Expansion Of Solid And Liquids Introduction Expansion Of Solids
WBBSE Class 11 Expansion of Solids and Liquids Notes
Usually, all solid substances expand on heating and contract on cooling. For a certain rise in temperature, this change is so small for solids, compared to that of liquids and gases, that the change is not always noticeable.
But with the help of proper experiments, it can be shown that solids expand on heating. This phenomenon of expansion with a change in temperature is called thermal expansion. Expansions in solids by the application of external forces have been discussed in the chapter Elasticity.
Thermal expansion in solids is of three types
- Linear expansion (a change in length),
- Surface or superficial expansion (the change in surface area) and
- Volume or cubical expansion (change in volume).
The thermal expansion of different solids, for the same rise in temperature, is different. For example, copper undergoes a greater thermal expansion than iron, for the same rise in temperature with respect to their initial length, surface or volume.
Generally, with the rise in temperature, a solid expands equally in all directions. But there are exceptions as well. A few crystals expand differently in different directions when heated. Again an alloy of iron and nickel, called invar, practically does not show any expansion with the rise in temperature.
Reason for thermal expansion of solids: From the simple considerations of the structure of a crystalline solid, it can be said that the atoms of the crystal are arranged in a regular array under the elastic force.
Between two adjacent atoms this elastic force behaves like an almost inextensible spring. At a fixed temperature, two atoms thus maintain an average distance between them and vibrate. This average distance increases with rise in temperature. Hence, solids expand with the rise in temperature.

Expansion Of Solid And Liquids – Coefficient Of Surface Or Superficial Expansion
Superficial Expansion Definition: The increase in surface area for a unit rise in temperature for a unit surface area of a solid is called the coefficient of surface expansion of the material of that solid.
Let S1 and S2 be the surface areas of a solid at temperatures t1 and t2 respectively, where t2 > t1
Proceeding in a way similar, we get, the coefficient of surface expansion,
⇒ \(\beta=\frac{S_2-S_1}{S_1\left(t_2-t_1\right)}\)
= \(\frac{\text { increase in area }}{\text { initial area } \times \text { rise in temperature }}\) …….(1)
or, \(S_2-S_1=S_1 \beta\left(t_2-t_1\right)\)
or, \(S_2=S_1\left\{1+\beta\left(t_2-t_1\right)\right\}\) ….(2)
If the initial temperature = 0 and the final temperature = t, we may write, St = S0 {1 + βt}……..(3)
where S0 = surface area at zero temperature.
- The coefficient of surface expansion β is not a constant For precise measurements of β, the surface area at 0°C is to be taken as the initial surface area.
- Value of β does not depend on the unit of surface area,
- Value of β depends on the unit of temperature.
Unit of β is °C or °F-1. The change in temperature by 1°F = 5/9°C change in temperature.
∴ \(\beta_F=\frac{5}{9} \beta_C \text {, where } \beta_F\) = value of 0 in Fahrenheit scale, and 0C = value of 0 in Celsius scale.
Expansion Of Solid And Liquids – Relation Among The Three Coefficients of Expansion Numerical Examples
Example 1. At 30°C the diameter of a brass disc is 8 cm. What will be the increase in surface area if it is heated to 80°C? a of brass = 18 x 10-6 °C-1.
Solution:
Given
At 30°C the diameter of a brass disc is 8 cm.
Increase in surface area = \(S_2-S_1=\beta S_1\left(t_2-t_1\right)\)
Here, \(\beta=2 \alpha=2 \times 18 \times 10^{-6}{ }^{\circ} \mathrm{C}^{-1} \text { and } S_1=\pi \times\left(\frac{8}{2}\right)^2 \mathrm{~cm}^2\)
Increase in temperature = t2– t1 = 80-30 = 50 °C
∴ Increase in surface area, \(S_2-S_1\)=\(2 \times 18 \times 10^{-6} \times \pi \times\left(\frac{8}{2}\right)^2 \times 50\)
= \(36 \times 10^{-6} \times 16 \pi \times 50=0.0905 \mathrm{~cm}^2 .\)
Example 2. A rectangular copper block measures 20 cm x 12 cm x 3 cm. What will be the change in volume of the block when it is heated from 0°C to 800°C? The coefficient of linear expansion of copper is 0. 16 x 10-4 °C-1.
Solution:
Given
A rectangular copper block measures 20 cm x 12 cm x 3 cm.
Initial volume of the block, V0 =20 X 12 X 3 = 720 cm³, increase in temperature = t2 – t1 = 800 – 0 = 800°C.
γ = 3α = 3 x 0.16 x 10-4 °C-1
Cubical expansion, \(V_{800}-V_0=V_0 \times \gamma \times(800-0)\)
= 720 x 3 x 0.16 x 10-4 x 800 = 27.65 cm3.
Example 3. A lead bullet has a volume of 2.5 cm3 at 0°C. Its volume increases by 0.021 cm³ when heated to 98°C. Find the coefficient of linear expansion of lead.
Solution:
Given
A lead bullet has a volume of 2.5 cm3 at 0°C. Its volume increases by 0.021 cm³ when heated to 98°C.
By definition, the coefficient of volume expansion of lead, \(\gamma=\frac{V_t-V_0}{V_0 t}\)
Given, \(V_t-V_0=0.021 \mathrm{~cm}^3, V_0=2.5 \mathrm{~cm}^3 \text { and } t=98^{\circ} \mathrm{C}\)
∴ \(\gamma=\frac{0.021}{2.5 \times 98}=85.7 \times 10^{-6}{ }^{\circ} \mathrm{C}^{-1}\)
∴ Coefficient of linear expansion of lead \(\alpha=\frac{\gamma}{3}=\frac{8.57 \times 10^{-6}}{3}=2.86 \times 10^{-6 \circ} \mathrm{C}^{-1}\)
Example 4. An aluminium sphere of diameter 20 cm Is heated from 0°C to 100°C. What will be its change in volume? Coefficient of linear expansion of aluminium = 23x 10-6 °C-1.
Solution:
Given
An aluminium sphere of diameter 20 cm Is heated from 0°C to 100°C.
The initial volume of the aluminium sphere,
= \(\frac{4}{3} \pi\left(\frac{20}{2}\right)^3=\frac{4}{3} \pi(10)^3 \mathrm{~cm}^3\)
Value of γ for aluminium =3 x α =3x23x 10-6 °C-1.
Hence, increase in volume, \(V_t-V_0=V_0 \gamma t=\frac{4}{3} \pi \times 10^3 \times 3 \times 23 \times 10^{-6} \times 100\)
= 28.9 cm³.
Example 5. A piece of metal weighs 46 g xg in air. When immersed in a liquid of relative density 1.24, kept at 27°C, its weight is 30 g x g. When the temperature of the liquid is raised to 42°C, the metal piece in it weighs 30.5 g x g. At 42°C, the relative density of the liquid is 1.20. Find the coefficient of linear expansion of the metal.
Solution:
Given
A piece of metal weighs 46 g xg in air. When immersed in a liquid of relative density 1.24, kept at 27°C, its weight is 30 g x g. When the temperature of the liquid is raised to 42°C, the metal piece in it weighs 30.5 g x g. At 42°C, the relative density of the liquid is 1.20.
The apparent loss in weight of the metal at 27°C = weight of an equal volume of the liquid = (46 – 30) g x g;
Thus the volume of the displaced liquid at 27 °C = \(\frac{46-30}{1.24}=\frac{16}{1.24} \mathrm{~cm}^3\) = volume of the metal piece at 27°C(= V1).
Similarly, the volume of the metal piece at 42 °C (= V2)
= \(\frac{46-30.5}{1.20}=\frac{15.5}{1.20} \mathrm{~cm}^3\)
∴ Coefficient of volume expansion of the metal,
⇒ \(\gamma=\frac{V_2-V_1}{V_1\left(t_2-t_1\right)}=\frac{1}{\left(t_2-t_1\right)}\left(\frac{V_2}{V_1}-1\right)\)
= \(\frac{1}{42-27}\left(\frac{15.5}{1.2} \times \frac{1.24}{16}-1\right)=\frac{1}{15}\left(\frac{961}{960}-1\right)\)
= \(\approx 6.94 \times 10^{-5 \circ} \mathrm{C}^{-1}\)
∴ The coefficient of linear expansion of the metal piece \(\alpha=\frac{\gamma}{3}=\frac{6.94 \times 10^{-5}}{3}{ }^{\circ} \mathrm{C}^{-1}=23.15 \times 10^{-6{ }^{\circ}} \mathrm{C}^{-1}\)
Change Of Density Of A Solid Due To Change Of Temperature
Understanding Thermal Expansion of Solids and Liquids
It is known that the density of a substance = \(\frac{\text { mass }}{\text { volume }}\).
With the change in temperature, while the mass of a solid remains the same, its volume changes. Hence, with the change in temperature, the density of a solid changes.
With the rise in temperature, volume increases, thus density decreases and with the decrease in temperature, volume decreases, thus density increases.
Let for a solid of mass m at temperature t1, the volume be V1 and density be D1; while at temperature t2, its volume becomes V2 and density becomes D2.
∴ \(D_1=\frac{m}{V_1} \text { and } D_2=\frac{m}{V_2}\)
∴ \(\frac{D_1}{D_2}=\frac{V_2}{V_1}\)
If the coefficient of volume expansion of the solid is γ, then
⇒ \(V_2=V_1\left[1+\gamma\left(t_2-t_1\right)\right] \quad \text { or, } \frac{D_1}{D_2}=\frac{V_1\left[1+\gamma\left(t_2-t_1\right)\right]}{V_1}\)
or, \(D_1=D_2\left[1+\gamma\left(t_2-t_1\right)\right]\)
or, \(D_2=\frac{D_1}{\left[1+\gamma\left(t_2-t_1\right)\right]}=D_1\left[1+\gamma\left(t_2-t_1\right)\right]^{-1}\)
or, \(D_2=D_1\left[1-\gamma\left(t_2-t_1\right)\right]\)
[neglecting higher powers of γ(t2 – t2), as γ is very small]
If t2 > t1, D2 < D1
If D0 and Dt are the densities of the solid at 0 and t degree temperatures respectively, equation (2) reduces to Dt = D0(1-γt).
Expansion Of Solid And Liquids – Change Of Density Of A Solid Due To Change Of Temperature Numerical Examples
Example 1. Density of glass at 10°C is 2.6 g · cm-3 and that at 60°C is 2.596 g • cm-3. What is the average value of the coefficient of linear expansion of glass between these two temperatures?
Solution:
Given
Density of glass at 10°C is 2.6 g · cm-3 and that at 60°C is 2.596 g • cm-3.
Using the equation D1 = D2 [1 +γ(t2 -t1)], and substituting the given values,
D1 = 2.6 g · cm-3, D1 = 2.596 g · cm-3, t1 = 10°C and t2 = 60°C,
we get, 2.6 = 2.596 [1 + γ(60 – 10)]
or, \(1+50 \gamma=\frac{2.6}{2.596} \quad \text { or, } 50 \gamma=\frac{2.6-2.596}{2.596}\)
or, 50γ = 1.00154 – 1
or, \(\gamma=\frac{0.00154}{50}=30.8 \times 10^{-6 \circ} \mathrm{C}^{-1}\)
∴ \(\alpha=\frac{\gamma}{3}=10.27 \times 10^{-6 \circ} \mathrm{C}^{-1}\)
| Class 11 Physics | Class 12 Maths | Class 11 Chemistry |
| NEET Foundation | Class 12 Physics | NEET Physics |
Unit 7 Properties Of Bulk Matter Chapter 5 Expansion Of Solid And Liquids Thermal Stress
A change in temperature causes a change in the length of a metal rod. But if the two ends of the rod are rigidly fixed at fixed supports, expansion or contraction of the rod gets obstructed. Hence, a large force is generated along the rod. This force, measured per unit area of the rod is called ther¬mal stress.
Experimental demonstration of thermal stress: A metal rod B is set within the gap of a heavy iron frame Y. One end of the rod B is threaded and two holes P1 and P2 are at the other end.
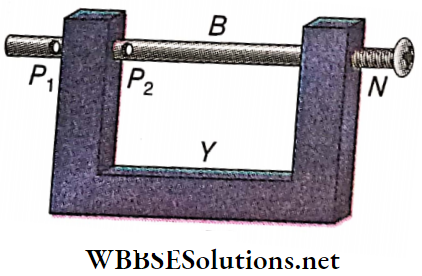
- A screw N is fitted at the threaded end of B. A cast iron pin is introduced through the hole P1 and the rod B is heated.
- When the rod expands, the pin at P1 is tightly fitted with the frame by adjusting the screw N.
- If the rod is cooled now, the pin obstructs the contraction of the rod developing a huge force which breaks the pin inserted through P1.
- Expansion of the rod, when obstructed, also generates a huge force. To demonstrate this, a pin is inserted through P2 and the rod is fixed rigidly by adjusting the screw N. If now the rod is heated, the pin P2 gets broken due to the force developed in the rod on expansion.
Magnitude of thermal stress: Let a rod of length l, cross-sectional area A, coefficient of linear expansion α be heated so that the rise in temperature is t. The rod, therefore expands by l∝t.
- Now if the two ends of the rod are rigidly fixed and it is cooled to its original temperature, it tends to contract back to its original length and this contraction is opposed by a force F (say).
- Therefore, the reaction to the force F, which is equal and opposite to F, is the thermal force developed in the rod due to expansion lα t.
From Hooke’s law, the Young’s modulus of the rod,
Y = \(\frac{\text { stress }}{\text { strain }}=\frac{\text { applied force } / \text { area }}{\text { change in length } / \text { initial length }}=\frac{F / A}{l a t / l}\)
∴ F = AYαt
Therefore, the thermal stress = F/A = Yαt
Clearly, thermal stress is independent of length or area of the cross-section of the rod (or a wire).
Expansion Of Solid And Liquids Thermal Stress Numerical Examples
Example 1. Two ends of a steel rod are rigidly fixed with two supports. At 30° C its area of cross-section is 4 cm². How much force will be exerted on the supports by the ends of the rod if the temperature of the rod is raised by 60°C? [Young’s modulus of steel = 2.1 x 1012 dyn · cm-2 and its coefficient of linear expansion is 12 x 10-6 °C-1
Solution:
Given
Two ends of a steel rod are rigidly fixed with two supports. At 30° C its area of cross-section is 4 cm².
In this case, A = 4 cm², Y = 2.1 x 1012 dyn · cm-2, α = 12 x 10-6 °C-1 and t = 60 – 30 = 30°C
∴ The force exerted
=AYαt =4×2.1 x 1012 x 12 x 10-6 x 30 = 3.024x 109 dyn.
Example 2. Two ends of a wire are rigidly clamped. If its temperature is decreased by 10°C, find the change in the tension of the wire. Area of cross-section of the wire =0.01 cm²; α= 16 x 10 -6 °C-1, Y = 20 x 1011 dyn · cm-2
Solution:
Given
Two ends of a wire are rigidly clamped. If its temperature is decreased by 10°C,
Area of cross-section of the wire =0.01 cm²; α= 16 x 10 -6 °C-1, Y = 20 x 1011 dyn · cm-2
Here A = 0.01 cm², Y = 20 x 1011 dyn · cm-2,α = 16 x 10-6 °C-1, t = 10°C
∴ Change in tension
AYαt = 0.01 x 20 x 1011 x 16 x 10-6 x 10 = 32 x 105 dyn.
Unit 7 Properties Of Bulk Matter Chapter 5 Expansion Of Solid And Liquids Apparent And Real Expansion Of Liquids
To heat a liquid, it has to be kept in a container. When heat is applied, the container also expands along with the liquid. As the liquid expands more than the container for the same change in temperature, expansion of the container is sometimes neglected and only the expansion of the liquid is recorded.
- Hence, the recorded expansion of the liquid, ignoring the expansion of the container, is less than the actual expansion of the liquid.
- The expansion of a liquid, ignoring the expansion of the container, is called the apparent expansion of the liquid.
- The sum of the apparent expansion of the liquid and the expansion of the container is called the real expansion of the liquid.
Experiment: Let, surface of the liquid in a flask rest at mark O. If the flask is heated from outside, at first the flask expands and the surface of the liquid comes down at mark A.
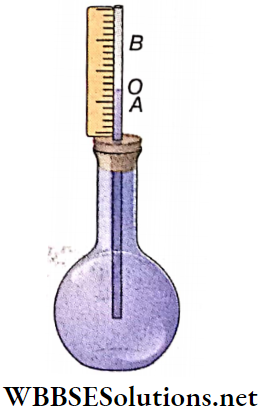
Thus the length OA represents expansion of the flask. Then the supplied heat reaches the liquid and surface of the liquid rises up to mark B. Therefore, volume of AB is the real expansion of the liquid.
On the other hand, if the first change of volume is unnoticed, it seems like the surface rises from initial position O to final position B. So, volume of OB is the apparent expansion of the liquid.
∴ For a liquid, real expansion = apparent expansion of the liquid + expansion of container
Coefficients of Apparent and Real Expansion of Liquids
Since liquid expansions are of two types, two separate coefficients of expansion are to be considered:
- Coefficient of apparent expansion and
- Coefficient of real expansion.
Coefficient of apparent expansion of a liquid Definition: The apparent expansion of unit volume of a liquid for a temperature rise of 1° is called the coefficient of apparent expansion (γ’) of the liquid.
Expression for γ’: Let the volume of a certain amount of liquid be V1 at temperature t1, and its apparent volume be V’2 at temperature t2.
∴ For a rise in temperature of (t2 – t1), apparent expansion of the liquid of volume \(V1 = (V2′ – Vx).V_1=\frac{V_2^{\prime}-V_1}{\left(t_2-t_1\right)}\)
∴ For a unit rise in temperature, the apparent expansion per unit volume = \(\frac{V_2^{\prime}-V_1}{V_1\left(t_2-t_1\right)}\)
By definition, \(\gamma^{\prime} =\frac{V_2^{\prime}-V_1}{V_1\left(t_2-t_1\right)}\)
= \(\frac{\text { apparent expansion }}{\text { initial volume } \times \text { rise in temperature }}\) ….(1)
From (1), we get, \(V_2^{\prime}=V_1\left\{1+\gamma^{\prime}\left(t_2-t_1\right)\right\}\)…(2)
It is important to note that, the coefficient of apparent expansion of a liquid is not an intrinsic property of the liquid. It depends on the material of the container. Hence, a liquid may have different values of γ’ when heated in containers of different materials.
Coefficient of real expansion of a liquid Definition: The actual or real increase of unit volume of a liquid for a temperature rise of 1° is called the coefficient of real expansion (γ) of the liquid.
Expression for γ: Let the volume of a fixed amount of a liquid at a temperature t1 be V1, and at a temperature t2 be V2.
Hence, volume increases by (V2-V1) for a rise (t2 – t1) in temperature.
∴ By definition, \(\gamma=\frac{V_2-V_1}{V_1\left(t_2-t_1\right)}\)
= \(\frac{\text { real expansion }}{\text { initial volume } \times \text { rise in temperature }}\)…(3)
∴ \(V_2 =V_1\left\{1+\gamma\left(t_2-t_1\right)\right\}\) …(4)
The coefficient of real expansion is an intrinsic property of the liquid and does not depend on the material of the container.
1. It is clear from equations (1) and (3) that the values of γ and γ’ are independent of the unit of volume but depend on scale of temperature used.
- For example, the coefficient of real expansion of mercury in the Celsius and the Fahrenheit scales are 18.18 x 10-5 °C-1 and 10.1 x 10-5 °F-1 respectively.
- The coefficient of volume expansion of a liquid is the same in the Celsius and the Kelvin scales but in the Fahrenheit scale it is 5/9 times that in Celsius and Kelvin scales.
2. It is assumed during the derivations of (2) and (4) that the value of the coefficient of expansion of a liquid is the same for all ranges of temperature. Precise observations show that the value changes, though the changes are very small.
Hence, the values deduced above are the average values of γ and γ’ for the temperature range between t1 and t2. However, in practice, the values of γ and γ’ of a liquid are taken as constants for all temperature ranges.
3. While defining γ or γ’, initial volume at any temperature is taken. But for finer measurements, volume at 0°C should be taken as the initial volume In practice, the difference is ignored.
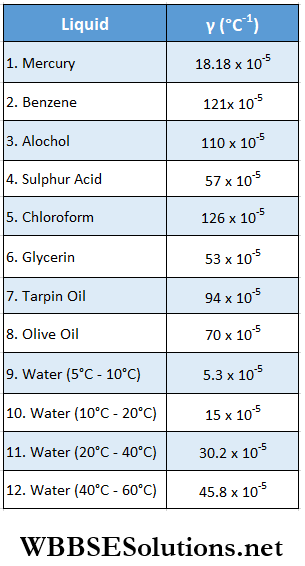
Values of the coefficient of real expansion of a few liquids are shown in the following table.
Relation between the Coefficients of Apparent and Real Expansions of Liquids
Let the volume of certain mass of a liquid in a container at a temperature t1 = V1. At a temperature t2, the apparent volume of that liquid = V’2 and its real volume = V2.
The part of the container, that contained the liquid at t1, has a volume V at t2.
∴ Apparent expansion of the liquid = V2‘ – V1 and real expansion = V2 – V1.
Expansion of the part of the container containing the liquid at t1 = V- V1
Since for a liquid, real expansion = apparent expansion + expansion of container, \(V_2-V_1=\left(V_2^{\prime}-V_1\right)+\left(V-V_1\right)\)
Dividing both sides by V1 t (where t = t2 – t1)
⇒ \(\frac{V_2-V_1}{V_1 t}=\frac{V_2^{\prime}-V_1}{V_1 t}+\frac{V-V_1}{V_1 t}\)
or, γ = γ’ + γg[γg = coefficient of volume expansion of the material of the container]
Hence, the coefficient of real expansion of a liquid = the coefficient of apparent expansion of the liquid + the coefficient of volume expansion of the material of the container.
Coefficient of Linear Expansion in Solids
Relation between Density and Coefficient of Real Expansion of Liquids: The volume of a liquid increases with the increase in temperature. Thus the density decreases. Water between 0°C and 4°C is an exception, and that will be discussed later. Let the mass of some liquid be m, the volume of that liquid be V1 and the density be ρ1, at temperature t1.
At temperature t1, its volume becomes V1 and density ρ2. Consider t2> t1.
Hence, \(m_1=V_1 \rho_1=V_2 \rho_2 \quad \text { or, } \frac{\rho_1}{\rho_2}=\frac{V_2}{V_1}\)…(1)
If the coefficient of real expansion of the liquid is γ, then
⇒ \(V_2 =V_1\left\{1+\gamma\left(t_2-t_1\right)\right\}\)
or, \(\frac{V_2}{V_1}=1+\gamma\left(t_2-t_1\right)\)…..(2)
From equations (1) and (2) we get,
⇒ \(\frac{\rho_1}{\rho_2}=\left\{1+\gamma^{\prime}\left(t_2-t_1\right)\right\}\)
or, \(\rho_1=\rho_2\left[1+\left(t_2-t_1\right)\right]\)….(3)
\(\rho_2 =\frac{\rho_1}{1+\gamma\left(t_2-t_1\right)}=\rho_1\left\{1+\gamma\left(t_2-t_1\right)\right\}^{-1}\)= \(\rho_1\left[1-\gamma\left(t_2-t_1\right)\right]\)
neglecting higher powers of γ, as it is very small.
Hence, the density of a liquid decreases with the increase in temperature.
Equations (3) and (4) both give the relation between the coefficient of real expansion and the density of the liquid.
Equation (4) can be written as \(\gamma=\frac{\rho_1-\rho_2}{\rho_1\left(t_2-t_1\right)}\)
Thus if the densities of a liquid at two different temperatures are known, its coefficient of real expansion can be found out.
Unit 7 Properties Of Bulk Matter Chapter 5 Expansion Of Solid And Liquids Apparent Loss In Weight Of A Solid Immersed In A Liquid At Different Temperatures
There is an apparent loss in the weight of a solid when it is immersed in a liquid. This loss in weight is due to the upthrust it receives in a liquid. This upthrust depends directly on the density of the liquid as well as the volume of the immersed portion of the body.
With the change in temperature, density of the liquid and volume of the solid, both change. Hence, the apparent weight of a solid will be different at different temperatures.
Let the weight of a solid in air = W.
Weight of the solid completely immersed in a liquid at temperature t1 = W1 and that at temperature t2 = W2.
Hence, the apparent loss in weight at temperature t1 = W- W1 = M1g, and that at t2 = W- W2 = M2g,
where M1 and M2 are the masses of the liquid displaced by the body.
If V1 and ρ1 are the volume and density at t1, and V2 and ρ2 are those at t2 respectively, then, \(M_1=V_1 \rho_1 \text { and } M_2=V_2 \rho_2\)
If the coefficient of real expansion of the liquid is γ and the coefficient of volume expansion of the material of the solid is γs, then,
⇒ \(\rho_1=\rho_2\left[1+\gamma\left(t_2-t_1\right)\right]\)
= \(\rho_2[1+\gamma t]\left[\text { Let } t_2-t_1=t\right]\)
and \(V_2=V_1\left(1+\gamma_s t\right)\)
∴ \(M_2 =V_2 \rho_2=\frac{\rho_1 V_1\left(1+\gamma_s t\right)}{1+\gamma t}\)
= \(\rho_1 V_1\left(1+\gamma_s t\right)(1+\gamma t)^{-1}\)
= \(\rho_1 V_1\left(1+\gamma_s t\right)(1-\gamma t)\)
[neglecting the higher powers of γ]
Coefficient of Volume Expansion in Liquids
= \(M_1\left[1-\left(\gamma-\gamma_s\right) t\right]\) neglecting the term \(\gamma \gamma_s t^2\)
Usually \(\gamma \gg \gamma_s and t=t_2-t_1>0\),
∴ \(M_1>M_2 \text { or, } M_1 g>M_2 g \text { i.e., } W_1<W_2 \text {. }\)
Hence, the apparent weight of a body immersed in a liquid increases with the increase in temperature of the liquid.
Unit 7 Properties Of Bulk Matter Chapter 5 Expansion Of Solid And Liquids Apparent Loss In Weight Of A Solid Immersed In A Liquid At Different Temperatures Numerical Examples
Example 1. A piece of metal weighs 50 g x g in air. It weighs45 g x g when immersed in a liquid at 25°C, and 45.1 g x g at 100°C. If the coefficient of linear expansion of the metal is 12 x 10-6 °C-1, find the coefficient of real expansion of the liquid.
Solution:
Given
A piece of metal weighs 50 g x g in air. It weighs45 g x g when immersed in a liquid at 25°C, and 45.1 g x g at 100°C. If the coefficient of linear expansion of the metal is 12 x 10-6 °C-1,
Apparent loss in weight at 25°C = M1g = (50 – 45) gxg = 5gxg Apparent loss in weight at 100°C
= \(M_1 g=(50-45) g \times g=5 g \times g\)
Apparent loss in weight at \(100^{\circ} \mathrm{C}\)
= \(M_2 g=(50-45.1) g \times g=4.9 \mathrm{~g} \times \mathrm{g}\)
As \(M_2=M_1\left[1-\left(\gamma-\gamma_s\right)\left(t_2-t_1\right)\right]\)
∴ 4.9 = \(5\left[1-\left(\gamma-12 \times 10^{-6} \times 3\right)(100-25)\right]\)
or, 4.9 = \(5\left[1-\left(\gamma-12 \times 10^{-6} \times 3\right) \times 75\right]\left[\right. as \left.\gamma_s=3 \times \alpha_s\right]\)
or, \(\left(\gamma-36 \times 10^{-6}\right) \times 75 \times 5=5-4.9\)
or, \(\gamma-36 \times 10^{-6}=\frac{0.1}{75 \times 5}\)
or, \(\gamma=36 \times 10^{-6}+2.67 \times 10^{-4}=3.03 \times 10^{-4}{ }^{\circ} \mathrm{C}^{-1}\).
Example 2. A glass rod weighs 90g x g In air. It weighs 49.6 g x g when immersed In n liquid at 12°C, and 51.9 g x g at 97°C. Wnd the real expansion coefficient of the liquid. Volume expansion coefficient of glass = 2.4 x 10-5 °C-1
Solution:
Given
A glass rod weighs 90g x g In air. It weighs 49.6 g x g when immersed In n liquid at 12°C, and 51.9 g x g at 97°C.
let the volume of the glass rod be V1, at 12°C, and density of the liquid be ρ1.
The mass of the displaced liquid at that temperature = 90-49.6 =40.4 g
∴ Volume of the glass rod at that temperature, \(V_1=\frac{40.4}{\rho_1}\)……(1)
Again at 97°C, mass of the displaced liquid = 90-51.9 = 38.1 g
Let at 97°C the volume of the glass rod be V2, and density of the liquid be ρ2.
∴ \(V_2=\frac{38.1}{\rho_2}\) ….(2)
From (1) and (2), \(\frac{V_2}{V_1}=\frac{\rho_1}{\rho_2} \times \frac{38.1}{40.4}\)….(3)
Now for the glass rod, V2 = V1 [1 + 2.4 x 10-5 x 85]
∴ \(\frac{V_2}{V_1}=1+2.4 \times 10^{-5} \times 85=1.00204\)
In case of liquid, \(\rho_1=\rho_2[1+\gamma \times 85]\)
∴ \(\frac{\rho_1}{\rho_2}=1+\gamma \times 85\)
From equation (3), \(1.00204=(1+\gamma \times 85) \times \frac{38.1}{40.4}\)
or, \(1+85 \gamma=\frac{1.00204 \times 40.4}{38.1}\)
or, \(85 \gamma=\frac{1.00204 \times 40.4}{38.1}-1=\frac{2.382}{38.1}\)
or, \(\gamma=7.35 \times 10^{-4 \circ} \mathrm{C}^{-1} .\)
Example 3. Apparent weights of a solid in a liquid are 50 g x g and 52 g x g at 25°C and 75°C respectively. If the coefficient of linear expansion of the solid Is ag αs = 6.6 x 10-6 °C-1, and γ for the liquid Is 7.3 x 10-4 °C-1, what Is the real weight of the solid In air?
Solution:
Given
Apparent weights of a solid in a liquid are 50 g x g and 52 g x g at 25°C and 75°C respectively. If the coefficient of linear expansion of the solid Is ag αs = 6.6 x 10-6 °C-1, and γ for the liquid Is 7.3 x 10-4 °C-1,
Let the real weight of the solid in air = M g x g.
Apparent loss in weight at \(225^{\circ} \mathrm{C}:-M_1 g=(M-50) g \times g\)
and apparent loss in weight at \(75^{\circ} \mathrm{C}=M_2 g \circ(M-52) \mathrm{g} \times g\)
∴ \(M_2=M_1\left|1-\left(\gamma-\gamma_s\right) \times t\right|\)
⇒ \((M-52)=(M-50) \mid 1-\left(7.3 \times 10^{-4}\right.\) \(-19.8 \times\left(0^{-6}\right) \times 501\)
= \((M-50)\left\{1-7.102 \times 10^{-4} \times 50\right\}\)
= \((M-50) \times 0.96449\)
M = 0.96449 M-48.2245+52
or, 0.03551 M=3.7755
M = \(\frac{3.7755}{0.03551}=106.32 \mathrm{~g}\)
∴ Real weigth of the solid is 106.32 g x g
Example 4. A sphere of mass 266.5 g and of diameter 7 cm floats on a liquid. When the liquid Is heated to 35°C the sphere sturts sinking In the liquid. If the density of the liquid at 0°C Is 1.527 g • cm-3, find the coefficient of volume expansion. Neglect the expansion of the sphere.
Solution:
Given
A sphere of mass 266.5 g and of diameter 7 cm floats on a liquid. When the liquid Is heated to 35°C the sphere sturts sinking In the liquid. If the density of the liquid at 0°C Is 1.527 g • cm-3,
Volume of the sphere = \(\frac{4}{3} \pi\left(\frac{7}{2}\right)^3 \mathrm{~cm}^3\)
∴ Volume of displaced liquid at 35°C = \(\frac{4}{3} \pi\left(\frac{7}{2}\right)^3 \mathrm{~cm}^3\)
If the density of the liquid at 35°C is ρ35, then the mass of the displaced liquid at 35°C = \(\frac{4}{3} \pi\left(\frac{7}{2}\right)^3 \times \rho_{35} g .\)
From the condition of floatation, \(\frac{4}{3} \pi\left(\frac{7}{2}\right)^3 \times \rho_{35}=266.5\)…(1)
Now, \(\rho_{35}=\frac{\rho_0}{1+\gamma \times 35}\)
= \(\frac{1.527}{1+\gamma \times 35}\)
From equations (1) and (2), we get, \(\frac{1.527}{1+\gamma \times 35}=\frac{266.5}{\frac{4}{3} \pi\left(\frac{7}{2}\right)^3}\)
or, \(1+35 \gamma=\frac{4 \times 22 \times(7)^3 \times 1.527}{3 \times 7 \times(2)^3 \times 266.5}\)
or, \(35 \gamma=1.029-1\)
or, \(\gamma=\frac{0.029}{35}=8.28 \times 10^{-4}{ }^{\circ} \mathrm{C}^{-1}\).
Example 5. A piece of metal weighs 46 g x g in air. It weighs 30 g x g in a liquid of specific gravity 1.24 at 27°C. At 42°C, when the specific gravity of the liquid is 1.20, the weight of the piece immersed in it is 30.5 g x g. Find the coefficient of linear expansion (α) of the metal.
Solution:
Given
A piece of metal weighs 46 g x g in air. It weighs 30 g x g in a liquid of specific gravity 1.24 at 27°C. At 42°C, when the specific gravity of the liquid is 1.20, the weight of the piece immersed in it is 30.5 g x g.
Mass of the displaced liquid at 27°C = 46-30 = 16 g
Volume of the displaced liquid at 27°C, \(V_{27}=\frac{16}{1.24} \mathrm{~cm}^3\)
Similarly volume of the displaced liquid at 42 °C, \(V_{42}=\frac{46-30.5}{1.20}=\frac{15.5}{1.20} \mathrm{~cm}^3\)
So the volume of the piece at 27 °C and 42° C are \(\frac{16}{1.24} \mathrm{~cm}^3\) and \(\frac{15.5}{1.20} \mathrm{~cm}^3\) respectively.
Now, \(V_{42}=V_{27}\{1+\gamma(42-27)\}\)
[where γ = coefficient of volume expansion of the metal]
or, \(\frac{15.5}{1.20}=\frac{16}{1.24}\{1+\gamma \times 15\} or, 1+15 \gamma=\frac{15.5 \times 1.24}{1.20 \times 16}\)
or, \(15 \gamma=1.001-1\)
or, \(\gamma=\frac{0.001}{15}=3 \alpha\)
∴ Coefficient of linear expansion of the metal \(\alpha=\frac{\gamma}{3}=\frac{0.001}{45}=2.2 \times 10^{-5 \circ} \mathrm{C}^{-1}\)
Unit 7 Properties Of Bulk Matter Chapter 5 Expansion Of Solid And Liquids Anomalous Expansion Of Water
When a liquid is heated, its volume increases and density decreases with rise in temperature. Exceptions are observed in case of water for a certain range of temperatures.
- When heated from 0°C to 4°C, the volume of water decreases and the density increases. Above 4°C, the volume of water increases again with the increase in temperature.
- Hence, water has a maximum density and a minimum volume at 4°C. Also, on cooling from 4°C to 0°C, the volume of water increases instead of decreasing. This exceptional behaviour of water in respect of expansion within the range of 0°C to 4°C, is called anomalous expansion of water.
- The two graphs below represent the change In volume and density of 1 g of water, with the Increase in temperature.
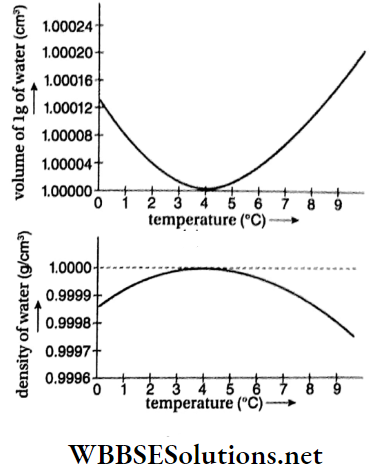
Conclusions from the two graphs are:
- Volume of water decreases as its temperature rises from 0°C to 4°C. Hence, expansion of water is anomalous and does not follow the general rule. Consequently, the coefficient of volume expansion of water is negative for this temperature range.
- Water has the least volume and the maximum density at 4°C.
- After 4°C, expansion of water takes place following the general rule. This means that, with the increase in tem-perature, the volume also starts increasing. The expan¬sion is no longer anomalous.
- The slope of the curves for volume or for density, at temperature close to 4°C is almost zero. So, there is practically no change in volume or density for a small variation of temperature at and around 4°C. Hence, the density of water at 4°C is taken as unity.
Effect of Anomalous Expansion of Water on Marine Life: Due to anomalous expansion of water, fishes and various living creatures can survive under frozen lakes, rivers or seas.
- In cold countries, with the fall in atmospheric temperature, upper surface of lakes, seas and various ponds gradully, cool. Water of the upper surface, then being denser and heavier, moves down.
- Water below it, being comparatively warmer and lighter, moves up. This convection process in water continues until the density of the water in the lower part becomes maximum i.e., the temperature of the lower water reaches 4°C.
- As the temperature of the upper surface decreases further below 4°C, density begins to decrease. So water cannot move down further. It then begins to cool further and at last turns into ice. As ice is lighter than water, a thick layer of ice, thus formed, floats over the surface of water.
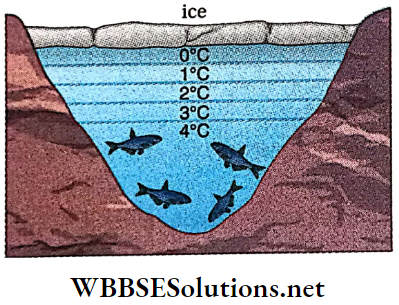
Both ice and water are bad conductors of heat. So, a negligible amount of heat can be conducted from the lower levels of water to the atmosphere outside. So, the entire vol¬ume of water (top to bottom) in a pond cannot freeze.
Unit 7 Properties Of Bulk Matter Chapter 5 Expansion Of Solid And Liquids Anomalous Expansion Of Water Numerical Examples
Applications of Thermal Expansion in Engineering
Example 1. The area of the cross-section of the capillary tube of a mercury thermometer is A0 and the volume of the bulb filled with mercury is V0 at 0°C. Find the length of the mercury column in the capillary tube as the bulb is heated to t°C. The coefficient of linear expansion of glass is α and coefficient of volume expansion of mercury is β.
Solution:
Given
The area of the cross-section of the capillary tube of a mercury thermometer is A0 and the volume of the bulb filled with mercury is V0 at 0°C.
The coefficient of linear expansion of glass is α and coefficient of volume expansion of mercury is β.
Let Vg and Vm be the volumes of the bulb and mercury at t°C.
∴ \(V_g=V_0\left(1+\gamma_g t\right)=V_0(1+3 \alpha t)\)
and \(V_m=V_0(1+\beta t)\)
Hence, volume of mercury entering the tube is \(V_m-V_g=V_0(1+\beta t)-V_0(1+3 \alpha t)=V_0 t(\beta-3 \alpha)\)
If the area of the cross-section of the capillary tube at t°C is At then At = A0(1+2αt).
If the length of the mercury column in the tube is l, then \(A_t \times l=V_0 t(\beta-3 \alpha)\)
∴ l = \(\frac{V_0 t(\beta-3 \alpha)}{A_0(1+2 \alpha t)}\)….(1)
= \(\frac{V_0 t(\beta-3 \alpha)(1+2 \alpha t)^{-1}}{A_0}=\frac{V_0}{A_0} t(\beta-3 \alpha)(1-2 \alpha t)\)
= \(\frac{V_0}{A_0}(\beta-3 \alpha) t\)…..(2)
[neglecting αβ and α² as they are very small]
Both the equations (1) and (2), indicate the length of mercury column in the capillary tube at t °C.
Example 2. A 1L flask contains some mercury. It is observed that the volume of air in the flask remains unchanged at all temperatures. What is the volume of mercury in the flask? The coefficient of linear expression of the material of the flask = 9 x 10-6 °C-1 and coefficient of real expansion of mercury = 1.8 x 10-4 °C-1
Solution:
Given
A 1L flask contains some mercury. It is observed that the volume of air in the flask remains unchanged at all temperatures.
The coefficient of linear expression of the material of the flask = 9 x 10-6 °C-1 and coefficient of real expansion of mercury = 1.8 x 10-4 °C-1
Due to expansion of the scale, the actual barometric reading will be greater than the apparent reading. Let the real reading be h and the apparent reading be H.
∴ h = \(H(1+\alpha t)=75.34\left(1+18 \times 10^{-6} \times 5\right)\)
= \(75.34\left(1+9 \times 10^{-5}\right)\)
h = \(75.34 \times 1.00009 \mathrm{~cm}\)
Again, mercury expands with increase in temperature. Hence, the barometric reading at 0°C will be less than that at 20°C. Let at 0°C, the barometric reading be H0.
∴ \(H_0=h(1-\gamma t)=75.34 \times 1.00009\left[1-18 \times 10^{-5} \times 25\right]\)
= 75.34 x 1.00009[1 -0.0045]
= 75.34 x 1.00009 x 0.9955 = 75.01 cm.
Example 4. Coefficients of volume expansion of benzene and wood are 1.2×10-3 °C-1 and 1.5 x 10-4 °C-1 respectively. Their respective densities at 0°C are 900 kg · m-3 and 880 kg · m-3. Find the temperature at which wood will just immerse in benzene.
Solution:
Given
Coefficients of volume expansion of benzene and wood are 1.2×10-3 °C-1 and 1.5 x 10-4 °C-1 respectively. Their respective densities at 0°C are 900 kg · m-3 and 880 kg · m-3.
The wood will just immerse in benzene at a temperature for which the densities are equal. Let the required temperature be t°C, when the density of both is ρ.
∴ 900 = \(\rho\left[1+1.2 \times 10^{-3} \times t\right]\)
and \(880=\rho\left[1+1.5 \times 10^{-4} \times t\right]\)
Dividing (1) by (2) we get, \(\frac{900}{880}=\frac{1+1.2 \times 10^{-3} t}{1+1.5 \times 10^{-4} t}\)
or, \(t\left(900 \times 1.5 \times 10^{-4}-880 \times 1.2 \times 10^{-3}\right)=880-900 or, \quad t=21.7^{\circ} \mathrm{C}\).
Example 5. A metal piece of density 8g · m-3 is suspended from a wooden hook by a weightless string. The tension in the string is 56 g x g. What will be the tension in the string, if the system is Immersed in a liquid at 40°C? The surrounding temperature during the experiment is 20°C. At 20°C the specific gravity of the liquid is 1.24. The coefficients of volume expansion of the liquid and the metal are 4 x 10-5 °C-1 and 8 x 10-4 °C-1 respectively.
Solution:
Given
A metal piece of density 8g · m-3 is suspended from a wooden hook by a weightless string. The tension in the string is 56 g x g.
The surrounding temperature during the experiment is 20°C. At 20°C the specific gravity of the liquid is 1.24. The coefficients of volume expansion of the liquid and the metal are 4 x 10-5 °C-1 and 8 x 10-4 °C-1 respectively.
Volume of the metal piece at 20°C, \(V_{20}=\frac{56}{8}=7 \mathrm{~cm}^3\)
∴ Volume at 40°C, \(V_{40}=V_{20}\left[1+8 \times 10^{-4} \times 20\right]\)
= \(7\left(1+8 \times 10^{-4} \times 20\right) \mathrm{cm}^3\)
Volume of the displaced liquid = V40
∴ Mass of displaced liquid = V40 x ρ40
[ρ40 = density of the liquid at 40°C]
i.e, weight of the displaced liquid = V40 x ρ40 x g
∴ Upthrust = \(V_{40} \times \rho_{40} \times g\)
= \(7\left[1+8 \times 10^{-4} \times 20\right] \times \frac{1.24}{1+20 \times 4 \times 10^{-5}} \times 980\)
= \(7 \times 1.24[1+0.016]\left[1+8 \times 10^{-4}\right]^{-1} \times 980 \)
= \(7 \times 1.24 \times 1.016[1-0.0008] \times 980\)
= \(7 \times 1.24 \times 1.016 \times 0.9992 \times 980=8.81 \times 980 \mathrm{dyn}\)
∴ Tension in the string = \((56-8.81) \times 980=4.625 \times 10^4 \mathrm{dyn} .\)
Example 6. A body, at 4°C, floats with 0.98 part of its volume immersed in water. At what temperature the body will just be immersed in water? Coefficient of real expansion of water = 3.3 x 10-4 ° C-1. Neglect expansion of the solid body.
Solution:
Given
A body, at 4°C, floats with 0.98 part of its volume immersed in water.
Coefficient of real expansion of water = 3.3 x 10-4 ° C-1.
Let the required temperature be t°C, and the volume of the body = V.
Let the densities of water at 4°C and t°C be d1 and d2 respectively.
∴ From the condition of floatation, Vx 0.98 x d1 = Vxd2
∴ \(\frac{d_1}{d_2}=\frac{1}{0.98}=\frac{50}{49}\)
As \(d_1=d_2\left\{1+3.3 \times 10^{-4}(t-4)\right\},\)
∴ \(\frac{d_2}{d_2}\left\{1+3.3 \times 10^{-4}(t-4)\right\}=\frac{50}{49}\)
∴ \(3.3 \times 10^{-4} \times(t-4)=\frac{50}{49}-1=\frac{1}{49}\)
∴ t = \(\frac{1}{49 \times 3.3 \times 10^{-4}}+4=61.84+4=65.84^{\circ} \mathrm{C} .\)
Real-Life Examples of Expansion in Solids and Liquids
Example 7. A solid at 0°C floats with 98% of its volume immersed in a liquid. The solid floats completely immersed when the temperature is raised to 25°C. If the coefficient of volume expansion of the solid is 2.6 x 10-6 °C-1, find the coefficient of real expansion of the liquid.
Solution:
Given
A solid at 0°C floats with 98% of its volume immersed in a liquid. The solid floats completely immersed when the temperature is raised to 25°C. If the coefficient of volume expansion of the solid is 2.6 x 10-6 °C-1
Let at 0°C, volume of the solid = V0, density of the liquid = ρ0; at 25°C, volume of the solid = V’ and density of the liquid = ρ’
Now, \(V^{\prime}=V_0\left[1+2.6 \times 10^{-6} \times 25\right]\)
and \(\rho_0=\rho^{\prime}[1+\gamma \times 25]\)
where γ = coefficient of real expansion of’tlre liquid.
From the condition of floatation, \(V_0 \times 0.98 \times \rho_0=V^{\prime} \rho^{\prime}\)
or, \(V_0 \times 0.98 \times \rho^{\prime}[1+\gamma \times 25]\)
= \(V_0\left[1+2.6 \times 10^{-6} \times 25\right] \times \rho^{\prime}\)
or, \(1+25 \gamma=\frac{1+0.000065}{0.98} \quad \text { or, } 25 \gamma=1.02047-1\)
∴ \(\gamma=8.19 \times 10^{-4 \circ \mathrm{C}^{-1} .}\)
Example 8. A mercury thermometer contains 0.4 cm³ of mercury at 0°C. The diameter of the capillary tube of the thermometer is 0.2 mm. What should be the length of the scale to measure temperatures between 0°C to 100°C? The coefficient of apparent expansion of mercury = 1.7 x 10-4 °C-1.
Solution:
Given
A mercury thermometer contains 0.4 cm³ of mercury at 0°C. The diameter of the capillary tube of the thermometer is 0.2 mm.
The apparent expansion of mercury due to increase in temperature from 0°C to 100°C = 0.4 x 1.7 x 10-4 x 100 = 0.0068 cm³
The cross-section of the capillary tube = π(0.01)² cm²
∴ The length of the temperature measuring scale = \(\frac{0.0068}{\pi(0.01)^2}=21.6 \mathrm{~cm}\)
Example 9. A and B are two thermometers made of glass and both contain the same liquid. Both thermometers have spherical bulbs. The internal diameter of the bulb of A is 7.5 mm and radius of the capillary tube is 1.25 mm. The corresponding values for B are 6.2 mm and 0.9 mm. Find the ratio of the lengths between two consecutive graduations in thermometers A and B.
Solution:
Given
A and B are two thermometers made of glass and both contain the same liquid. Both thermometers have spherical bulbs. The internal diameter of the bulb of A is 7.5 mm and radius of the capillary tube is 1.25 mm. The corresponding values for B are 6.2 mm and 0.9 mm.
Let the separations between two consecutive graduations for 1° in thermometers A and B be x cm and y cm respectively.
Hence, for the thermometer A, volume expansion of liquid in the bulb due to an increase of 1°C in temperature = vol¬ume of x cm length in the tube,
⇒ \(\frac{4}{3} \pi\left(\frac{0.75}{2}\right)^3 \times \gamma^{\prime} \times 1=x \times \pi(0.125)^2\)…..(1)
where γ’ is the coefficient of apparent expansion of the liquid.
Similarly for the thermometer B, \(\frac{4}{3} \pi\left(\frac{0.62}{2}\right)^3 \times \gamma^{\prime} \times 1=y \times \pi \times(0.09)^2\)….(2)
Dividing equation (1) by (2) we get,
⇒ \(\frac{x \times \pi \times(0.125)^2}{y \times \pi \times(0.09)^2}=\frac{\frac{4}{3} \pi\left(\frac{0.75}{2}\right)^3 \times \gamma^{\prime} \times 1}{\frac{4}{3} \pi\left(\frac{0.62}{2}\right)^3 \times \gamma^{\prime} \times 1}\)
or, \(\frac{x}{y}=\frac{(0.75)^3 \times(0.09)^2}{(0.62)^3 \times(0.125)^2}=0.92 \text { (approx.). }\)
Example 10. A container is filled up to the brim with 500 g of water and 1000 g of mercury. When 21200 cal of heat is supplied to the system, 3.52 g of water flows out of the container. Neglecting the expansion of the container, find the coefficient of real expansion of mercury. Given, the volume expansion coefficient of water =1.5x 10-4 °C-1, density of mercury ss 13.6 g · cm-3, density of water 1 g · cm-3 and speciflc heat capacity of mercury = 0.03 cal · g-1 · °C-1.
Solution:
Given
A container is filled up to the brim with 500 g of water and 1000 g of mercury. When 21200 cal of heat is supplied to the system, 3.52 g of water flows out of the container. Neglecting the expansion of the container,
Given, the volume expansion coefficient of water =1.5x 10-4 °C-1, density of mercury ss 13.6 g · cm-3, density of water 1 g · cm-3 and speciflc heat capacity of mercury = 0.03 cal · g-1 · °C-1.
Due to the application of heat, 3.52 g i.e., 3.52 cm³ of water flows out of the container.
∴ The total expansion of mercury and water = 3.52 cm³.
Let the rise in temperature = t°C.
Heat absorbed by mercury + heat absorbed by water = 21200
∴ 500 x 1 x t + 1000 x 0.03 x t= 21200 or, t = 40°C
Expansion of water = 500 x 1.5 x 10-4 x 40 =3 cm3
∴ Expansion of mercury = (3.52-3) = 0.52 cm3 1000
∴ \(\frac{1000}{13.6} \times \gamma \times 40=0.52\)
[γ = volume expansion coefficient of mercury] 7
∴ γ= 1.768 x 10-4 C-1.
Example 11. A glass bulb is filled in at 0°C by 350 g of mercury. When a few steel balls are put in the bulb, it can then hold only 265 g of mercury. When the bulb is heated to 100°C, with the steel balls in mercury, 5 g of mercury flows out. Find the coefficient of linear expansion of steel. Given, the coefficient of real expansion of mercury = 18 x 10-5 °C-1. Neglect expansion of glass.
Solution:
Given
A glass bulb is filled in at 0°C by 350 g of mercury. When a few steel balls are put in the bulb, it can then hold only 265 g of mercury. When the bulb is heated to 100°C, with the steel balls in mercury, 5 g of mercury flows out.
The coefficient of real expansion of mercury = 18 x 10-5 °C-1. Neglect expansion of glass.
Let ρ0 and ρ100 be the densities of mercury at 0°C and 100°C respectively.
∴ Volume of mercury in the glass bulb at 0°C = \(\frac{350}{\rho_0}\)
Volume of mercury in the glass bulb after the steel balls are put = \(\frac{265}{\rho_0}\)
∴ Volume occupied by the steel balls at 0°C = \(\frac{350}{\rho_0}-\frac{265}{\rho_0}=\frac{85}{\rho_0}\)
Expansion of mercury at 100°C = \(\frac{265}{\rho_0} \times 18 \times 10^{-5} \times 100=\frac{4.77}{\rho_0}\)
Expansion of the steel balls at 100°C = \(\frac{85}{\rho_0} \times \gamma_s \times 100=\frac{8500 \gamma_s}{\rho_0}\)
[γs = coefficient of volume expansion of steel]
Now, expansion of mercury + expansion of the steel balls = volume of mercury expelled
i.e., \(\frac{4.77}{\rho_0}+\frac{8500 \gamma_s}{\rho_0}=\frac{.5}{\rho_{100}}\)
or, \(4.77+8500 \gamma_s=\frac{5 \rho_0}{\rho_{100}}=\frac{5 \rho_{100}\left(1+18 \times 10^{-5} \times 100\right)}{\rho_{100}}\)
= \(5 \times 1.018\)
⇒ \(\left[because \rho_0=\rho_{100}\left(1+18 \times 10^{-5} \times 100\right)\right]\)
or, \(8500 \gamma_s=0.32\) or, \(\gamma_s=\frac{0.32}{8500}=37.65 \times 10^{-6}{ }^{\circ} \mathrm{C}^{-1}\).
∴ Coefficient of linear expansion, \(\alpha_s=\frac{\gamma_s}{3}=\frac{37.65}{3} \times 10^{-6}=12.55 \times 10^{-6{ }^{\circ}} \mathrm{C}^{-1}\)
Example 12. Between two similar thermometers, one is filled with mercury and another with alcohol of the same volume at 0°C. The gap for each degree in the mercury thermometer is l and that in the alcohol thermometer is l’. Show that \(\frac{l}{l^{\prime}}=\frac{\gamma-3 \alpha}{\gamma_1-3 \alpha}\)
coefficient of real expansion of mercury, γ1 = coefficient of real expansion of alcohol and a = coefficient of linear expansion of glass.
Solution:
Given
Between two similar thermometers, one is filled with mercury and another with alcohol of the same volume at 0°C. The gap for each degree in the mercury thermometer is l and that in the alcohol thermometer is l’.
In both thermometers, let the area of the cross-section of the tube be A and the volume of the bulb be V.
For 1° rise in temperature in mercury thermometer, apparent expansion of mercury = volume of length l of the tube
i.e., \(V(\gamma-3 \alpha) \times 1=l \times A \quad\left[because \gamma^{\prime}=\gamma-\gamma_g\right]\)….(1)
Similarly, for 1° rise in temperature in alcohol thermometer,
∴ \(V\left(\gamma_1-3 \alpha\right) \times 1=l^{\prime} \times A\)….(2)
Dividing (1) by (2) we get,
⇒ \(\frac{l \times A}{l^{\prime} \times A}=\frac{V(\gamma-3 \alpha)}{V\left(\gamma_1-3 \alpha\right)} \text { or, } \frac{l}{l^{\prime}}=\frac{\gamma-3 \alpha}{\gamma_1-3 \alpha}\)
Example 13. A cylindrical container contains some liquid. The coefficient of linear expansion of the material of the container is a. When the container is heated it is observed that the liquid level inside the container remains unchanged. What is the volume expansion coefficient of the liquid?
Solution:
Given
A cylindrical container contains some liquid. The coefficient of linear expansion of the material of the container is a. When the container is heated it is observed that the liquid level inside the container remains unchanged.
Let height of the liquid level inside the container be x and area of the cross-section be A.
Now, the level of liquid surface inside the container will remain unchanged when volume expansion of the container due to an increase in temperature t = volume expansion of the liquid due to increase in temperature t.
∴ xA x 3α x t = xA x γ x t [y = volume expansion coefficient of the liquid]
∴ γ = 3α
Short Answer Questions on Thermal Expansion
Example 14. A body weighs W0 in air. Its apparent weights in a liquid at t1 °C and t2 °C are W1 and W2 respectively. If the coefficient of volume expansion of the material of the body is γ, find the coefficient of real expansion of the liquid.
Solution:
Given
A body weighs W0 in air. Its apparent weights in a liquid at t1 °C and t2 °C are W1 and W2 respectively. If the coefficient of volume expansion of the material of the body is γ,
Weight of displaced liquid at t1 °C = W0-W1
Let V1 and V2 be the volumes of the body and d1 and d2 be the densities of the liquid at t1°C and t2°C respectively.
We have, \(W_0-W_1=V_1 d_1 g\)….(1)
and \(W_0-W_2=V_2 d_2 g\)
Dividing equation (1) by equation (2) we get,
⇒ \(\frac{W_0-W_1}{W_0-W_2}=\frac{V_1 d_1}{V_2 d_2} .\)
Now \(V_2=V_1\left[1+\gamma\left(t_2-t_1\right)\right] and d_1=d_2\left[1+\gamma_l\left(t_2-t_1\right)\right]\),
where \(\gamma_l\) is the coefficient of real expansion of the liquid.
∴ \(\frac{W_0-W_1}{W_0-W_2}=\frac{1+\gamma_l \times t}{1+\gamma \times t} [where \left.t=\left(t_2-t_1\right)\right]\)
or, \(1+\gamma_l t=\frac{W_0-W_1}{W_0-W_2} \times(1+\gamma t)=\frac{W_0-W_1}{W_0-W_2}+\frac{\gamma t\left(W_0-W_1\right)}{W_0-W_2}\)
or, \(\gamma_l t=\frac{W_2-W_1}{W_0-W_2}+\frac{\gamma t\left(W_0-W_1\right)}{W_0-W_2}\)
or, \(\left(W_0-W_1\right)(1+\gamma t)=\left(W_0-W_2\right)\left(1+\gamma_l \times t\right)\)
or, \(\gamma_l=\frac{W_2-W_1}{\left(W_0-W_2\right) t}+\frac{\gamma\left(W_0-W_1\right)}{W_0-W_2}\)
Example 15. Using two different containers A and B, the coefficients of apparent expansion of a liquid are found to be γ1 and γ2 respectively. If the coefficient of linear expansion of the material A is α, find that of the material B.
Solution:
Given
Using two different containers A and B, the coefficients of apparent expansion of a liquid are found to be γ1 and γ2 respectively. If the coefficient of linear expansion of the material A is α,
We have, γ = γ’ + γg or, γ = γ’ + 3α
[γ’ = Coefficient of apparent expansion of the liquid, γ = coefficient of real expansion of the liquid, γg = coefficient of volume expansion of the material of the container.]
For container A, \(\gamma=\gamma+\gamma_g=\gamma_1+3 \alpha\)
and for container B, \(\gamma=\gamma_2+3 \alpha^{\prime}\)
[α’ = coefficient of linear expansion of the material of the container B ]
∴ \(\gamma_2+3 \alpha^{\prime}=\gamma_1+3 \alpha\)
or, \(3 \alpha^{\prime}=\gamma_1+3 \alpha-\gamma_2 \quad or, \alpha^{\prime}=\frac{1}{3}\left(\gamma_1-\gamma_2\right)+\alpha\).
Example 16. A hollow iron ball floats completely immersed in water at 10°C temperature. What will happen if the temperature of both of them is raised to 50°C?
Solution:
Given
A hollow iron ball floats completely immersed in water at 10°C temperature.
The iron ball will sink in water if the temperature of the ball and of water is raised to 50°C from 10°C.
Let dy be the density of water and be the volume of the iron ball at 10°C.
So, the apparent loss in weight of the ball at 10°C = V1d1g.
Again, if d2 is the density of water and V2, is the volume of the iron ball at 50°C, the apparent loss in weight of the bull at 50°C = V2d2g
Now, \(V_2=V_1\left[1+\gamma_i(50-10)\right]\)
[\(\gamma_i=\) coefficient of volume expansion of iron]
and \(d_2=d_1\left[1-\gamma_w(50-10)\right]\)
⇒ \(\gamma_w\). coefficient of volume expansion of water]
∴ \(V_2 d_2=V_1 d_1\left[1+40 \gamma_i\right]\left[1-40 \gamma_w\right]\)
= \(V_1 d_1\left[1-40\left(\gamma_w-\gamma_i\right)\right] \text { (approx.) }\)
Since \(\gamma_w>\gamma_i, V_2 d_2 g<V_1 d_1 g\).
Therefore, the loss in weight of the ball at 50°C temperature is less. Hence its apparent weight increases. So the ball will sink in water.
Unit 7 Properties Of Bulk Matter Chapter 5 Expansion Of Solid And Liquids Useful Relations For Solving Numerical Problems
1. If the length of a rod at temperature is t1 is l and that at temperature t2 is l2 then,
coefficient of linear expansion, \(\alpha=\frac{l_2-l_1}{l_1\left(t_2-t_1\right)}\)
2. If the initial length of the rod at temperature 0 is l0 and the, final length of the rod at temperature t is lt, then, \(l_t=l_0(1+\alpha t)\)
3. If the values of α in Celsius and Fahrenheit scales are αC and αF then, \(\alpha_F=\frac{5}{9} a_C\)
If the surface area of a solid at temperature t1, is S1 and that at temperature t2, is S2, then,
coefficient of surface expansion, \(\beta=\frac{S_2-S_1}{S_1\left(t_2-t_1\right)}\)
⇒ \(\beta_F=\frac{5}{9} \beta_C\)
If the volume of a solid body at temperature t1 is V1 and that at temperature t2 is V2 then,
coefficient of volume expansion, \(\gamma=\frac{V_2-V_1}{V_1\left(t_2-t_1\right)}\)
⇒ \(\gamma_F=\frac{5}{9} \gamma_C\)
⇒ \(\alpha=\frac{\beta}{2}=\frac{\gamma}{3}\)
If the density of a solid at temperature t1 is D1 and that at temperature t2 is D2 then, \(D_2=D_1\left\{1+\gamma\left(t_2-t_1\right)\right\}^{-1} \approx D_1\left\{1-\gamma\left(t_2-t_1\right)\right\}\)
If area of the cross-section of a rod is A, rise in temperature is t, Young’s modulus for the material is Y, coefficient of linear expansion is a and the force developed inside the rod, when its length is kept unchanged, is F then,
thermal stress = \(\frac{F}{A}=Y \alpha t\)
Real expansion of a liquid = apparent expansion of the liquid + expansion of the container.
Coefficient of real expansion of a liquid = coefficient of apparent expansion of the liquid + coefficient of volume expansion of the material of the container, i.e., \(\gamma=\gamma^{\prime}+\gamma_g \)
⇒ \(\gamma=\frac{\rho_1-\rho_2}{\rho_1\left(t_2-t_1\right)}\) [where ρ1 = density of the liquid at the temperature t1, ρ2 = density of the liquid at the temperature t2]
Apparent loss in weight of a solid body immersed completely in a liquid is M2 = M1{1-(γ-γs)t}
where M1g= apparent loss in weight of the body at the temperature t1, M2g = apparent loss in weight of the body at the temperature t2,t = t2-t1, γ = coefficient of real expansion of the liquid and γs = coefficient of volume expansion of the immersed solid.
Unit 7 Properties Of Bulk Matter Chapter 5 Expansion Of Solid And Liquids Very Short Answer Type Questions
Question What is the unit of the coefficient of linear expansion of a substance?
Answer: °C-1
Question 2. What is the relation between the coefficient of linear expansion (α) and the coefficient of volume expansion (γ) of a solid?
Answer: γ = 3α
Question 3. A bimetallic strip made up of brass and iron remains linear at 20 °C. When the temperature is decreased to 0°C, the strip bends. Which material remains on the convex side of the bent strip?
Answer: Iron
Question 4. Coefficient of linear expansion of platinum is 9 x 10-6 °C-1. What will be the value of this coefficient when the temperature is expressed in Fahrenheit unit?
Answer: 5 x10-6 °F-1
Question 5. If the coefficient of linear expansion of iron is 0. 0000067 °F-1, then what is its value in the Celsius scale?
Answer: 0.000012°C-1
Question 6.Due to rise in temperature, if each side of a copper cube increases by 0.1%, then find out the increase in volume of that cube.
Answer: 0.3%
Question 7.Write down the variation of density of a solid with increase in temperature.
Answer: Decreases
Question 8. Thermal expansion of invar is _______ than that of all other metals or alloys.
Answer: Less
Question 9.Between the coefficient of apparent expansion (γ’) and the coefficient of real expansion (γ) of a liquid which one is a characteristic property of the liquid?
Answer: y
Question 10. A glass vessel is filled with water up to its brim. Now what will happen if temperature is increased?
Answer: Water will overflow
Question 11. At what temperature density of water will be the maximum?
Answer: 4°C
Question 12. How does the density of a liquid change with temperature?
Answer: Density decreases
Question 13. At what temperature under standard atmospheric pressure, the density of pure water will be the maximum?
Answer: 4°C
Question 14. Real expansion of a liquid = apparent expansion of the liquid + volume expansion of the _______.
Answer: vessel
Question 15. Coefficient of real expansion of water from 0°C to 4°C is ___________.
Answer: Negative
Question 16. ‘Usually, the thermal expansion of a liquid is greater than the thermal expansion of an equal volume of a solid.’ State whether the statement is true or false.
Answer: True
Question 17. ‘Usually, the thermal expansion of a liquid is greater than
the thermal expansion of an equal volume of a gas.’ State whether the statement is true or false.
Answer: False
Question 18. What is the density of pure water at 4°C in SI?
Answer: 1000 kg • m-3
Unit 7 Properties Of Bulk Matter Chapter 5 Expansion Of Solid And Liquids Assertion Reason Type Question And Answers
Direction: These questions have statement 1 and statement 2. Of the four choices given below, choose the one that best describes the two statements.
- Statement 1 is true, statement 2 is true; statement 2 is a correct explanation for statement 1.
- Statement 1 is true, statement 2 is true; statement 2 is not a correct explanation for statement 1.
- Statement 1 is true, statement 2 is false.
- Statement 1 is false, statement 2 is true.
Question 1.
Statement 1: A brass disc just fits in a hole in a steel plate. The system must be cooled to loosen the disc from the hole.
Statement 2: The coefficient of linear expansion for brass is greater than the coefficient of linear expansion for steel.
Answer: 1. Statement 1 is true, statement 2 is true; statement 2 is a correct explanation for statement 1.
Question 2.
Statement 1: A beaker is completely filled with water at 4 °C. It will overflow, both when heated or cooled.
Statement 2: There is expansion of water below and above 4°C.
Answer: 1. Statement 1 is true, statement 2 is true; statement 2 is a correct explanation for statement 1.
Question 3.
Statement 1: The coefficient of real expansion of the liquid is independent of the nature of container.
Statement 2: ϒr = γa + γv where γv = coefficient of real expansion, γa = coefficient of expansion and γv = coefficient of apparent expansion of vessel.
Answer: 2. Statement 1 is true, statement 2 is true; statement 2 is not a correct explanation for statement 1.
Question 4.
Statement 1: The coefficient of apparent expansion can be negative.
Statement 2: Coefficient of real expansion of a liquid can be less than the coefficient of expansion of vessel.
Answer: 1. Statement 1 is true, statement 2 is true; statement 2 is a correct explanation for statement 1.
Question 5.
Statement 1: A solid and a hollow sphere of same material when heated through the same temperature, wall expand by the same amount.
Statement 2: The change in volume is independent of the original mass but depends on original volume.
Answer: 1. Statement 1 is true, statement 2 is true; statement 2 is a correct explanation for statement 1.
Unit 7 Properties Of Bulk Matter Chapter 5 Expansion Of Solid And Liquids Match Column 1 With Column 2.
Question 1. A piece of metal of density ρ1 floats on mercury of density ρ2. The coefficients of expansion of the metal and mercury are. γ1 and γ2 respectively. The temperatures of both mercury and metal are increased by ΔT.
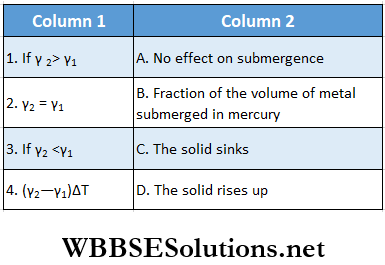
Answer: 1. C, 2. A, 3. D, 4. B
Question 2.
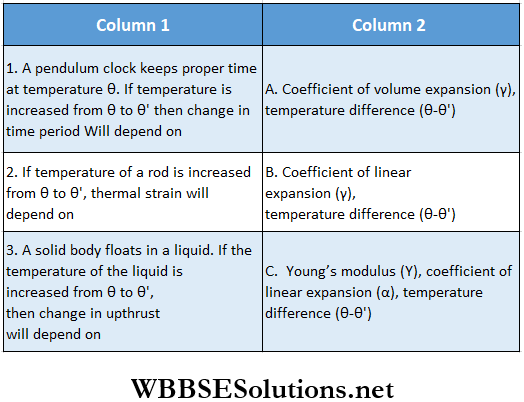
Answer: 1. B, 2. C, 3. A
Unit 7 Properties Of Bulk Matter Chapter 5 Expansion Of Solid And Liquids Comprehension Type Question And Answers
Read the following passages carefully and answer the questions at the end of them.
Question 1. A copper collar is to fit tightly about a steel shaft that has a diameter of 6 cm at 20 °C. The inside diameter of the copper collar at that temperature is 5.98 cm.
1. To what temperature must the copper collar be raised so that it will just slip on the steel shaft, assuming the steel shaft remains at 20 °C? (αcopper= 17 x 10-6 K-1)
- 324 °C
- 21.7 °C
- 217 °C
- 32.4 °C
Answer: 3. 217 °C
2. The tensile stress in the copper collar when its temperature returns to 20 °C is (Y = 11 x 1010 N • m-2)
- 1.34 x 105 N • m-2
- 3.68 x 10-12 N • m-2
- 3.68 x 108 N • m-2
- 1.34 x 10-12 N • m-2
Answer: 3. 3.68 x 108-12 N • m-2
3. If the breaking stress of copper is 230 N • m-2, at what temperature will the copper collar break as it cools?
- 20 °C
- 47 °C
- 94 °C
- 217 °C
Answer: 3. 94 °C
Unit 7 Properties Of Bulk Matter Chapter 5 Expansion Of Solid And Liquids Integer Type Question And Answers
In this type, the answer to each of the questions is a single-digit integer ranging from 0 to 9.
Question 1. A composite rod is made by joining a copper rod, end to end, with a second rod of a different material but of same cross-section. At 25 °C, the composite rod is lm in length of which the length of the copper rod is 30 cm. At 125 °C the length of the composite rod increases by 1.91 mm. The coefficient of linear expansion of copper is α = 1.7 x 10-5 °C-1 and that of the second rod is β = n x 10-5 °C-1. Find the value of n
Answer: 2
Question 2. The volume of a metal sphere increases by 0.24% when its temperature is raised by 40 °C. The coefficient of linear expansion of the metal is n x 10-5 °C-1.Find the value of n.
Answer: 2
Question 3. The coefficient of real expansion of mercury is 18 x 10-6 °C-1. A thermometer has a bulb of volume 10-6 m³ and the cross-section of the stem is 0.002 cm². Assuming the bulb to be filled with mercury at 0 °C, find the length (in cm) of the mercury column at 100 °C.
Answer: 9
Question 4. A thin copper wire of length l increases in length by 1 % when heated from 0 °C to 100 °C. If a thin copper plate of area 2lx l is heated from 0 °C to 100 °C, find the percentage increase in its area.
Answer: 2
