Kinetic Theory Of Gases
Interpretation Of Temperature From Kinetic Theory
WBBSE Class 11 Kinetic Interpretation of Temperature Overview
Total energy (E) of gas molecules: According to the kinetic theory, the potential energy of gas molecules = 0; the entire energy of the gas comes from the kinetic energy of the molecules.
∴ E = \(\frac{1}{2} m c_1^2+\frac{1}{2} m c_2^2+\cdots+\frac{1}{2} m c_N^2\)
[m = mass of each molecule; c1, c2,….. cN = velocity of the N molecules in the container]
= \(\frac{1}{2} m\left(c_1^2+c_2^2+\cdots+c_N^2\right)=\frac{1}{2} m N \frac{c_1^2+c_2^2+\cdots+c_N^2}{N}\)
As mN = M = mass of the gas,
and c = \(\sqrt{\frac{c_1^2+c_2^2+\cdots+c_N^2}{N}}\)
= rms speed of the molecules,
∴ E = \(\frac{1}{2} M c^2=\frac{1}{2} M \cdot \frac{3 p}{\rho}\left[\text { As } p=\frac{1}{3} \rho c^2, \text { we have } c^2=\frac{3 p}{\rho}\right]\)
Again, as \(\frac{M}{\rho}\) = V = volume of the gas, we get
E = \(\frac{3}{2} p V\)…(1)
This is the expression for the total energy of a gas.
Relation between pressure and energy density: Energy density of a gas (u) = energy per unit volume = \(\frac{E}{V}\)
From relation (1), \(\frac{E}{V}=\frac{3}{2} p\)…(1)
or, \(u=\frac{3}{2} p \quad \text { or, } \quad p=\frac{2}{3} u\)…(2)
So, the pressure of a gas is \(\frac{2}{3}\)rd of its energy density. This relation (2) is a fundamental one, valid for all ideal gases.
Effect of heat absorbed by a gas: when a gas absorbs heat from its surroundings, two effects occur simultaneously.
- The temperature of the gas increases.
- Heat is converted to some other form of energy inside the gas. It is nothing but the kinetic energy of the molecules. This means that the kinetic energy of the molecules increases.
These two effects suggest that the temperature of a gas and the kinetic energy of its molecules are intimately related with each other. The definition of temperature in kinetic theory comes from this concept.

Definition of Kinetic Temperature for Class 11
Idea of temperature: Temperature (T) is a property of a gas, which is proportional to the kinetic energy of the gas molecules.
So, T ∝ E or, T = aE where a is a constant.
The constant a may have any value. Different values of a will give different temperature scales. Usually, the value of a is so chosen that the temperature scale is an exact match with the experimental Kelvin scale of temperature.
Let us take 1 mol of an ideal gas. Then E is the kinetic energy of the molecules present in 1 mol of the gas. Then we choose \(a=\frac{2}{3 R}\), where
R = universal gas constant = 8.31 J · mol · K-1
Then, for 1 mol of an ideal gas, \(T=\frac{2}{3 R} E \quad \text { or, } \quad E=\frac{3}{2} R T\)….(3)
With this choice of a, the quantity T becomes exactly the same as the Kelvin temperature.
- We know that the potential energy of an ideal gas molecule is zero. So the total internal energy is essentially the kinetic energy of the molecules. But for real gases and also for liquids and solids, the molecular potential energy is not zero.
- Then the total internal energy becomes the sum of the molecular kinetic and potential energies. Here, it must be noted that the temperature is taken to be proportional to the kinetic energy only of the molecules.
- In this way, the concept of temperature is extended to all gases, liquids, and solids. This means that temperature is a property of all substances and is proportional to the kinetic energy of the molecules in the substance.
The proportionality constant is taken in such a way that the values of temperature exactly match with the values of the Kelvin scale. For this, the required absolute constant is the Boltzmann constant.
The idea of absolute zero of temperature: By definition, T ∝ E; so E = 0 when T = 0. This defines the absolute zero of temperature. It is the temperature at which the internal energy of the gas becomes zero, i.e., the molecular motions stop entirely.
Variation of the rms speed of gas molecules: Let M = molecular weight of a gas. Then, mass of 1 mol of the gas = M g.
The density of the gas is ρ = \(\frac{M}{V}\); so M = ρV.
Now, p = \(\frac{1}{3} \rho c^2 \text { or, } c^2=\frac{3 p}{\rho}=\frac{3 p V}{\rho V}=\frac{3 R T}{M}\)
∴ c = \(\sqrt{\frac{3 R T}{M}}\)….(3)
As R = constant and for a particular gas, M = constant, we get c ∝ √T. So, the rms speed of gas molecules is proportional to the square root of the temperature of the gas.
Average Kinetic Energy and Temperature Relationship
Most probable velocity of gas molecules: The kinetic theory assumes that a gas molecule may have a velocity between zero and infinity. But in reality, the number of molecules with very low or very high velocities is extremely small The majority of molecules have intermediate velocities.
- Maxwell analysed the phenomenon with his velocity distribution curve. This curve has a peak at P and the point P corresponds to a velocity cm.
- Among all velocities, the velocity cm is possessed by the highest number of gas molecules. This cm is known as the most probable velocity.
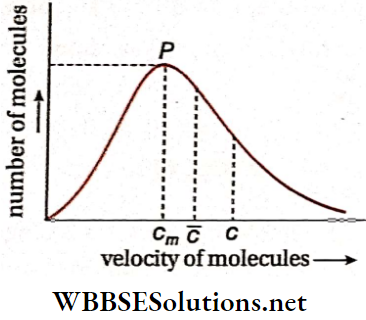
The most probable velocity of gas molecules Definition: The velocity which is possessed by the highest number of gas molecules in a container is called the most probable velocity.
Shows that, in general, cm is less than both the mean velocity \(\bar{c}\) and the rms speed c of gas molecules.
Actually, cm< \(\bar{c}\) <c.
Theoretically, we get an absolute temperature of T,
⇒ \(c_m=\sqrt{\frac{2 R T}{M}}, \bar{c}=\sqrt{\frac{8 R T}{\pi M}}, c=\sqrt{\frac{3 R T}{M}}\)
So, \(c_m: \bar{c}: c=\sqrt{2}: \sqrt{\frac{8}{\pi}}: \sqrt{3}=1: \frac{2}{\sqrt{\pi}}: \sqrt{\frac{3}{2}} .\)
| Class 11 Physics | Class 12 Maths | Class 11 Chemistry |
| NEET Foundation | Class 12 Physics | NEET Physics |
Interpretation Of Temperature From Kinetic Theory Numerical Examples
Examples of Kinetic Interpretation of Temperature
Example 1. At what temperature will the rms speed of molecules of nitrogen gas be twice of that at 0°C?
Solution:
T1 = 0°C = 273 K; rms speed at 0°C = c1
At temperature T2, rms speed = c2 = 2 c1.
As \(c=\sqrt{\frac{3 R T}{M}}\),
∴ \(c \propto \sqrt{T}\), we have \(\frac{c_1}{c_2}=\sqrt{\frac{T_1}{T_2}}\)
or, \(T_2 =T_1\left(\frac{c_2}{c_1}\right)^2=273 \times\left(\frac{2}{1}\right)^2=1092 \mathrm{~K}\)
= \((1092-273)^{\circ} \mathrm{C}=819^{\circ} \mathrm{C} .\)
Example 2. The temperature of a gas rises from 27°C to 327°C. Show that the rms speed of the gas molecules would be √2 times its initial value at die final higher temperature.
Solution:
Given
The temperature of a gas rises from 27°C to 327°C.
T1 = 27°C = (27 + 273) K = 300 K;
T2 = 327°C = (327 + 273) K = 600 K.
As \(c \propto \sqrt{T}\), we have \(\frac{c_1}{c_2}=\sqrt{\frac{T_1}{T_2}}\)
or, \(c_2=c_1 \sqrt{\frac{T_2}{T_1}}=c_1 \sqrt{\frac{600}{300}}=\sqrt{2} c_1\)
Mathematical Formulas for Kinetic Energy and Temperature
Example 3. The rms speed of oxygen gas molecules at STP is 4.5 x 104 cm · s-1. Find out the same for carbon dioxide gas molecules at STP. Given, the molecular weights of oxygen and carbon dioxide are 32 and 44, respectively.
Solution:
Given
The rms speed of oxygen gas molecules at STP is 4.5 x 104 cm · s-1.
We know, c = \(\sqrt{\frac{3 R T}{M}}\)
As the temperature is the same for both the gases, \(c \propto \frac{1}{\sqrt{M}}\)
So, \(\frac{c_{\mathrm{O}_2}}{c_{\mathrm{CO}_2}}=\sqrt{\frac{M_2}{M_1}}\)
[M1 = molecular weight of O2, M2 = molecular weight of CO2]
or, \(c_{\mathrm{CO}_2}=c_{\mathrm{O}_2} \sqrt{\frac{M_1}{M_2}}=4.5 \times 10^4 \times \sqrt{\frac{32}{44}}\)
= \(3.84 \times 10^4 \mathrm{~cm} \cdot \mathrm{s}^{-1} .\)
Example 4. Find out the kinetic energy of 2g of nitrogen gas at 27°C, Given, R = 8.3 x 107 erg mol-1 K-1
Solution:
The Kinetic energy of the molecules of 1 mol gas = 3/2 RT
Here, T = 27°C = (27 + 273) K = 300 K ; mass of 1 mol nitrogen gas = 28 g.
So, the kinetic energy of the molecules in 2 g of nitrogen gas
= \(\frac{2}{28} \times \frac{3}{2} R T=\frac{3}{28} R T=\frac{3 \times\left(8.3 \times 10^7\right) \times 300}{28}\)
= \(2.668 \times 10^9 \mathrm{erg}=2.668 \times 10^2 \mathrm{~J}=266.8 \mathrm{~J} .\)
Example 5. At what temperature the average kinetic energy of the molecules of a perfect gas be doubled than that at 20°C?
Solution:
Here, T1 = 20 °C = 293 K.
As the average kinetic energy of gas molecules is proportional to the absolute temperature of the gas, the required temperature,
T2 = 2 x 293 = 586 K = (586 – 273) °C = 313 °C
Kinetic Interpretation of Temperature in Gases
Question 6. Find out the temperature at which the molecular rms speed of a gas would be 1/3rd its value at 100°C.
Solution:
Let the required temperature be T2K, molecular rms speed at this temperature be c2, and that at 100°C be c1.
According to the question, \(c_2=\frac{1}{3} c_1\)
Here, \(T_1=100^{\circ} \mathrm{C}=(100+273) \mathrm{K}=373 \mathrm{~K}\)
As \(c \propto \sqrt{T}\), we have, \(\frac{c_1}{c_2}=\sqrt{\frac{T_1}{T_2}}\)
or, \(T_2=T_1\left(\frac{c_2}{c_1}\right)^2 =373 \times\left(\frac{1}{3}\right)^2=\frac{373}{9}=41.44 \mathrm{~K}\)
= \((41.44-273)^{\circ} \mathrm{C}=-231.56^{\circ} \mathrm{C}\).
