. Identify A, B, C, and D In the following reactions:

Organic Nitrogen Compounds
Answer:

Question 33. Mention the reagents for the following conversion in a single step:![]()
Answer:

The reagent is trifluoroacetic acid (CF3CO3H).
Question 34. Consider the compounds and answer the questions:

Which on treatment with aqueous NaNO2 dill. HCI followed by heating with SnCI2 /cone. HCI produces a hydrazine derivative? Write the structure of the hydrazine derivative.
Answer:

Organic Nitrogen Compounds
Organic Nitrogen Compounds
Question 35. Write the structures of A to C In the following reaction:

Answer:

Question 36. An organic compound (A) is soluble in water. Its aqueous solution liberates carbon dioxide from NaHCO3, forms a white precipitate with aqueous BaCl2 solution, and responds to azo-dye test. Which is (A) among the following—

Organic Nitrogen Compounds
Answer: 3. ![]()
Question 37. Which is produced when benzene-diazonium chloride is coupled with phenol in an alkaline medium—

Answer: 1. ![]()
Question 38. An organic compound (A) of molecular formula C7H7NO, on treatment with P2O5 provides (B). The reaction of both (A) and (B) with LiAlH4 gives (C). Acid hydrolysis of both (A) and (B) affords benzoic acid. Identify (A), (B), and (C) with reason.
OR, Identify A, B, C, D, E, and F in the following reactions:

Organic Nitrogen Compounds
Answer: 1.

Organic Nitrogen Compounds
Question 39. Which of the following compounds will be formed when aniline reacts with H2S2O5—

Answer: 3. ![]()
Question 40. Arrange the following compounds in decreasing order of their basicity:

Organic Nitrogen Compounds
Write the arrow-head equation for the following reaction: Aniline is refluxed with glacial acetic acid or, Write the organic products in the following reactions:

Answer:

Organic Nitrogen Compounds
Question 41. Which of the following is to not an aminium salt
- CH3CH2N+H3Cl–
- (CH3CH2)2N+H2Br–
- (CH3)3N+HI–
- (CH3)4N+Br–
Answer: 4. (CH3)4N+Br–
Question 42. Write down the reagents for the following reactions:

Answer:

Organic Nitrogen Compounds
Question 43. Which of the following compounds is the most basic

Answer: 4
Question 44. An organic compound (A) of molecular formula C7H5N in reaction with alkaline H2O2 furnishes (B). Acid hydrolysis of both (A) and (B) produces the same aromatic acid (C) of molecular formula C7H6O2. The reaction of both (A) and (B) with LiAlH4 gives a primary amine (B) of the molecular formula C7H9N. Identify (A), (B), (C), and (D). Write arrowhead equations for the formation of (B) from (A) and (D) from (A).
OR, Write the reagents for the following conversions:

Distinguish between the following two compounds by a chemical reaction:![]() Answer:
Answer:

Organic Nitrogen Compounds
Question 45. Write chemical equations for the following conversions:
- Nitrobenzene to benzoic acid
- Benzyl chloride to 2-phenylethanolamine
- Aniline to benzyl alcohol
Answer:

Question 46. Arrange in the decreasing order of basic strength in aqueous solutions: CH3NH2. (CH3)2NH, (CH3)3N, NH3.
Answer: NH3<(CH3)3N<CH3NH2<(CH3)2NH
Question 47. The conversion of primary aromatic amines into diazonium salts is known as
Answer: Diazotisation.
Question 48. Account for the following: Aniline does not undergo Friedel-Crafts reaction.
Answer:
Aniline is a Lewis base and reacts with a Lewis acid, AlCl3 to form a salt. As a result, the N-atom of aniline + acquires a positive charge. The —N+H3 group is a deactivating group for electrophilic substitution reaction. C6H5NH2 + AlCl3 → C6H5-N+H2– A–lCl3 Hence, aniline does not undergo Friedel-Craft’s reaction.
Question 49. Give the structures of A, B, and C:

Answer:

Question 50. Complete the reaction: C6H5N2Cl + H3PO2 + H2O→
Answer:

Question 51. Arrange the following in increasing order of basic strength: Aniline, p-nitroaniline, and p-toluidine
Answer: p-nitroaniline < Aniline < p-toluidine
Question 52. Write the IUPAC name of the given compound:
Answer: 2,4,6-tribromoaniline / 2,4,6-tribromobenzenamine
Question 53. Write the structures of A, B, and C In the following:

Answer:

Organic Nitrogen Compounds
Question 54. Write the IUPAC name of the given compound:![]()
Answer: N-phenylethylamine
Question 11. Complete the following reactions:

Answer:

Question 55. Write the IUPAC name of the following compound: (CH3CH2)NCH3
Answer: N-ethyl-N-methylethanamine.
Question 56. Give reasons:
- Acetylation of aniline reduces its activation effect.
- CH3NH2 is more basic than C6H5NH2.
- Although — NH2 iS o/p directing group, aniline on nitration gives a significant amount of m-nitroaniline.
Answer: Due to resonance, the lone pair of electrons on the nitrogen of acetanilide gets delocalized towards the carbonyl group.

- Hence the electrons are less available for donation to the benzene ring by resonance. Therefore, the activation effect of aniline is reduced.
- Nitration is carried out in an acidic medium. In an acidic medium, aniline is protonated to form the anilinium ion (which is mem-directing).
- For this reason, aniline on nitration gives a substantial amount of nitroaniline.
Question 57. Write the structure of 2,4-dinitrochlorobenzene.
Answer:
Question 58. Write the IUPAC name of the following compound: CH3NHCH(CH3)2
Answer: N-methyldopa-2-amine
Question 59. Write the IUPAC name of the following compound: (CH3)2N—CH2CH3
Answer: N, N-dimethylethanamine.
Question 60. Write the structures of compounds A, B, and C In the following reactions:

Answer:
- A = CH3CONH2, B = CH3NH2, C = CH3NC
- A = C6H5NO2, B = C6H5NH2, C = C6H5NHCOCH3
Question 61. Classify the following amines as primary, secondary, or tertiary:

Organic Nitrogen Compounds
Answer:
- Primary,
- Tertiary,
- Primary,
- Secondary
Question 62. Write structures of different isomeric amines corresponding to the molecular formula, C4HnN.
- Write the IUPAC names of all the isomers.
- What type of isomerism is exhibited by different pairs of amines?
Answer:
Primary amines:

IUPAC Names:
- Butan-1-amine;
- Butan-2-amine;
- 2-methylpropan-l-amine;
- 2-methylpropan-2-amine;
- N-methylpropan-l-amine;
- N-methylpropan-2-amine;
- N-ethylethanamine;
- N, N-dimethylethanolamine;
- Position Isomers: 1 and 2; 5 and 6
- Chain Isomers: 1 and 3; 1 and 4; 2 and 3; 2 and 4
- Metamers: 5 and 7; 6 and 7
Functional isomers: All primary amines are functional isomers of secondary and tertiary amines and vice versa. On the other hand, secondary and tertiary amines are functional isomers of each other.
Question 63. Write chemical equations for the following reactions:
- Reaction of ethanolic NH3 with C2H5Cl.
- Ammonolysis of benzyl chloride and the reaction of amine so formed with two moles of CH3Cl.
Answer:

Organic Nitrogen Compounds
Question 64. Write chemical equations for the following conversions:
- CH3—CH2—Cl into CH3 —CH2—CH2—NH2
- C6H5—CH2—Cl into C6H5—CH2—CH2—NH2
Answer:

Question 65. Write structures and IUPAC names of
- The amide which gives propanamide by Hofmann bromamide reaction,
- The amine produced by the Hofmann degradation of benzamide
Organic Nitrogen Compounds
Answer:
- In the Hofmann bromamide reaction, R—CONH2 is converted to R—NH2.
- Thus the structure of the amide that produces propanamine (CH3CH2CH2NH2) is CH3CH2CH2CONH2 and its IUPAC name is butanamide.
- Benzamide is C6H5CONH2. So the amine produced by Hofmann degradation ofbenzamide is C6H5NH2 and its IUPAC name is aniline or benzenamine.
Question 66. How will you convert— O Benzene into aniline,
- Benzene into N, N-dimethylaniline,
- Cl—(CH2)4—Cl into hexan-1, 6-diamine?
Answer:

Question 67. Arrange the following in decreasing order of their basic strength: C6H5NH2, C2H5NH2. (C2H5)2NH, NH3
Answer:
- Out of the given amines, C6H5NH2 is the weakest base because the lone pair on the amino nitrogen is involved in delocalization with the aromatic ring.
- The presence of two electron-donating C2H5 groups on the amino nitrogen of diethylamine makes it a stronger base than ethylamine (which contains only one C2H5 group).
- Basicity of NH3 is less than that of ethylamine and diethylamine. Thus, the order of basicity is (C2H5)2NH > C2H5NH2 > NH3 > C6H5NH2.
Question 68. Arrange the following in increasing order of their basic strength:
- C2H5NH2, C6H5NH2, NH3> C6H5CH2NH2 and (C2H5)2NH
- C2H5NH2> (C2H5)2NH, (C2H5)3N, C6H5NH2
- CH3NH2, (CH3)2NH, (CH3)3N, C6H5NH2, C6H5CH2NH2
Answer:
- C6H5 —NH2 is the weakest base because the lone pair on amino nitrogen is involved in resonance with the aromatic ring. (C2H5)2NH is stronger than C2H5NH2 due to the presence of an additional C2H5 group (+1 effect).
- NH3 is weaker than either of these amines due to the absence of an alkyl group.
- C6H5CH2NH2 is weaker than NH3 because of the electron-withdrawing -I effect of the C6H5 group. However, it is stronger than aniline because the C6H5 group is not directly attached to the amino group.
- Thus the sequence of basicity is, C6H5NH2 < C6H5CH2NH2 < NH3 < C2H5NH2 < (C2H5)2NH.
- The basic, strength of 1°, 2° and 3° alkylamines in an aqueous medium is determined by two factors: (a) electron donation by increasing the number of alkyl groups tends to increase the basicity, (b) stability through solvation of the conjugate acid formed by uptake of a proton (which decreases in the sequence: C2H5NH3 > (C2H5)2NH2 > (C2H5)3NH ).
- Combining these two factors, the sequence of basicity is found to be, (C2H5)2NH > (C2H5)3N > C2H5NH2. Thus the order of basicity is, C6H5NH2 < C2H5NH2 < (C2H5)3N<(C2H5)2NH.
- Alkyl amines (1°, 2°, and 3°) are stronger bases than C6H5CH2NH2 because the latter contains electron withdrawing —C6H5 group. Based on the electron-donating +1 effect of alkyl groups on the amino nitrogen and relative stabilization of the conjugate acids of the alkyl amines, it is found that the basicity follows the sequence: (CH3)2NH > CH3NH2 > (CH3)3N.
Thus the order ofbasicity is, C6H5NH2 < C6H5CH2NH2 < (CH3)3N < CH3NH2 < (CH3)2NH .
Question 69. Complete the following acid-base reactions and name the products:
- CH3CH2CH2NH2+ HCl→
- (C2H5)3N + HCl→
Organic Nitrogen Compounds
Answer:

Question 70. Write reactions of the final alkylation product of aniline with excess methyl iodide in the presence of sodium carbonate solution.
Answer:


Question 71. Write the chemical reaction of aniline with benzoyl chloride and write the name of the product obtained.
Answer:

Organic Nitrogen Compounds
Question 72. Write structures of different isomers corresponding to the molecular formula, C3HgN. Write IUPAC names of the isomers that will liberate nitrogen gas on treatment with nitrous acid.
Answer:
C3H9N has four isomers:

Only 1° amines are found to react with HNO2 to liberate N2 gas, according to the equation:
⇒ \(\mathrm{R}-\mathrm{NH}_2+\mathrm{HNO}_2 \rightarrow \mathrm{ROH}+\mathrm{N}_2 \uparrow+\mathrm{H}_2 \mathrm{O}\)
Thus the isomers that liberate nitrogen gas are— CH3CH2CH2NH2 (Propan-1-amine) and

Question 73. How will you convert 4-nitrotoluene to 2-bromobenzoic acid?
Answer:

Question 74. Convert:
- 3-methylaniline into 3-nitrotoluene.
- Aniline Into 1,3,5-tribromobenzene.
Answer:

Organic Nitrogen Compounds
Question 75. Write IUPAC names of the following compounds and classify them into primary, secondary, and tertiary amines.
- (CH3)2CHNH2
- CH3(CH2)2NH2
- CH3NHCH(CH3)2
- (CH3)3CNH2
- C6H5NHCH3
- (CH3CH2)2NCH3
- m-BrC6H4NH2
Answer:

Question 76. Give one chemical test to distinguish between the following pairs of compounds.
- Methylamine and dimethylamine
- Secondary and tertiary amines
- Ethylamine and aniline
- Aniline and benzylamine
- Aniline and Nmethylaniline.
Answer:
When heated with an alcoholic solution of KOH and chloroform (CHCl3), methylamine (1° amine) gives an offensive smell of methyl isocyanide (CH3NC). Dimethylamine (2° amine) does not respond to this test.

2° amines react with HNO2 (i.e, NaNO2 + HCl ) to give yellow coloured oily N-nitrosoamines.
R2NH + HNO2 → R2N—N=O + H2O
- Nitrosoamine, when warmed with phenol and cone. H2SO4 gives a green solution that turns deep blue when made alkaline with aq. NaOH. It becomes red on dilution with water. Tertiary amines do not respond to this test.
- It is similar to the distinction between methylamine and aniline (see ‘Distinguish between’ section).
- See the ‘Distinguish between’ section.
- When heated with an alcoholic solution of KOH and CHCl3, aniline (1° amine) gives an offensive smell of phenyl isocyanide. N-methyl aniline (2° amine) does not respond to this test.
Question 77. Account for the following:
- pKb of aniline is more than that of methylamine.
- Ethylamine is soluble in water whereas aniline is not.
- Methylamine In water reacts with ferric chloride to precipitate hydrated ferric oxide.
- Although the amino group Is o- and p -directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of m-nitroaniline.
- Aniline does not undergo Friedel-Crafts reaction.
- Diazonium salts of aromatic amines are more stable than those of aliphatic amines.
- Gabrlelphthalimide synthesis is preferred for synthesizing primary amines.
Answer:
- In aniline, the lone pair on amino nitrogen is involved in delocalization with an aromatic ring so it is less available for combination with a proton. In contrast, in CH3NH2, the +I effect of the CH3 group increases the electron density on the N-atom.
- So the lone pair of N-atom is more available for protonation. Thus, aniline is a weaker base and its pKb value is greater than that of methylamine.
- Ethylamine is soluble in water because it can form intermolecular H-bonds with H2O molecules:

- But in the case of aniline, due to the large lyophobic part (i.e., hydrocarbon part), the extent of intermolecular H-bonding with water decreases considerably, and hence, aniline is almost insoluble in water.
- Methylamine reacts with water forming OH-ions according to the following acid-base equilibrium:
![]()
These OH- ions combine with Fe3+ ions to form a brown precipitate of hydrated ferric oxide:
![]()
- Nitration is usually carried out using mixed acid consisting of a mixture of cones. HNO3 + cone. H2SO4. In the presence of these acids, a large portion of the aniline molecule gets protonated and forms an anilinium ion, and an equilibrium is established.
- Aniline in which the —NH2 group is ortho/para-orienting undergoes nitration and forms a mixture of ortho- and para-nitro aniline, while anilinium ion in which the —N+H3 group is meta-orienting undergoes nitration to form the meta isomer.

- In the Friedel-Crafts reaction, AlCl3 is used as a catalyst. This is a Lewis acid that combines with the basic compound aniline (substrate) and forms a complex, in which the amino nitrogen becomes positively charged.
- Consequently, the aromatic ring gets highly deactivated and the Friedel-Crafts reaction (a type of electrophilic substitution) fails.

Organic Nitrogen Compounds
Diazonium salts derived from aromatic amines are more stable due to the dispersal of the +ve charge through delocalization involving the aromatic ring system.

Gabriel-phthalimide synthesis involves nucleophilic attack of the N-atom of potassiophthalimide on the alkyl group of an alkyl halide to form N-alkyl phthalimide (in fact, the alkyl group gets attached to the attacking nitrogen atom).
This N-atom (which is not attached to any H-atom) can no longer be involved in further nucleophilic attack on a second molecule of alkyl halide, and hence, pure primary amines (devoid of any secondary or tertiary amines) are formed in this reaction.
Organic Nitrogen Compounds
Question 78. Arrange the following:
- In decreasing order of pKb values: C2H5NH2, C6H5NHCH3, (C2H5)2NH and C6H5NH2
- In increasing order of basic strength: C6H5NH2, C6H5N(CH3)2, (C2H5)2NH and CH3NH2 In increasing order of basic strength: (a) Aniline, p -nitroaniline and p-toluidine (b) C6H5NH2, C6H5NHCH3, C6H5CH2NH2
- In decreasing order of basic strength in gas phase: C2H5NH2< (C2H5)2NH, (C2H5)3N and NH3 In increasing order of boiling point: C2H5OH, (CH3)2NH, C2HgNH2 In increasing order of solubility in C6H5NH2, (C2H5)2NH, C2H5NH2.
Answer:
- Aromatic amines are weaker bases than aliphatic amines because the lone pair on the N-atom of the former is involved in delocalization with the aromatic ring system and hence, less available for protonation.
- C6H5NHCH3 is a stronger base than C6H5NH2 because the +1 effect of the CH3 group causes an increase in electron density on the N-atom.
- Similarly (C2H5)2NH is stronger than C2H5NH2 because of greater electron donation by an additional C2H5 group in the former. Thus the order of increasing basic strength or decreasing pKb values is, C6H5NH2, C6H5NHCH3, C2H5NH2, (C2H5)2NH.
- It has already been explained in Ans. Q that the basic strength of (C2H5)2NH is greater than that of C6H5NH2. C6H5N(CH3)2 is a stronger base than CgHgNH2 because the +1 effects of two CH3 groups cause a greater increase in electron density on the N-atom of the former.
- But (C2H5)2NH is more basic than CH3NH2 primarily due to the greater +1 effect of the two C2H5 groups (in the former) over one CH3 group (in the latter). Thus increasing order of basic strength of the given compounds is, C6H5NH2 <C6H5N(CH3)2 <CH3NH2 <(C2H5)2NH
Organic Nitrogen Compounds
In aromatic amines, the presence of an electron-withdrawing group in the ring system causes a decrease in basic strength, while the presence of an electron-donating group causes an increase in basic strength.
- Thus, p-p-nitroaniline (containing electron-withdrawing —NO2) is a weaker base than aniline, while p-toluidine (containing electron-donating CH3 group) is a stronger base than aniline.
- Thus, the order of basicity is, p-nitroaniline < aniline < p-toluidine (i.e., p methylaniline).
- In C6H5NH2 and C6H5NHCH3, the amino nitrogen is directly attached to the benzene ring. So the lone pair of electrons on the N-atom is delocalized over the ring system.
- Thus both C6H5NH2 and C6H5NHCH3 are weaker bases than C6H5CH2NH2 (in which the N-atom is attached to the ring via a CH2 group).
- Due to the +1 effect of the CH3 group, C6H5NHCH3 is a stronger base than C6H5NH2. So the basic strength increases in the order: C6H5NH2 < C6H5NHCH3 < C6H5CH2NH2.
- In the gas phase, we need not consider solvent effects since, there is no question of stabilization of the conjugate acids through solvation involving, H-bonding.
So basic strengths of the given aliphatic amines will be determined by the number of electron-donating alkyl groups on the amino nitrogen.
- The basic strength increases as the number of alkyl groups on the amino nitrogen increases. In other words, basic strength in the gas phase decreases in the sequence: (C2H5)3N > (C2H5)2NH > C2H5NH2.
- Since oxygen is more electronegative than nitrogen, alcohols form stronger H-bonds than amines. So the boiling points of alcohols are higher than those of amines having comparable molecular masses. Therefore, C2H5OH (MW = 46) has a higher boiling point than (CH3)2NH and C2H5NH2 (each having MW = 45).
- The extent of H-bonding depends on the number of H-atoms on the N-atom. Therefore, C2H5NH2 (having two H-atoms attached to N) has a higher boiling point than (C2H5)2NH (having one H-atom attached to N). Thus boiling points of the given compounds follow the sequence: (CH3)2NH < C2H5NH2 < C2H5OH.
- Solubility of amines decreases with an increase in molecular mass due to an increase in the size of the hydrophobic hydrocarbon part and also with a decrease in the number of H-atoms (attached to N-atom) that take part in H-bonding.
- Thus, C6H5NH2 (MW = 93) has the lowest solubility. Out of (C2H5)2NH and C2H5NH2, the latter has higher solubility because the extent of H-bonding is greater, and also the size of the hydrophobic hydrocarbon part is smaller. In other words, the solubility increases in the order: of C6H5NH2 < (C2H5)2NH < C2H5NH2.
Organic Nitrogen Compounds
Question 79. How will you convert:
- Ethanoic acid into methenamine
- Hexanenitrile into 1-amino pentane
- Methanol to ethanoic acid
- Ethanamine into methenamine
- Ethanoic acid into propanoic acid
- Methenamine into ethanolamine
- Nitromethane Into dimethylamine
- Propanoic acid into ethanoic acid?
Answer:

Organic Nitrogen Compounds
Question 80. Accomplish the following conversions:
- Aniline to 2,4,6-tribromofluorobenzene
- Benzyl chloride to 2-phenylethanolamine
- Chlorobenzene to p-chloroaniline
- Aniline to p-bromoaniline
- Benzamide to toluene
- Aniline to benzyl alcohol.
Answer:

Organic Nitrogen Compounds
Question 81. Give the structures of A, B, and C in the following reactions:

Answer:

Organic Nitrogen Compounds
Question 82. An aromatic compound ‘A’ on treatment with aqueous ammonia and heating forms compound ‘B’ which on heating with Br2 and KOH forms a compound ‘C’ of molecular formula C6H7N. Write the structures and IUPAC names of compounds A, B, and C.
Answer:
Compound ‘C’ (C6H7N) is obtained by heating compound ‘B’ with Br2 and KOH. This suggests that the reaction is Hofmann degradation and ‘C’ is a primary amine. Its molecular formula shows that it is aniline (C6H5NH2). So ‘B’ is an amide with the formula C6H5CONH, (benzamide).

Organic Nitrogen Compounds
Since the treatment of compound ‘A’ with aqueous ammonia, followed by heating gives the amide B (C6H5CONH2), it indicates that ‘A’ must be benzoic acid (C6H5COOH).
Question 83. Complete the following reactions:
- C6H5NH2 + CHClg + alc.KOH→
- C6H5N2CI + H3PO2 + H2O→
- C6H5NH2 + H2SO4(conc.)→
- C6H5N2Cl + C2H5OH→
- C6H5NH2 + Br2(aq)→
- C6H5NH2 + (CH3CO)2O→

Answer:


Organic Nitrogen Compounds
Question 84. Why cannot aromatic primary amines be prepared by Gabriel-phthalimide synthesis?
Answer:
The most important step in Gabriel phthalimide synthesis is the SN2 reaction in which the nucleophile, phthalimide anion displaces the halide ion from alkyl halide to form Nalkylphthalimide. This upon subsequent hydrolysis gives the corresponding aliphatic primary amine.

Since aryl halides are reluctant to undergo nucleophilic substitution reactions, aromatic primary amines (i.e., aryl amines) cannot be prepared by Gabriel-phthalimide synthesis.

Question 85. Write the reactions of
- Aromatic and
- Aliphatic primary amines with nitrous acid.
Answer:
Aromatic 1° amines react with HNO2 in the presence of HCl or H2SO4 at 0-5°Cto form diazonium salts.

Aliphatic primary amines also react with HNO2 at 0-5°C to form diazonium salts, which are unstable and thus decompose readily to form alcohol and N2 gas.

Question 86. Give a plausible explanation for each of the following:
- Why are amines less acidic than alcohols of comparable molecular masses?
- Why do primary amines have higher boiling points than tertiary amines?
- Why are aliphatic amines stronger bases than aromatic amines?
Organic Nitrogen Compounds
Answer:
- Since oxygen is more electronegative than nitrogen, the conjugate base (RO–)of an alcohol (ROH) is more stable than the conjugate base (RNH–) of an amine (RNH2).
- So, the loss of a proton from alcohol is more favorable than that from an amine. Hence, alcohols are more acidic than amines. Conversely, amines are less acidic than alcohols.

- Due to the presence of two H-atoms on the N-atom of primary amines, they undergo association through the intermolecular H-bonding.
- However tertiary amines do not undergo such association because they cannot form intermolecular H-bonds due to the absence of a H-atom on the N-atom. Thus, primary amines have higher boiling points than tertiary amines of comparable molecular masses.

- In aromatic amines, the lone pair on the amino nitrogen is involved in delocalization with the aromatic ring and hence, is less available for protonation.
- In aliphatic amines, the lone pair on the amino nitrogen is not delocalized and hence, fully available for protonation. Because of this reason, aliphatic amines are stronger bases than aromatic amines.
Question 87. What is the role of HNO3 in the nitrating mixture used for the nitration of benzene?
Answer:
It acts as a base in the nitrating mixture and generates the effective electrophile, NO2 (nitronium ion).

Organic Nitrogen Compounds
Question 88. Why is the NH2 group of aniline acetylated before carrying out nitration?
Answer:
- Nitration is carried out using mixed acid (cone. HNO2 cone. H2SO4 ). If aniline is subjected to nitration directly, it undergoes protonation to form an anilinium cation, in e which the -NH3 group is m-directing.
- This causes the formation of m-nitro derivatives mainly, instead of o-and p-nitro derivatives. So, before carrying out the nitration of aniline, its basic character is eliminated by subjecting it to an acetylation reaction.
![]()
—NHCOCH3 group is o -Ip -directing and moderately activating. C6H5NHCOCH3 undergoes nitration by mixed acid to give a mixture of o and pnitroacetanilides. These on hydrolysis produce o- and p-nitroanilines.
Question 89. What is the product when C6H5CH2NH2 reacts with HNO2?
Answer: C6H5CH2NH2 (an aryl-substituted aliphatic 1° amine) reacts with HNO2 to give C6H5CH2OH.
Question 90. What is the best reagent to convert nitrile to primary amine?
Answer: Na+C2H5OH, or LiAlH4
Question 91. Give the structure of ‘A’ in the following reaction.

Answer:

Question 92. Under what reaction conditions (acidic/basic), the coupling reaction of aryldiazonium chloride with aniline is carried out?
Answer:
- The reaction is not carried out under basic conditions because even under weakly basic conditions a few diazonium cations form diazohydroxide, thereby lowering the concentration of the diazonium cations.
- The reaction cannot be carried out under strongly acidic conditions, because most of the aniline undergoes protonation to form anilinium cation, and hence, the coupling reaction fails.

Organic Nitrogen Compounds
Therefore the coupling reaction is carried out under mild acidic conditions (pH≅4-5 ) so that the concentration of Ar-N+2 is maximum and also the concentration of C6H5NH2 (very weak base) is sufficiently high. Under such a condition coupling occurs smoothly.
Question 92. Predict the product of the reaction of aniline with bromine in a non-polar solvent such as CS2.
Answer:

Explanation: In a non-polar solvent (CS2), the contribution of the resonance structures of aniline involving separation of charge is small, i.e., in a non-polar solvent, the activating effect of the —NH2 group of aniline is reduced. Hence, only mono-bromoanilines are formed.
Question 93. Arrange the following in increasing order of dipole moment. CH3CH2CH3, CH3CH2NH2, CH3CH2OH
Answer:
- Hydrocarbon molecules are almost non-polar and hence, propane (CH3CH2CH3) has the least dipole moment.
- Out of CH3CH2NH2 and CH3CH2OH, the latter has a greater dipole moment because O is more electronegative than N.
- So dipole moment increases in the sequence: CH3CH2CH3 < CH3CH2NH2 < CH3CH2OH.
Question 94. How will you carry out the following conversions?
- Toluene → p-toluidine
- P-toluidine diazonium chloride → p-toluic acid
Answer:

Organic Nitrogen Compounds
Question 95. Write the conversions:
- Nitrobenzene → acetanilide
- Acetanilide → p-nitroaniline.
Answer:

Organic Nitrogen Compounds
Question 96. A solution contains 1 gmol. each of p-toluene- diazonium chloride and p-nitrophenyl diazonium chloride. To this 1gmol. of an alkaline solution of phenol is added. Predict the major product. Explain.
Answer:
- In an alkaline medium, phenol is converted to phenoxide ion, which undergoes a coupling reaction (a type of electrophilic substitution) with diazonium cation.
- The reaction will be favored by increasing the strength of the electrophile (diazonium cation).
- Due to the electron-withdrawing -I and -R effects of the NO2 group, the electrophilic character of p-nitro-benzene diazonium chloride favors the formation of p’-nitrobenzene as the major product.

Question 97. How will you bring out the following conversion?

Answer:

Organic Nitrogen Compounds
Question 98. How will you carry out the following conversion?
![]()
Answer:

Question 99. How will you carry out the following conversion?
Answer:

Question 100. How will you carry out the following conversions?

Answer:

Organic Nitrogen Compounds
Organic Nitrogen Compounds Long Answers Questions And Answers
Question 1. Alkyl cyanides undergo hydrolysis in both uikuiinc and acidic media but hydrolysis of alkyl isocyanides occurs only In acidic medium. Explain
Answer:
In alkyl cyanide, due to electron deficiency in the carbon atom of the protonated alkyl cyanide (acid medium), the Catom becomes susceptible to nucleophilic attack (H2O). So, hydrolysis is feasible. In an alkaline medium, the partially positively charged carbon atom in the alkyl cyanide is attacked by the strong nucleophile OH– which makes the hydrolysis feasible.

However, in alkyl isocyanide due to electron deficiency in the carbon atom of the protonated alkyl isocyanide (acid medium) carbon is easily attacked by a nucleophile (H2O). So, hydrolysis is feasible. But in an alkaline medium, the negatively charged carbon atom or N-atom with its octet filled with electrons is not attacked by the nucleophile and hence, hydrolysis is not possible.

Question 2. Why are diazo reactions carried out at low temperatures (0-5°C)?
Answer:
Diazonium salts are not sufficiently stable and decompose very fast at ordinary temperatures liberating N2 gas. So, the reactions are carried out at low temperatures (0-5°C).

Question 3. How will you identify whether a compound is aniline hydrochloride or not?
Answer:
A compound can be identified as aniline hydrochloride if it fulfills the following criteria:
- It should be soluble in water.
- It should form a curdy white precipitate of silver chloride (AgCl) when silver nitrate solution is added to the aqueous solution of the compound.
- It must respond to the azo-dye test. In this test a brilliant scarlet red azo-dye is produced when a cold solution of sodium nitrite (NaNO2) is added to an aqueous solution of the compound under consideration (0-5°C), followed by the addition of this cold solution to an alkaline solution of β-naphthol (coupling reaction).
The reasons for the above observations are as follows:
- Aniline hydrochloride is an ionic compound that readily dissolves in water.
- The chlorine atom in aniline hydrochloride exists as a Clion. So in an aqueous solution, the free Clion reacts with silver nitrate to give a white precipitate of silver chloride:

Aniline hydrochloride is a salt of weak base & strong acid. It gets hydrolyzed in aqueous solution to produce HCl.
![]()
- So, an aqueous solution of aniline hydrochloride undergoes a diazotization reaction in the presence of NaNO2 only (without adding any acid from an external source).
- The diazonium chloride produced reacts with an alkaline solution of β-naphthol to give a red azo dye.
- Organic Nitrogen Compounds
Question 4. Starting with a suitable reactant how will you prepare methylamine by Curtius rearrangement and Lossen rearrangement reaction?
Answer:
Preparation of methylamine by Curtius rearrangement reaction: When acetyl azide (CH3CON3) is heated using benzene or chloroform as a solvent, methyl isocyanate is produced, which on hydrolysis gives methylamine.

Preparation of methylamine by Lossen rearrangement reaction: When ethane hydroxamic acid is heated with a cone. HCl, methyl isocyanate is obtained, which on hydrolysis gives methylamine.

Question 5. Arrange in the correct order of basicity with proper reasons. Aniline, p -nitroaniline and m -nitroaniline.
Answer:
The correct order of basicity of the given compounds:- Aniline > m -nitroaniline > p -nitroaniline
- This is because the —NO2 group is an electron-attracting group. If it occupies para-position in the ring concerning —NH2, then with the help of -R and -I effects it can draw the lone pair of electrons on nitrogen and bond pair of electrons in the C—N bond, respectively.
- As a result, the electron density of N-atom in the —NH2 group is so drastically reduced that the basicity of p -nitroaniline becomes much less than that of aniline.
- But if the —NO2 group resides in the m-position concerning the —NH2 group, it causes a decrease in electron density on the N-atom with the help of the -I effect only. Due to the lack of proper conjugation between the two groups, the -R effect does not come into play.
- Consequently, the electron density of N-atom in the — NH2 group does not decrease appreciably. In other words, the electron density diminishes only to some extent.
- Thus, the basicity of m -nitroaniline is somewhat less than that of aniline but much more than that of p -nitroaniline.

Organic Nitrogen Compounds
Question 6. In the reaction between aniline and nitrous acid in cold conditions, diazonium salt is produced while ethanol is obtained in the reaction between nitrous acid and ethylamine. Explain.
Answer:
Benzene diazonium cation attains its stability through resonance. So, it does not decompose easily evolving N2 gas. So, benzene diazonium salt (C6H5NCl–) is prepared by carrying out the reaction with HNO2 in cold conditions.

On the other hand, ethyl diazonium cation (C2H5N+2) finds no scope for stabilization through resonance. So it is very unstable. It decomposes easily evolving N2 gas and forming ethyl cation. This cation reacts readily with water to produce ethyl alcohol.

Question 7. Two isomeric compounds A and B have molecular formula C3H5N. Determine the structural formula of A and B based on the information given below:
- A and B do not react with HNO2 and CH3COCl.
- On refluxing A and B with diLHCl, two monocarboxylic acids C and D are respectively obtained.
- The molecular mass of D is 74.
- C reduces Tollens’ reagent but D does not. Write the structural formula of C and D. What compounds will be produced when A and B are reduced?
Answer:
Two isomers A and B (C3H5N) do not react with HNO2 or CH3COCl. So the compounds are not amino compounds. A and B when refluxed with dilute HCl give two monobasic carboxylic acids C and D, respectively. So the compounds may be alkyl cyanide or isocyanide.
The molecular mass of monobasic carboxylic acid obtained due to hydrolysis of B is 74. Suppose, the molecular formula of D is CnH2n + 1COOH.
∴ 12 × n +(2n + 1) + 12 + 2 × 16 +1 = 74
or, 14n + 46 = 74 or n = 2
- So the monobasic carboxylic acid D is C2H5COOH or CH3CH2COOH (propionic acid).
- Since hydrolysis of B (C3H5N), produces D (CH3CH2COOH), B is an alkyl cyanide having structural formula CH3CH2CN (ethyl cyanide).
- Since the monobasic carboxylic acid, C reduces Tollens’ reagent, C is formic acid (HCOOH).
- Hydrolysis of A produces formic acid (C) and A is an isomer of B (CH3CH2CN). Hence, ‘A’ is an alkyl isocyanide having the structural formula CH3CH2NC (ethyl isocyanide).
Reactions Involving Hydrolysis Of A and B :

Reduction Reactions Of A and B:

Question 8. A compound ‘A’ with molecular formula C3H9N, is dissolved in HCl and is reacted with NaNO2 solution. A colorless, odorless, and noninflammable gas is evolved. After completion of the reaction, the mixture on distillation produces a liquid organic compound (B). The compound (B) when heated with I2 and NaOH solution gives a yellow crystalline precipitate. Identify the compound A and write the equations of the reactions which occurred.
Answer:
The compound ‘A’ dissolves in HCl. Hence, it is a basic compound. This basic compound containing carbon, hydrogen, and nitrogen reacts with (NaNO2 + HCl) liberating a colorless, odorless, and non-inflammable gas (possibly N2). Therefore, ‘A’ is a primary amine. Two primary amines can be expressed with the help of the formula C3H9N:
Organic Nitrogen Compounds
![]()
- The liquid compound ‘B’, produced besides N2, in the reaction with HNO2, responds to the iodoform reaction.
- Hence, the compound having the structural formula does not represent the compound ‘ A ’ because the reaction of with HNO2, N2, and CH3CH2CH2OH (which does not respond to iodoform reaction) is obtained.
- On the other hand, ‘A’ can be expressed with the structural formula because, in the reaction of HNO2, N2, and CH3—CH(OH)CH3 (which gives a positive iodoform test) is obtained.

Question 9. Why is it not possible to prepare tertiary butylamine by reaction of ammonia with tertiary butyl bromide?
Answer:
Preparation of tertiary butylamine from tertiary butyl bromide, (a tertiary butyl halide) is a nucleophilic substitution reaction.
To eliminate the steric effect, tertiary butyl halides undergo an elimination reaction rather than a substitution reaction under the influence of a basic reactant like NH3, to produce an alkene as the major product and form tertiary butylamine as a minor product in a displacement reaction.

Organic Nitrogen Compounds
Question 10. A nitro compound with molecular formula C3H7O2N (A) reacts with nitrous acid producing a nitroso compound. The product dissolves in alkali turning the solution red. Identify the starting compound.
Answer:
- Primary nitroalkane (RCH2NO2) reacts with nitrous acid to give a nitroso compound which develops a red color on dissolution in alkali.
- From this, it is evident that the compound A, having the molecular formula C3H7O2N is a primary nitroalkane that stands for only one primary nitroalkane which is— CH3CH2CH2NO2 (1-nitropropane)

Question 11. A nitro compound ‘A’ with molecular formula C4H902N reacts with nitrous acid forming a nitroso compound that dissolves in alkali turning the solution blue. Identify the starting compound.
Answer:
Secondary nitroalkanes![]() react with nitrous acid (HNO2) to give nitroso compounds which dissolve in alkali resulting in a blue solution. Hence, the compound ‘A ‘ with molecular formula C4H9O2N is a secondary nitroalkane. Only one secondary nitroalkane (2°) can be expressed with the formula C4H9O2N which may be represented as CH3CH(NO2)CH2CH3 (2-nitrobutane).
react with nitrous acid (HNO2) to give nitroso compounds which dissolve in alkali resulting in a blue solution. Hence, the compound ‘A ‘ with molecular formula C4H9O2N is a secondary nitroalkane. Only one secondary nitroalkane (2°) can be expressed with the formula C4H9O2N which may be represented as CH3CH(NO2)CH2CH3 (2-nitrobutane).

Organic Nitrogen Compounds
Question 12. A, B, C, D, E, F, G, H—These amine compounds form hydrochloride salts, the chlorine content of each being 32.42%. In the reaction with nitrous acid A, B, C, and D liberate N2 but E, F, G, and H do not. Write the structure of each of the amino compounds from A to H, giving a reasonable explanation.
Answer:
The general formula of primary, secondary, and tertiary amines is CnH2n+3 N. So the general formula of the hydrochloride salts of amines is CnH2n+4 NCl. The percentage of chlorine in these hydrochloride salts is—
⇒ \(=\frac{35.5 \times 100}{12 \times n+(2 n+4)+14+35.5}=\frac{35.5 \times 100}{14 n+53.5}\)
According to the question, \(\frac{35.5 \times 100}{14 n+53.5}=32.42\) or,n=4
∴ The molecular formula of the amines is C4H11N.
A, B, C, and D are primary amines as they liberate N2 gas in reaction with
![]()
Thus, the structural formulae of these four primary amines viz. A, B, C, and D are—
- CH3CH2CH2CH2NH2(Butan-1-amine (A))
- CH3CH2CH(CH3)NH2(Butan-2-amine (B))

The amines E, F, G, and H do not evolve N2 gas reacting with HNO2. Hence, they are either secondary amines or tertiary amines. So the structural formula of these are—

Organic Nitrogen Compounds
Question 13. An organic compound ‘A’ (C3HgN) when boiled with NaOH solution liberates NH3 along with the formation of the sodium salt ‘B’ of a carboxylic acid, (C3H6O2). On reduction of ‘A’, ‘C’ (C3H9N) is produced. ‘C’ on reaction with HNO2, gives ‘D’. Identify A, B, C, D.
Answer:

- An organic compound containing carbon, hydrogen, and nitrogen ‘A’ (C3H5N) on boiling with NaOH solution liberates ammonia gas (NH3) and forms sodium salt of the carboxylic acid B.
- Hence, ‘ A’ is an alkyl cyanide (RCN) and B is the sodium salt of a carboxylic acid (RCOONa). The only alkyl cyanide having the formula C3H5N is CH3CH2CN i.e., the compound A is ethyl cyanide (CH3CH2CN). The compound B produced by hydrolysis of compound A is sodium propanoate (CH3CH2COONa).
- The compound C obtained by reduction of alkyl cyanide must be a 1° amine. Thus compound’ C’ is CH3CH2CH2NH2 (n-propylamine).
- In the reaction between nitrous acid and 1° amine, a 1° alcohol with the same number of C-atoms and N2 gas is produced. So the compound D is CH3CH2CH2OH (n-propyl alcohol). The chemical reactions involved are—

Question 14. An organic compound with molecular formula C6H5O2N when reduced by Sn and HCl gives another compound B whose molecular formula is C6H7N. A cold solution of NaNO2 is added to the compound B dissolved in HCl. The product obtained in the solution is first made to react with KCN in the presence of CuCN and then subjected to hydrolysis, when the compound C is obtained. The compound, if heated with soda lime yields benzene. Identify the compounds A, B, and C.
Answer:
- The ultimate product obtained from the compound ‘A ‘ through a series of chemical reactions is benzene. Thus compound A, is a benzene derivative containing nitrogen.
- From the given formula C6H5O2N, it can easily be understood that the compound is nitrobenzene (C6H5NO2). Nitrobenzene is reduced by Sn and HCl to form aniline (C6H5NH2).
- So the compound B with molecular formula C6H7N is aniline. When a cold solution of NaNO2 is added to aniline dissolved in a cold dilute solution of HCl, benzenediazonium chloride is formed, which reacts with KCN in the presence of CuCN to yield cyanobenzene or phenyl cyanide (C6H5CN).
- Hydrolysis of phenyl cyanide produces benzoic acid. So compound C is benzoic acid. Thus, A : Nitrobenzene (C6H5NO2), B: Aniline (C6H5NH2), C: Benzoic acid (C6H5COOH)
Reactions Involved:

Organic Nitrogen Compounds
Question 15. A compound of molecular formula C6H8NCl is water soluble. Its aqueous solution turns blue litmus red and gives a curdy white precipitate with AgNO3. If the alkaline solution of β-naphthol is added to an aqueous solution of the compound, premixed with NaNO2 solution, a red precipitate is obtained. Identify the compound.
Answer:
- As the compound in an aqueous solution gives a curdy white precipitate with stiver nitrate (AgNO3) solution, the compound is a water-soluble chloride salt. Its aqueous solution is acidic as it turns blue litmus paper red.
- This shows that the compound is a salt of a strong acid HCl and a weak base. Since one of the constituents of the weak organic base is nitrogen, so it is a hydrogen chloride salt of a weak organic base, which on hydrolysis produces an acidic solution.
- The reaction between the aqueous solution of the compound and NaNO2 and β-naphthol indicates that the compound in question is a primary aromatic amine.
- Since, it contains six carbon atoms and one nitrogen atom, the given compound is aniline (C6H5NH2) i.e., the compound with molecular formula C6H8NCl is aniline hydrochloride.
- This structural formula conforms with the reactions given by the compound. For instance, when NaNO2 is added to an aqueous solution of aniline hydrochloride, nitrous acid is produced.
- It converts free aniline into benzene diazonium chloride. When a solution of benzene diazonium chloride is added to an alkaline β-naphthol solution, it undergoes coupling and gives red azo-dye.

Question 16. Organic compound ‘A’ of molecular formula C2H3N is reduced to give compound ‘B’ which reacts with NaNO2 solution producing ethanol. When ‘B’ is heated in the presence of chloroform and ethanolic KOH, compound ‘C with a foul smell is evolved. Identify A, B, and C.
Answer:

- ‘B’ reacts with HNO2 to produce ethyl alcohol (CH3CH2OH). Therefore ‘B’ is a 1° amine whose formula is CH3CH2NH2 (ethanamine).
- ‘B’ on heating with chloroform and alcoholic KOH (arylamine test) generates compound ‘C’ having an unpleasant smell.
- Thus, the compound C is an alkyl isocyanide having the structural formula CH3CH2NC (ethyl isocyanide). Since, compound A (C2H3N) is composed of C, H, and N, which on reduction gives ethylamine, ‘A ‘ is an alkyl cyanide, represented by formula CH3C=N (methyl cyanide).
Organic Nitrogen Compounds

Organic Nitrogen Compounds
Question 17. An optically active organic compound (A) with molecular formula C4HnN dissolves in dilute HCl. It liberates N2 if it is allowed to react with nitrous acid. Determine the structural formula of the compound A.
Answer:
- The given organic compound dissolves in HCl and hence, it is basic. Since the compound consisting of C, H, and N reacts with HNO2 to evolve N2, it is a 1° amine.
- Moreover, an optically active compound should contain an asymmetric C-atom. Thus, only 1° amine of molecular formula C4H11N and having an asymmetric C-atom is butan-2-amine.

Question 18. A compound with molecular formula C4HnN reacts with HNO2 liberating N2 and produces the compound B having molecular formula C4H10O. B responds to the iodoform test. Will compound C obtained due to the reaction between p-toluene sulphonyl chloride and ‘A’ be soluble in KOH solution?
Answer:
Compound ‘A’ comprising carbon, hydrogen, and nitrogen (C4H11N), reacts with nitrous acid to liberate nitrogen gas. So it is a primary aliphatic amine. The probable structural formulae of the compound are—

Another compound ‘B’ produced besides N2 in the reaction of ‘A ‘ with HNO2 responds to the iodoform test. So compound B must contain the isopropyl [CH3—CH(OH) ] group. So the compound, indicated by a structural formula is ‘A’.

Organic Nitrogen Compounds
Thus, A and Bare respectively CH3CH(NH2)CH2CH3 (Butan-2-amine) and CH3CH(OH)CH2CH3 (Butan-2-ol). The reaction between p-toluene sulphonyl chloride & ‘A ‘is—

The compound, obtained in this reaction is soluble in KOH as N-alkyl sulphonamide (C) contains a replaceable H atom at N-atom.

Organic Nitrogen Compounds
Question 19. Write the structures of A, B, C, and D.

Organic Nitrogen Compounds
Answer:

Question 20. A hydrocarbon ‘A’ (C4H8) on reaction with HCl gives a compound ‘B’, (C4H9Cl) which on reaction with 1 mol of NH3 gives compound ‘C’ (C4H11N). On reacting with NaNO2 and HCl followed by treatment with water, compound ‘C’ yields optically active alcohol, ‘D’. Ozonolysis of ‘A’ gives 2 mols of acetaldehyde. Identify compounds1A’ to ‘D’. Explain the reactions involved.
Answer:
![]() , this implies that ‘A’ is CH3-CH=CH-CH3,
, this implies that ‘A’ is CH3-CH=CH-CH3,![]() which implies that ‘B’ is an alkyl chloride.
which implies that ‘B’ is an alkyl chloride.![]() this implies that the N-atom in B is substituted by the NH2 group.
this implies that the N-atom in B is substituted by the NH2 group. 
optically active alcohol (D); this shows that ‘C’ is a primary amine and ‘D’ contains an asymmetric C-atom. Based on the structure of ‘A’, the structures of the other compounds and the reactions involved can be represented as follows:
Organic Nitrogen Compounds

Question 21. A colorless substance (C6H3N) is sparingly soluble in water and gives a water-soluble compound ‘B’ on treatment with mineral acid. On reacting with CHCl3 and alcoholic potash ‘A’ produces an obnoxious smell due to the formation of compound ‘C’. The reaction of ‘A’ with benzene sulphonyl chloride gives compound ‘D’ which is soluble in alkali. With NaNO2 and HCl, ‘A’ forms compound ‘E’ which reacts with phenol in an alkaline medium to give an orange dye ‘F’. Identify compounds ‘A’ to ‘F’.
Answer:
From the molecular formula of A’, it is identified as aniline (C6H5NH2) and the other compounds are as follows:

Organic Nitrogen Compounds

Organic Nitrogen Compounds
Question 22. Predict the reagent or the product in the following reaction sequence.
Answer:

Class 12 Chemistry Unit 13Organic Nitrogen Compounds MCQ’s
Question 1. An equimolar mixture of toluene and chlorobenzene is treated with a mixture of cones. H2SO4 and cone. HNO3. Indicate the correct statement—
- P-nitrotoluene is formed in excess
- Equimolar amounts of p-nitrotoluene and
- P-nitrochlorobenzene are formed
- P-nitro chlorobenzene is formed in excess
- M-nitro chlorobenzene is formed in excess
Answer: 1. P-nitrotoluene is formed in excess
Question 2. Treatment of D with NaNH2 /Liq. NH3 gives—
D with NaNH2 /Liq. NH3 gives—

Answer: 4

Organic Nitrogen Compounds
Question 3. When aniline is nitrated with the nitrating mixture in ice-cold conditions, the major product obtained is—
- P-nitroaniline
- 2,4-dinitroaniline
- O-nitroaniline
- M-nitroaniline
Answer: 4. M-nitroaniline
In a nitrating mixture (cone. HNO3 + conc. H2SO4), aniline forms an anilinium cation (C6H5N+H3) with an H+ ion. As —N+H3 is a meta-orienting group, thus at the given reaction condition, meta-nitroanilinium cation i.e., metanitroaniline will be formed as a major product.
Organic Nitrogen Compounds
Question 4. The reagent with which the following reaction is best accomplished is—

- H3PO2
- H3PO3
- H3PO4
- NaHSO3
Answer: 1. H3PO2
Question 5. An amine C3H9N reacts with benzenesulphonyl chloride to form a white precipitate which is insoluble in aq-NaOH. The amine is—

Answer: 2

Organic Nitrogen Compounds
Question 6.  The product of the above reaction is—
The product of the above reaction is—

Answer: 3
Organic Nitrogen CompoundsOrganic Nitrogen Compounds
Question 7. Identify the correct method for the synthesis of the compound shown below from the following alternative-

Organic Nitrogen Compounds
Answer: 2

Organic Nitrogen Compounds
Question 8. The correct order of basicity of the following compound is-
![]()
Answer: 3
- Basicity increases with an increase in the stability of the conjugate acids. Therefore, guanidine (4) is the most basic as it has three equivalent resonance structures (of the conjugate acid).
- Acetamidine (3) is less basic as its conjugate acid has two equivalent resonance structures. Aldimine (2) is less basic compared to ethylamine (1) as the N-atom of aldimine is bonded to the sp2-C-atom. Thus the basicity order is—2 < 1 < 3 < 4.
Question 9. The yield of acetanilide in the reaction (100% conversion) of 2 mol of aniline with 1 mol of acetic anhydride is—
- 270 g
- 135 g
- 67.5 g
- 177 g
Answer: 2. 135 g

Organic Nitrogen Compounds
- The initial and final mole numbers ofaniline, acetic anhydride, acetanilide, and acetic acid are 2, 1, 0, 0 and 1, 0, 1, 1 respectively.
- As acetic anhydride is a limiting reagent in this reaction, only 1 mol of acetanilide (C8H9NO, M.W = 135 g/mol) will be produced at the end of the reaction. Hence yield of acetanilide is 135g.
Question 10. Among Me3N, C5H5N, and MeCN (Me = methyl group) the electronegativity of N is in the order—
- MeCN>C5H5N>Me3N
- C5H5N>Me3N>MeCN
- Me3N > MeCN > C5H5N
- Electronegativity same in all
Answer: 1. MeCN>C5H5N>Me3N
Organic Nitrogen Compounds
Electronegativity increases with an increase in the character of the N-atom. Among the given compounds MeCN, C5H5N, and Me3N are sp, sp2, and sp3-hybridised respectively. Thus the increasing order of electronegativity—
Question 11. For the reaction below:

Answer: 2

Question 12. The possible product(s) to be obtained from the reaction of cyclobutyl amine with HNO2 is/are—

Answer: 1 and 3

Organic Nitrogen Compounds
Question 13. Identify ‘M’ in the following sequence of reactions—


Answer: 2

Organic Nitrogen Compounds
Question 14. In the reaction:


Answer: 1

Question 15. Which of the following compounds will form a significant amount of meta-product during the mono-nitration reaction

Organic Nitrogen Compounds
Answer: 3

In an acidic medium, the —NH2 group converts into the —NH3 group, which is meta-orienting. On the contrary, the groups present in acetanilide, phenol, and phenyl acetate are o-/p- orienting. Due to this reason during mononitration of aniline significant amount of m-product will be obtained.
Question 16. The increasing order of basicity of the following compounds is—
Answer:

Answer: 1
- The conjugate acid is the most stable among the four compounds as it has two equivalent resonance structures. The conjugate acids of compound 4 do not have any resonance stability.
- However, the methyl group attached to the N-atom of amine in compound 1 increases the electron density on the N-centre.
- Compound 2 is least basic as here the N-atom ofamine is bonded to a sp2-carbon atom. Thus the correct order is— 2 < 1 < 4< 3.
Question 17. Which of the following compounds is the most basic—

Answer: 2. Aliphatic amines are more basic than aromatic amines.
Organic Nitrogen Compounds
Question 18. An organic compound (A) C3H9N, when treated with nitrous acid, gave an alcohol, and N2 gas was evolved. (A) on warming with CHCl3 and caustic potash gave (C) which on reduction gave isopropyimethylamine. Predict the structure of (A)—
- CH3CH2—NH—CH3
- (CH3)3N
- CH3CH2CH2—NH2
- (CH3)2CH—NH2
Answer: 4. (CH3)2CH—NH2
Organic Nitrogen Compounds

Question 19. In the reaction: A is—
A is—
- H+/H2O
- HgSO4/H2SO4
- CU2Cl2
- H3PO2 and H2O
Answer: 4. H3PO2 and H2O

Question 20. The method by which aniline cannot be prepared is—
- Hydrolysis of phenyl isocyanide with acidic solution
- Degradation of benzamide with bromine in alkaline solution
- Reduction of nitrobenzene with H2/Pd in ethanol
- Potassium salt of phthalimide treated with chlorobenzene followed by hydrolysis with aqueous NaOH solution
Organic Nitrogen Compounds
Answer: 4. Potassium salt of phthalimide treated with chlorobenzene followed by hydrolysis with aqueous NaOH solution

Due to the resonance of the Cl-atom with the benzene ring, chlorobenzene becomes inactive towards nucleophilic substitution reaction.
Organic Nitrogen Compounds
Question 21. Which one of the following nitro compounds does react with nitrous acid—

Answer: 4. Due to the absence of or-H tertiary nitroalkanes do not react with nitrous acid.
Question 22. In pyrrole the electron density is maximum
the electron density is maximum
- 2 and 5
- 1 and 3
- 3 and 4
- 2 and 4
Answer: 1. 2 and 5

The negative charges on C2 and C5 are the most stable as there is no adjacent positive charge, unlike C3 and C4.
Organic Nitrogen Compounds
Question 23. The correct statement regarding the basicity of arylamines is—
- Arylamines are generally more basic than alkylamines because the nitrogen atom in arylamines is sp-hybridized
- Arylamines are generally less basic than alkylamines because the lone pair of electrons on nitrogen are delocalized by interaction with the aromatic ring π-electron system
- Arylamines are generally more basic than alkylamines because the lone pair of electrons on nitrogen are not delocalized by interaction with the aromatic ring n electron system
- Arylamines are generally more basic than alkylamines because of the aryl group
Answer: 2. Arylamines are generally less basic than alkylamines because the lone pair of electrons on nitrogen are delocalized by interaction with the aromatic ring π-electron system
Question 24. Which of the following reactions is appropriate for converting acetamide to methenamine—
- Hoffmann hypobromamide reaction
- Stephens reaction
- Gabriel phthalimide synthesis
- Carbylamine reaction
Answer: 1. Hoffmann hypobromite reaction
Hoffmann bromamide reaction is only applicable for converting acetamide to methanamine.
Question 25. ![]() The final product {U) is—
The final product {U) is—
- C6H5CH2CH2NH2
- C6H5CH2CONH2
- C6H5CH2NH2
- C6H5CH2NHCH3
Organic Nitrogen Compounds
Answer: 1. C6H5CH2CH2NH2

Organic Nitrogen Compounds
Question 26. Which is the major product formed when C6H5CONHC6H5 undergoes nitration—

Organic Nitrogen Compounds
Answer: The right attached to the nitrogen atom in benzamide is strongly activated towards an electrophilic substitution reaction. Hence, nitration occurs nt p-position to the ring attached to the N-atom.

Question 27. Which of the following does not give nitroalkanes—

Answer: 1. Teritry amines are not oxidized by KMnO4.
Organic Nitrogen Compounds
Question 28. Which of the following will give the Carbylamine test—
- CH3NH2
- CH3NHCH3
- CH3N(CH3)CH3
- CH3CONH2
Answer: 1. CH3NH2 Only primary amines will give a Carbylamine test
![]()
Question 29. The reaction of aniline with HNO2 followed by treatment of dilute acid gives—
- C6H5NHOH
- C6H5OH
- C6H5NHNH2
- C6H6
Answer: 2. C6H5OH
NaNO2 + HCl → NaCl + HNO2

Organic Nitrogen Compounds
Question 30. Which one of the following forms propane nitrile as the major product—
- Propyl bromide + alcoholic KCN
- Ethyl bromide + alcoholic KCN
- Ethyl bromide + alcoholic AgCN
- Propyl bromide + alcoholic AgCN
Answer: 2. Ethyl bromide + alcoholic KCN

Question 31. The major organic product formed in the following reaction is—


Answer: 2

Organic Nitrogen Compounds
Question 32. Which amine amongst the following will positively the Carbylamine test—
- C6H5-NH-CH3

- C6H5-NH-C4H9
- C6H5-N(C2H5)2
Answer: 2. ![]()
Only aliphatic 1° amines and aromatic 1° amines ![]() in the given cave give positive Carbylamlne test
in the given cave give positive Carbylamlne test
Question 33. Which of the following reagents cannot be used for the given Conversion

- Sn-HCl
- Fe-HCl
- LiAlH4
- Pd/C
Answer: 3. With LiAH4 nitroarenes give azo compounds
Organic Nitrogen Compounds
![]()
Question 34. What is the correct order of basicity among the following compounds—

- 2 > 1 > 3 > 4
- 1 > 2 > 3 > 4
- 3 > 1 > 2 > 4
- 1 > 3 > 2 > 4
Answer: 4. 1 > 3 > 2 > 4

- The conjugate add formed by addition of a proton to compound 1 is stabilised by two equivalent resonance structures and hence, compound 1 is the most basic.
- Further, 2° amines 3 are more basic than 1 amine 2 while amides 4 are least basic due to the delocalization of a lone pair of nitrogen with the —group. Thus, the order is: 1 > 3 > 2 > 4.
Question 35. Which of the following reactions gives the wrong product—

Answer: 3
Organic Nitrogen Compounds
Question 36. Which of the following a 3° amine—
- 1-methyl cyclohexylamine
- Triethylamine
- Tert-butylamine
- N-methyl aniline
Answer: 2. Triethylamine
Organic Nitrogen Compounds

Question 37. The correct IUPAC name for CH2=CHCH2NHCH3 is-
- Allylmethylamine
- 2-amino-4-pentene
- 4-amino pent-1-ene
- N-methyl prop-2-en-1-amine
Answer: 4. N-methyl prop-2-en-1-amine
Explanation: 
Question 38. Amongst the following, the strongest base in aqueous medium Is—
- CH3NH2
- NCCH2NH2
- (CH3)2NH
- C6H5NHCH3
Answer: 3. (CH3)2NH
Explanation: The presence of electron-donating groups enhances basicity, while the presence of electron-withdrawing -I or -R groups lowers basicity
Question 39. Which of the following is the weakest Brdnsted base—

Answer: 1
Explanation: The lone pair on the N-atom of aniline is involved in delocalization with the aromatic ring and hence, less available for donation to a proton.
Organic Nitrogen Compounds
Question 40. Benzylamine may be alkylated as shown in the equation: C6H5CH2NH2 + R—X—C6H5CH2NHR Which of the following alkyl halides is best suited for this reaction through SN1 mechanism—
- CH3Br
- C6H5Br
- C6H5CH2Br
- C2H5Br
Answer: 3. C6H5CH2Br
Explanation: C6H5CH2Br on ionisation produces resonance stabilised benzyl cation (C6H5CH2). So it is best suited for SN1 reaction with C6H5CH2NH2.
Question 41. Which of the following reagents would not be a good choice for reducing an aryl nitro compound to an amine—
- H2 (excess)/Pt
- LiAlH4 in ether
- Fe and HCI
- Sn and HCl
Answer: 2. LiAlH4 in ether
Explanation: LiAlH4 reduces aromatic nitro compounds to azo compounds.

Organic Nitrogen Compounds
Question 42. To prepare a 1° amine from an alkyl halide with the simultaneous addition of one CH2 gr. in a C-chain, the reagent used as a source of nitrogen is
- Sodium amide, NaNH2
- Sodium azide, NaN3
- Potassium cyanide, KCN
- Potassium phthalimide, C6H4(CO)2N–K+
Answer: 3. Potassium cyanide, KCN

Question 43. The source of nitrogen in Gabriel’s synthesis of amines is
- Sodium azide, NaN3
- Sodium nitrite, NaNO2
- Potassium cyanide, KCN
- Potassium phthalimide, C6H4(CO)2N–K+
Answer: 4. Potassium phthalimide, C6H4(CO)2N–K+
Question 44. Amongst the given set of reactants, the most appropriate for preparing 2° amine is—
- 2°R—Br + NH3
- 2°R —Br + NaCN followed by H2/Pt
- 1°R—NH2 + RCHO followed by H2/Pt
- 1°R—Br (2 mol) + potassium phthalimide followed by H3O+ /heat
Answer: 3. 1°R—NH2 + RCHO followed by H2/Pt ![]()
Organic Nitrogen Compounds
Question 45. The best reagent for converting 2-phenylpropanolamine into 2-phenylpropanolamine is—
- Excess H2
- Br2 in aqueous NaOH
- Iodine in the presence of red phosphorus
- LiAlH4 in ether
Answer: 4. LiAlH4 in ether

Organic Nitrogen Compounds
Question 46. The best reagent for converting 2-phenylpropanolamine into 1-phenylethanolamine is—
- ExcessH2/Pt
- NaOH/Br2
- NaBH4/methanol
- LiAlH4/ether
Answer: 2. NaOH/Br2

Question 47. HofmannBromamide Degradation is shownby—
- ArNH2
- ArCONH2
- ArNO2
- ArCH2NH2
Answer: 2. ArCONH2
Explanation: Acetamides undergo Hofmann degradation.
Question 48. The correct increasing order of basic strength for the following compounds is

- 2 < 3 < 1
- 3 < 1 < 2
- 3 < 2 < 1
- 2 < 1 < 3
Answer: 4. 2 < 1 < 3
Explanation: The presence of an electron-donating group (for example., —CH3) in the ring system of aryl amines increases the basicity, whereas the electron-withdrawing group decreases the basicity.
Organic Nitrogen Compounds
Question 49. Methylamine reacts with HNO2 to form
- CH3 — O — N=O
- CH3 — O — CH3
- CH3OH
- CH3CHO
Answer: 3. CH3OH
Explanation: 1° amines react with HNO2 to form alcohol
Question 50. The gas evolved when methylamine reacts with nitrous acid is
- NH3
- N2
- H2
- C2H6
Answer: 2. N2
Explanation: 1° amines react with HNO2to form alcohol and N2 gas
Organic Nitrogen Compounds
Question 51. In the nitration of benzene using a mixture of cone. H2SO4 and cone. HNO3, the species which initiates the reaction is
- NO2
- NO+
- NO+2
- NO–2
Answer: 3. NO+2
![]()
Organic Nitrogen Compounds
Question 52. The reduction of aromatic nitro compounds using Fe and HCl gives
- Aromatic oxime
- Aromatic hydrocarbon
- Aromatic primary amine
- Aromatic amide
Answer: 3. Aromatic primary amine![]()
Question 53. Most reactive amine towards dilute HCl is

Answer: 2
Explanation: The secondary aliphatic amine [(CH3)2NH] is the most basic and most reactive towards dilute HCl.
Organic Nitrogen Compounds
Question 54. Acid anhydrides in reaction with primary amines give
- Amide
- Imide
- 2° amine
- Inline
Answer: 1. Amide

Question 55. The reaction![]() is named as
is named as
- Sandmeyer reaction
- Gattermann reaction
- Claisen reaction
- Carbylamine reaction
Answer: 2. Gattermann reaction
Question 56. The best method for preparing primary amines from alkyl halides without changing the number of carbon atoms in the chain is—
- Hofmann Bromamide reaction
- Gabriel phthalimide synthesis
- Sandmeyer reaction
- Reaction with NH3
Answer: 2. Gabriel phthalimide synthesis
Organic Nitrogen Compounds
Question 57. Which of the following compounds will not undergo azo coupling reaction with benzene diazonium chloride—
- Aniline
- Phenol
- Anisole
- Nitrobenzene
Answer: 4. Nitrobenzene
Explanation: Azo coupling is an electrophilic substitution where the diazonium cation is the electrophile and the other component is an aromatic compound having an electron-donating group.
C6H5N2Cl fails to undergo coupling with nitrobenzene containing a highly deactivated ring system.
Question 58. Which of the following compounds is the weakest Bronsted base—

Answer: 3
Explanation: Aniline and phenol are weaker bases than the corresponding hexahydro derivatives as in former compounds lone pairs on N and O undergo delocalization with a benzene ring and the latter is the weaker base as O is more electronegative than N.
Question 59. Among the following amines, the strongest Bronsted base is

Answer: 4
Explanation: Pyrrole is not at all basic as the lone pair on N is used for making aromatic sextet. Aniline is, however, a weaker base than NH3 and pyrrolidine as the lone pair on N is involved in delocalization with the benzene ring and hence, less available for donation to a proton. Further, the secondary amine pyrrolidine is a stronger base than NH3.
Organic Nitrogen Compounds
Question 60. The correct decreasing order of basic strength of the following species is—H2O, NH3, OH-, NH2.
- NH–2 > OH– > NH3 > H2O
- OH– > NH–2 > H2O > NH3
- NH3 >H2O > NH–2 > OH–
- H2O > NH3 > OH– > NH–2
Answer: 1. NH–2 > OH– > NH3 > H2O
Explanation: NH3 is more basic than H2O:, as N is less electronegative than O. Anionic bases, (NH–2 and OH–), are stronger than the neutral bases NH3 and H2O respectively. Further, NH2 is a stronger base than OH- because N is less electronegative than O. Thus, the sequence of basicity is NH–2 > OH– > NH3 > H2O:
Question 61. Which of the following should be most volatile
- CH3CH2CH2NH2
- (CH3)3N

- CH3CH2CH3
- 2
- 4
- 1
- 3
Answer: 2. 4
Explanation: 1°and 2° amines are associated through intermolecular H-bonding, so they have higher b.pts than (CH3)3N and CH3CH2CH3.
- Due to the polarity of the C—N bond, dipole-dipole interactions are involved among the molecules of (CH3)3N.
- In CH3CH2CH3, there are only weak van der Waals forces of attraction among the molecules. So it has the lowest b.p. and is the most volatile.
Question 62. Which of the following methods of preparation of amines will not give the same number of carbon atoms in the chain of amines as in the reactant—
- Reaction of nitrite with LiAlH4
- The reaction of the amide with LiAlH4 followed by treatment with water
- Heating alkyl halide with potassium salt of phthalimide followed by hydrolysis
- Treatment of amide with Br2 in aq. solution of NaOH
Answer: 4. Treatment of amide with Br2 in aq. solution of NaOH
Organic Nitrogen Compounds

Question 63. Which of the following cannot be prepared by Sandmeyer’s reaction—
- Chlorobenzene
- Bromobenzene
- Iodobenzene
- Fluorobenzene
Answer: 3 and 4
Question 64. Reduction of nitrobenzene by which of the following reagents gives aniline—
- Sn/HCl
- Fe/HCl
- H2-Pd
- Zn/NH4OH
Answer: 1, 2 and 3
Question 65. Which of the following species are involved in the arylamine test—
- R—NC
- CHCl3
- COCl2
- NaNO2 + HCl
Answer: 1 and 2
Organic Nitrogen Compounds
Question 66. The reagents that can be used to convert benzene diazonium chloride to benzene are
- SnCl2/HCl
- C2H5OH
- H3PO2
- LiAlH4/ether
Answer: 2 and 3
Organic Nitrogen Compounds

Question 67. The product of the reaction is

Answer: 1 and 2
Organic Nitrogen Compounds
Question 68. Arenium ion involved in the bromination of aniline is—

Answer: 1,2 and 3

Question 69. Which of the following amines can be prepared by Gabriel synthesis—
- Isobutyl amine
- 2-phenylethylamine
- N-methyl benzylamine
- Aniline
Answer: 1 and 2
Organic Nitrogen Compounds
Explanation: Aliphatic primary amines [but not aromatic 1° amines and 2° amines (C6H5CH2—NHCH3)] can be prepared by Gabriel’s method].
Question 70. Which of the following reactions is correct—

Answer: 1 and 3
Explanation: Alkyl halides react with NH3 to give 1° amines. Alkyl RCl reacts with aq. KOH to give alcohol. Cycloalkyl chloride reacts with ale. KOH to give corresponding alkene. 1° amines react with HNO2 to give alcohol with the same no. of C-atoms.
Organic Nitrogen Compounds
Question 71. Under which of the following reaction conditions, aniline gives p -nitro derivative as the major product—
- Acetyl chloride/pyridine followed by reaction with cone. H2SO4 + cone. HNO3.
- Acetic anhydride/pyridine followed by cone. H2SO4 + cone. HNO3.
- dil. HCl followed by reaction with cone. H2SO4 + cone. HNO3.
- Reaction with cone. HNO3+ cone. H2SO4
Answer: 1 and 2

Organic Nitrogen Compounds
Question 72. Which of the following reactions belong to electrophilic aromatic substitution—
- Bromination of acetanilide
- Coupling reaction of aryldiazonium salts
- Diazotisation of aniline
- Acylation of aniline
Answer: 1 and 2
Explanation: Bromination of acetanilide and coupling reactions of diazonium salts are electrophilic aromatic substitutions as they involve substitution with ring C-atoms. However, diazotization of aniline or acylation of aniline does not involve ring C-atoms. So these are not aromatic electrophilic substitutions.
Question 73. 1°, 2° and 3° nitroalkanes are Identified by—
- HNO2 + NaOH (aq)
- CHCl3 + NaOH (aq)
- CHCl3 + KOH(n/c)
- None of these
Answer: 1. HNO2 + NaOH (aq)
Organic Nitrogen Compounds
Question 74. Which one is correct for methylamine—
- Strongly acidic
- Less basic than NH3
- More basic than NH3
- Forms salt on reaction with base
Answer: 3. More basic than NH3
Organic Nitrogen Compounds
Question 75. Ethyiamine, on oxidation by KMnO4, followed by hydrolysis forms—
- Acid
- Alcohol
- Aldehyde
- N -oxide
Answer: 3. Aldehyde
Question 76. The correct order of basicity in aprotic solvent is—
- 3°>2°>1°>NH3
- 3°<2°<1°<NH3
- 2° < 3° < 1° < NH3
- None of these
Answer: 1. 3°>2°>1°>NH3
Organic Nitrogen Compounds
Question 77. Urea reacts with malonic ester to form—
- Cinnamic acid
- Butyric acid
- Barbituric acid
- Crotonic acid
Answer: 3. Barbituric acid
Question 78. Which one of the following liberates CO2 from NaHCO3 —
- CH3CONH2
- CH3NH2
- (CH3)4N+OH–
- (CH3)4N+H3Cl–
Answer: 4. (CH3)4N+H3Cl–
Question 79. Methyl iodide in its reaction with ammonia produces—
- Methylamine
- Dimethylamine
- Trimethylamine
- All of these
Answer: 4. All of these
Organic Nitrogen Compounds
Question 78. Which one of the following reacts with ethylamine to give a colorless, odorless, and non-inflammable gas—
- NaOH
- CH3COCl
- NaNO2 + HCl
- H2SO4
Answer: 3. NaNO2 + HCl
Question 79. The product obtained when aniline is heated with glacial acetic acid in the presence of anhydrous ZnCl2 is—
- Acetamide
- Acetanilide
- Phenyl acetamide
- Chlorobenzene
Answer: 2. Acetanilide
Question 80. CH3NO2 is acidic concerning which one of the following—
- Na2CO3
- NaOH
- Alcohol
- Liquid NH3
Answer: 2. NaOH
Question 81. Which one of the following reacts with COCl2 to produce phenyl isocyanate—
- Aniline
- Aminophenol
- Nitrobenzene
- Chlorobenzene
Answer: 1. Aniline
Organic Nitrogen Compounds
Question 82. The product obtained in the reaction of aniline with alcoholic KOH and CS2 is—
- Thiourea
- Schiff’s base
- Phenol
- N, N-diphenyl thiourea
Answer: 4. N, N-diphenyl thiourea
Question 83. Which of the following has the highest value of pKb—
- P-methoxy aniline
- P-chloroaniline
- P-nitroaniline
- P-methyl aniline
Answer: 3. p-nitroaniline
Organic Nitrogen Compounds
Question 84. Reduction of nitrobenzene by Al-Hg leads to the formation of—
- Azobenzene
- Aniline
- Azoxybenzene
- Phenylhydroxylamine
Answer: 4. Phenylhydroxylamine
Question 85. Which one of the following does not participate in the diazotization reaction—

Organic Nitrogen Compounds
Answer: 2
Question 86. Quaternary ammonium hydroxide on heating gives
- Primary amine
- Secondary amine
- Mixture of 1° and 2° amines
- Tertiary amines
Answer: 4. Tertiary amines
Question 87. The compound which can not be detected by the Mike Barker test is—

Answer: 1
Question 88. The product obtained in the reaction of POCl3 with benzamide is—
- Aniline
- Benzonitrile
- Chlorobenzene
- Benzylamine
Answer: 2. Benzonitrile
Question 89. Which of the following cannot react with HNO2 —
- CH3CONH2
- (CH3)3CNO2
- (CH3CH2)2NH
- CH3CH2NH2
Organic Nitrogen Compounds
Answer: 2. (CH3)3CNO2
20.![]()
- Nitrochloromethane
- Ethanenitrile
- Chloropicrin
- None of these
Answer: 3. Chloropicrin
Question 90. In the diazo coupling reaction of benzene diazonium chloride and aniline, the pH of the medium should be—
- 1-2
- 9-10
- 4-5
- 7-8
Answer: 3. 4-5
Organic Nitrogen Compounds
Question 91. Which of the following compounds responds to arylamine reaction—
- N-methyl aniline
- P-toluidene
- Phenyl-N-butylamine
- N, N-diethylaniline
Answer: 2. P-toluidene
Question 92. Ethyl cyanide is converted into ethyl isocyanide on reaction with which of the following reagents—
- H3O+,LiAlH4, Red P/I2,AgCN
- LiAlH4, NaNO2/HCl, KCN
- H3O+, NH2, P2O5
- None of these
Answer: 1. H3O+,LiAlH4, Red P/I2,AgCN
Organic Nitrogen Compounds
Question 93. The most suitable reagent for the preparation of primary amine from alkyl isocyanide is—
- H2/Pt
- Zn/HCl
- Acidic hydrolysis
- None of these
Answer: 3. Acidic hydrolysis
Question 94. Which of the following compounds in reaction with nitrous acid at a low temperature produces an oily nitrosamine compound—
- Methylamine
- Ethylamine
- Triethylamine
- Diethylamine
Answer: 4. Diethylamine
Question 95. Electrolytic reduction of nitrobenzene in mild acidic solution forms—
- Aniline
- Nitrosobenzene
- N-phenylhydroxylamine
- P-hydroxy aniline
Answer: 1. Aniline
Organic Nitrogen Compounds
Question 96. In the reaction of acetaldehyde with aniline, the product formed is—
- Schiff’s base
- Carbylamine
- Imine
- None of these
Answer: 1. Schiff’s base
Question 97. Which one of the following influences Beckmann’s rearrangement reaction—
- Sulphuric acid
- Polyphosphoric acid
- PCl5
- All of these
Answer: 4. All of these
Question 98. Nitrosamine (R2N — N =O) reacts with cone. H2SO4, to produce a secondary amine. The reaction is—
- Ubermann reaction
- Etard reaction
- Fries reaction
- Perkin reaction
Answer: 1. Ubermann reaction
Question 99. The order of basicity of amines in aqueous medium is—
- C2H5NH2 > (C2H5)2NH > (C2H5)3N
- (C2H5)2NH>(C2H5)3N>C2H5NH2
- (C2H5)3N > (C2H5)2NH > C2H5NH2
- (C2H5)2NH>C2H5NH2>(C2H5)3N
Answer: 2. (C2H5)2NH>(C2H5)3N>C2H5NH2
Organic Nitrogen Compounds
Question 100. Before nitration of aniline, acetylation is carried out because—
- Due to acetylation, the reactivity of the —NH2 group decreases
- Oxidation can be prevented
- The o- and p-products are obtained in greater yield
- All of these are correct
Answer: 4. All of these are correct
Question 101. In the catalytic reduction of 1° alkyl isocyanide, the product formed is—
- 1° amine
- 3° amine
- N-alkene alkanamine
- N-methyl alkanamine
Answer: 4. N-methyl alkanamine
Question 102. Which of the following is the most basic—
- 2, 4, 6-trinitroaniline
- 2, 4, 6 -trimethylamine
- Aniline
- N, N-dimethylaniline
Answer: 2. 2, 4, 6-trimethylamine
Question 102. In an alkaline medium, the reaction of benzene diazonium chloride with phenol produces a red azo dye. This reaction is—
- Electrophilic substitution reaction
- Nucleophilic substitution reaction
- Oxidative coupling
- Free radical reaction
Answer: 1. Electrophilic substitution reaction
Organic Nitrogen Compounds
Question 103. The product obtained due to the reaction between hypophosphorous acid and benzene diazonium hydrogen sulfate is—
- Phenyl hydrazine
- Azobenzene
- Phenol
- Benzene
Answer: 4. Benzene
Organic Nitrogen Compounds
Question 104. Hydrolysis of a mixture of ethanenitrile and ethanol in the presence of a cone. H2SO4 yields—
- Ethyl ethanoate
- Butyraldehyde
- Methyl propanoate
- 2 -butanone
Answer: 1. Ethyl ethanoate
Question 105. Which of the following reactions can directly convert an amide into a 10 amine—
- Claisen reaction
- Perkin reaction
- Schmidt reaction
- Reduction by LiAlH4
Answer: 4. Reduction by LiAlH4
Organic Nitrogen Compounds
Question 106. In which of the following cases aniline is produced—
- C6H5NO2 + K2Cr2O7/H2SO4
- C6H5Cl + NH3 + Cu2O
- C6H5NO2 + Sn/HCl
- C6H5CHO + NH3 + Cu2O
Answer: 2 and 3
Question 107. Which of the following on reduction yield 1° amine—
- Alkyl isocyanide
- Alkyl cyanide
- Acetamide
- Primary nitroalkane
Answer: 2,3 and 4
Question 108. CH3CH2COOH → CH3CH2NH2. The most effective reagent for the above conversion is—
- NH3 (excess), heat, Na/C2H5OH
- N3H, cold cone. H2SO4
- NH3 (excess), heat, NaOH, Br2
- NH2 — NH2/H+; NaNO2/HCl; OH–/H2O
Answer: 2,3 and 4
Organic Nitrogen Compounds
Question 109. Acylation of which of the following amines is not possible—
- Trimethylamine
- Sec-butylamine
- N, N-dimethylaniline
- N-ethylethanamine
Answer: 1 and 3
Question 110. Which of the following compounds do not respond to arylamine reaction—
- N, N-dimethylaniline
- 2, 4-dimethylaniline
- N-methyl-o-methyl aniline
- P-methyl benzylamine
Answer: 1 and 3
Organic Nitrogen Compounds
Question 111. Which of the following statements is correct—
- Most amines in aq. solutions are more basic than NH3
- pka value of Me3NH is more than NH4
- The ammonium ion is stabilized through polarization
- CH3NH2 has a higher pkb value than NH3
Answer: 1,2 and 3
Question 112. In which of the following cases, N-atom is sp2-hybridised—
- Nitrobenzene
- Trimethylamine
- Pyrrole
- Acetaldoxime
Answer: 1,3 and 4
Organic Nitrogen Compounds
Question 113. Identify the correct orders—
- (CH3)3C-NH2<CH3-NHCH3 (Basicity in aqueous medium)
- CH3CH2CH2NH2 > (CH3)3N (Basicity in aqueous medium)
- CH3-CH(CH3)-NH2 < CH3NHCH2CH3 (Basicity in gaseous medium)
- C2H5NH2 < C6H5NH2 (Basicity in aqueous medium)
Answer: 1,2 and 3
Organic Nitrogen Compounds Match The Following Questions And Answers
Question 1.

Organic Nitrogen Compounds
Answer: 1-D, 2-C, 3-A, 4-B
Organic Nitrogen Compounds
Question 2.

Answer: 1-B, 2-A, 3-D, 4-C
Class 12 Chemistry Unit 13 Organic Nitrogen Compounds Assertion-Reason Type
In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
- Both assertion and reason are false.
Question 1. Assertion (A): Acylation of amines gives a mono-substituted product whereas alkylation of amines gives a polysubstituted product.
Reason (R): Acyl group sterically hinders the approach of further acyl groups.
Answer: 3. A is true but R is false.
Organic Nitrogen Compounds
Question 2. Assertion (A): Hofmann’s bromamide reaction is given by primary amines.
Reason (R): Primary amines are more basic than secondary amines.
Answer: 3. A is true but R is false.
Question 3. Assertion (A): N-ethylbenzene sulphonamide is soluble in alkali.
Reason (R): Hydrogen attached to nitrogen in sulphonamide is strongly acidic.
Answer: 1. Both A and R are true and R is the correct explanation of A.
Organic Nitrogen Compounds
Question 4. Assertion (A): N, N-Diethylbenzene sulphonamide is insoluble in alkali.
Reason (R): Sulphonyl gr. attached to the nitrogen atom is a strong electron-withdrawing group.
Answer: 2. Both A and R are true but R is not the correct explanation of A.
Organic Nitrogen Compounds
Question 5. Assertion (A): Only a small amount of HCl is required in the reduction of nitro compounds with iron scrap and HC1 in the presence of steam.
Reason (R): FeCl2 formed gets hydrolyzed to release HCl during the reaction.
Answer: 1. Both A and R are true and R is the correct explanation of A.
Question 6. Assertion (A): Aromatic 1° amines can be prepared by Gabriel Phthalimide Synthesis.
Reason (R): Aryl halides undergo nucleophilic substi¬tution with anion formed by phthalimide.
Answer: 5. Both assertion and reason are false.
Question 7. Assertion (A): Acetanilide is less basic than aniline.
Reason (R): Acetylation of aniline results in a decrease of electron density on nitrogen.
Answer: 1. Both A and R are true and R is the correct explanation of A.
Class 12 Chemistry Unit 13 Organic Nitrogen Compounds Fill In The Blanks
Question 1. Alkyl isocyanides are hydrolyzed by
Answer: Dilute acid
Question 2. Nitroalkane and alkyl nitrite are_____
Answer: Functional group
Question 3. _____amines respond to arylamine test.
Answer: Primary
Organic Nitrogen Compounds
Question 4. _____ amines are detected by the Libermann nitroso test.
Answer: Secondary
Question 5. Nitrobenzene on reduction in ____ phenylhydroxylamine.
Answer: Neutral
Question 6. Removal of the —NH2 group from aniline is known as _____
Answer: Deamination
Question 7. In the reaction between secondary amine and HNO2, a yellow, oily substance _____ is formed.
Answer: Nitrosamine
Organic Nitrogen Compounds
Question 8. In ____ state, the order of basicity of amine is 3° > 2° > 1°.
Answer: Gaseous
Question 9. Solubility of CH3NC in aq. medium is _____ CH3CN.
Answer: less than
Question 10. In the Mulliken-Barker test, a precipitate of ____ formed.
Answer: Phenylhydroxylamine
Organic Nitrogen Compounds Warm-Up Exercise
Question 1. How will you separate methyl cyanide from a mixture containing methyl isocyanide as an impurity?
Answer:
The impure sample of methyl cyanide (containing methyl isocyanide as the impurity) is shaken with dil. HCl. As a result, CH3NC undergoes hydrolysis to give a mixture of CH3NH2 and HCOOH. CH3NH2 dissolves in HCl to form a water-soluble salt. HCOOH also dissolves in H2O. From this mixture, unreacted CH3CN is extracted by shaking with ether. On evaporation, ether is removed and CH3CN is obtained in a pure state.
Question 2. In the reduction of alkyl cyanide, 1° amine is obtained whereas 2° amine is produced in the reduction of alkyl isocyanide—why?
Answer:
In alkyl cyanide, the alkyl group is directly attached to a carbon atom of the cyano group. Thus on reduction, primary amine (RCH2NH2) is obtained. On the other hand, in alkyl isocyanide, the alkyl group is attached to a nitrogen atom for which on reduction it gives secondary amine (RNHCH3).
Organic Nitrogen Compounds
Question 3. What happens when nitroalkane is allowed to react with the following reagents?
- Concentrated HCl,
- HNO2,
- LiAlH4
Answer:![]()
Question 3. What happens when m -dinitrobenzene is heated with KOH in the presence of K3[Fe(CN)6] and the residual mixture is subsequently acidified?
Answer:

Question 4. Which out of nitrobenzene and aniline is soluble in dilute HCI?
Answer: A basic amino group is present in aniline for which it is soluble in dilute HCl.
Question 5. Identify the following amines as primary, secondary, and tertiary amines:
- CH3CH(NH2)CH3
- CH3CH2-N(CH2CH3)CH3


Organic Nitrogen Compounds
Answer:
- 1, 3 Primary amine
- 2 Tertiary amine
- 4 Secondary amine.
Organic Nitrogen Compounds
Question 6. Write the structure and IUPAC name of the following:
- An amide compound that gives ethanamine in the Hofmann bromamide reaction.
- An amide whichgivesanilineonHofmanndegradation.
Answer:

Question 7. Prepare N,N-dimethylaniline from benzene, Hexan-1 , 6 -diaminefrom Cl(CH2)4Cl, 1, 3, 5- tribromobenzenefrom aniline; 3-nitrotoluenefrom 3-methylaniline.
Answer:

Organic Nitrogen Compounds
Question 8. An aromatic compound (A) on heating with an aqueous solution of ammonia gives a compound (B). ‘B’ on heating with Br2 and KOH solution produces a compound “C’ having molecular formula C6H7N. Identify the compounds A, B, and C.
Answer: A: C6H5COCl; B: C6H5CONH2; C: C6H5NH2
Question 9. What will be the final product obtained by the alkylation of aniline by adding an excess methyl iodide in the presence of an aqueous solution of sodium carbonate?
Answer:
![]()
Question 10. Why does methylamine give ferric hydroxide precipitate in reaction with ferric chloride in an aqueous solution?
Answer:

