Expansion Of Gases Volume And Pressure Coefficients Of A Gas
WBBSE Class 11 Volume and Pressure Coefficients Overview
In general, both the volume and the pressure of a fixed mass of a gas change due to any change in its temperature. However, for convenience, we at first consider the two extreme types of heating:
- Either by keeping its pressure constant or
- By keeping its volume constant.
It is not possible to change the temperature of a gas keeping both pressure and volume constant. Hence, there are two coefficients of a gas. When a gas is heated, keeping the pressure constant, its volume increases and we get the volume coefficient at constant pressure (γp).
Again when the gas is heated, keeping the volume constant, its pressure increases and we get the pressure coefficient at constant volume (γv).
Volume coefficient (γp): The volume coefficient of a fixed mass of a gas at a constant pressure is the increment of its volume when the temperature of a unit volume is raised by 1°C from 0°C.
Let at constant pressure, the volume of a specific amount of gas be V0 at 0°C and Vt at t °C.
∴ Increase in volume = Vt-VQ and increase in temperature = t-0 = t°C
∴ Increase in volume for 1°C rise in temperature of a unit volume of the gas = \(\frac{V_t-V_0}{V_0 t}\)
i.e., the volume coefficient \(\gamma_p=\frac{V_t-V_0}{V_0 t}\)….(1)
or, Vt =V0(1 + γpt)….(2)
Pressure coefficient (γv): The pressure coefficient of a fixed mass of a gas at a constant volume, initially at unit pressure, is the increment of its pressure when its temperature is raised by 1°C from 0°C.
Let at a constant volume the pressure of a specific amount of gas be p0 at 0°C and pt at t °C.
Increase in pressure = pt – p0 and increase in temperature = t-0 = t°C
∴ Increase in pressure for 1°C rise in temperature per unit
initial pressure of the gas = \(\frac{p_t-p_0}{p_0 t}\)…(1)
i.e., pressure coefficient, \(\gamma_\nu=\frac{p_t-p_0}{p_0 t}\)….(3)
or, pt=p0(l + γvt)….(4)
Relationship between the two coefficients of expansion of a gas: Suppose at 0°C a fixed mass of gas has volume V0 and pressure p0. The gas is heated to t °C. We can perform this increase in temperature in any of the two ways.
1. Keeping the volume V0 constant when pressure increases to pt from p0.
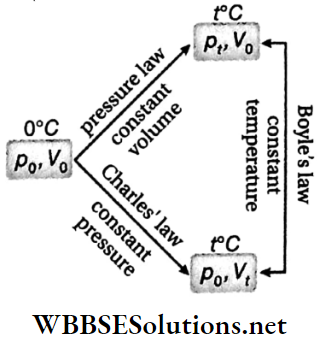
2. Keeping the pressure P0 constant when volume increases to Vt from V0.
Following pressure law in the first case, \(p_t=p_0\left(1+\gamma_\nu t\right)\)
In the second case as per Charles’ law, \(V_t=V_0\left(1+\gamma_p t\right)\)
Since the final temperature is t°C in both cases, as per Boyle’s law
⇒ \(p_t V_0=p_0 V_t \quad \text { or, } p_0 V_0\left(1+\gamma_v t\right)=p_0 V_0\left(1+\gamma_p t\right)\)
∴ \(\gamma_v=\gamma_p\)
Hence, for any ideal gas, the coefficient of volume expansion is equal to the coefficient of pressure expansion.
A comparison of equations (2) and (4) respectively with the corresponding expressions derived from Charles’ law and pressure law shows that
⇒ \(\gamma_p=\frac{1}{273} \text { or, } 0.00366^{\circ} \mathrm{C}^{-1}\)
and also \(\gamma_\nu=\frac{1}{273} or, 0.00366^{\circ} \mathrm{C}^{-1}\)
Definition of Volume Expansion Coefficient in Gases
Therefore,
- The pressure coefficient (γv) and the volume coefficient (γp) have the same value which is the same for all gases, though different solids and liquids have different values for the coefficients of volume expansion.
- Gases are heated in a container like liquids. However, the volume expansion of a gas is much higher (near about 100 times) than the corresponding expansion of the container. Unless much accuracy is required, two separate expansion coefficients (real and apparent) are not needed. Practically, the apparent expansion coefficient of a gas is the same as the real expansion coefficient of the gas.
- To find the volume coefficient (γp) of a gas, the initial volume is to be taken at 0°C and o to find the pressure coefficient of a gas, initial pressure is to be taken at 0°C.
To illustrate 3 or 4 the following example may be considered:
Let the initial volume V0 of a fixed mass of a gas at 0°C, be 273 cm³.
According to Charles’ law, volume at 100°C, \(V_{100} =V_0\left(1+\frac{100}{273}\right)\)
= \(273\left(1+\frac{100}{273}\right)=373 \mathrm{~cm}^3\)
and volume at 150°C, \(V_{150}=V_0\left(1+\frac{150}{273}\right)\)
= \(273\left(1+\frac{150}{273}\right)=423 \mathrm{~cm}^3\)
In the case of solids and liquids magnitudes of expansion coefficients are too small. So to find volume expansion in the case of solids and liquids it is not always necessary to take the initial volume at 0°C. Values of expansions do not differ much if we consider initial volume at some temperature other than 0°C.

Pressure Expansion Coefficient Explained for Class 11
Expansion Of Gases Volume And Pressure Coefficients Of A Gas Numerical Examples
Example 1. The volume of a gas is doubled by raising its temperature at constant pressure. The initial temperature of the gas was 13°C. Find the final temperature.
Solution:
Given
The volume of a gas is doubled by raising its temperature at constant pressure. The initial temperature of the gas was 13°C.
As the pressure is constant, using Charles’ law we have \(\frac{V_1}{T_1}=\frac{V_2}{T_2}\)
Here V1 = xcm³ (suppose), T1 = 273+ 13 = 286 K and V2 = 2x cm³.
∴ \(\frac{x}{286}=\frac{2 x}{T_2}\) or, T2 = 572 K = (527-273)°C = 299°C.
| Class 11 Physics | Class 12 Maths | Class 11 Chemistry |
| NEET Foundation | Class 12 Physics | NEET Physics |
Example 2. The volume of fixed mass of a gas at 47°C is 640cm³ and its pressure is 75 cm of Hg. To which temperature should the gas be raised at constant volume to make its pressure double?
Solution:
Given
The volume of fixed mass of a gas at 47°C is 640cm³ and its pressure is 75 cm of Hg.
As volume is a constant, we get, using pressure law \(\frac{p_1}{T_1}=\frac{p_2}{T_2}\)
Here p1 = 75 cmHg, T1 = 273 + 47 = 320 K
and p2 = 2 x 75 = 150 cmHg.
⇒ \(\frac{75}{320}=\frac{150}{T_2}\)
or, T2 = 640 K = (640 – 273)°C = 367°C.
Mathematical Formulas for Volume and Pressure Coefficients
Example 3. The volume of a fixed mass of gas is 300 cm³ at STP. When the temperature is raised to 50°C at constant volume, the pressure exerted by the gas becomes 900 mmHg. What is the pressure coefficient of the gas?
Solution:
Given
The volume of a fixed mass of gas is 300 cm³ at STP. When the temperature is raised to 50°C at constant volume, the pressure exerted by the gas becomes 900 mmHg.
Here, pt = 900 mmHg, p0 = 760 mmHg and t = 50°C.
⇒ \(p_t=p_0\left(1+\gamma_\nu t\right)\)
∴ \(\gamma_\nu=\frac{p_t-p_0}{p_0 t}\)
or, \(\gamma_\nu=\frac{900-760}{760 \times 50}=\frac{140}{760 \times 50}=0.00368^{\circ} \mathrm{C}^{-1}\)
Example 4. At constant pressure, if the volume of a fixed mass of gas at temperature 80°C is 500 cm³ and that at 150°C is 600 cm3, what is the coefficient of volume expansion (γp) of the gas?
Solution:
Given
At constant pressure, if the volume of a fixed mass of gas at temperature 80°C is 500 cm³ and that at 150°C is 600 cm3,
We have, Vt = V0(1 + γpt)
Using the given conditions we get,
500 = V0(1 +γp x 80) ….(1)
and 600 = V0 (1 + γp x 150)…(2)
Dividing (2) by (1), we get,
⇒ \(\frac{6}{5}=\frac{1+150 \gamma_p}{1+80 \gamma_p} \text { or, } 6+480 \gamma_p=5+750 \gamma_p\)
or, \(270 \gamma_p=1 or, \gamma_p=\frac{1}{270}{ }^{\circ} \mathrm{C}^{-1}\).
Example 5. If heated to 35 °C at constant pressure, the volume of gas increases from 5 L at 0°C, by 640 cm³. What should be the value of absolute zero for this gas in the Celsius scale?
Solution:
Given
If heated to 35 °C at constant pressure, the volume of gas increases from 5 L at 0°C, by 640 cm³.
Let the absolute zero temperature for that gas be -T°C.
So, 0°C = TK = T1, 35°C = (T+35) K = T2,
V1 = 5000 cm³ and V2 = 5000 + 640 = 5640 cm³.
As per Charles’ law, \(\frac{V_1}{T_1}=\frac{V_2}{T_2}\) at constant pressure.
∴ \(\frac{5000}{T}=\frac{5640}{T+35}\)
or, 500(T+35)=564 T
64 T =17500,
T = \(\frac{17500}{64}=273.43\)
Hence, absolute zero in the Celsius scale =-273.43°C.
Applications of Volume and Pressure Coefficients in Real Life
Example 6. A hydrogen cylinder can withstand an internal pressure of 7 x 106 Pa. The pressure of hydrogen in a cylinder at 15°C is 1.7 x 106 Pa. At what minimum temperature an explosion may take place?
Solution:
Given, p1 = 1.7 x 106 Pa and T1 = 273 + 15 = 288 K
A hydrogen cylinder can withstand an internal pressure of 7 x 106 Pa. The pressure of hydrogen in a cylinder at 15°C is 1.7 x 106 Pa.
The explosion may occur at a pressure p2 = 7 x 106 Pa
As volume is constant in the cylinder, according to pressure law,
⇒ \(\frac{p_1}{T_1}=\frac{p_2}{T_2} \text { or, } T_2=\frac{p_2 T_1}{p_1}\)
∴ \(T_2=\frac{7 \times 10^6 \times 288}{1.7 \times 10^6}=1185.9 \mathrm{~K}\)
= \((1185.9-273)^{\circ} \mathrm{C}=927^{\circ} \mathrm{C}\)
Example 7. A glass vessel is filled with air at 30 °C. Up to which temperature should the vessel be heated keeping the pressure constant so that 1/3rd of the initial volume of air is expelled? \(\gamma_p=\frac{1}{273}^{\circ} \mathrm{C}^{-1}\).
Solution:
Given
A glass vessel is filled with air at 30 °C.
Let the initial volume of air = V1
∴ The final volume of an equal mass of air, \(V_2=V_1+\frac{V_1}{3}=\frac{4}{3} V_1\)
[as the volume of expelled air = 1/3 V1]
Initial temperature, T1 = 273 + 30 = 303 K.
Let the required temperature be T2 K.
As pressure is constant,
⇒ \(\frac{V_1}{T_1}=\frac{V_2}{T_2} \text { or, } \frac{V_1}{303}=\frac{\frac{4}{3} V_1}{T_2}\)
or, \(T_2=\frac{4}{3} \times 303=404 \mathrm{~K}=(404-273)^{\circ} \mathrm{C}=131^{\circ} \mathrm{C} .\)
Example 8. At 27°C, and a pressure of 76 cmHg 100 cm³ of a gas is collected over the water surface. The space occupied by the gas is saturated with water vapour. Maximum vapour pressure of water at 27°C is 17.4 mmHg. What will be the volume of dry gas at STP?
Solution:
Given
At 27°C, and a pressure of 76 cmHg 100 cm³ of a gas is collected over the water surface. The space occupied by the gas is saturated with water vapour. Maximum vapour pressure of water at 27°C is 17.4 mmHg.
Let the volume of the dry gas at STP = V2 cm³, pressure p2 = 76 cmHg and temperature T2 = 0°C = 273 K.
Given V1 = 100 cm³, p1 = 76- 1.74 = 74.26 cmHg and T1 = 27 + 273 = 300K.
Hence, from the equation of state, \(\frac{p_1 V_1}{T_1}=\frac{p_2 V_2}{T_2} \text { or, } \frac{100 \times 74.26}{300}=\frac{V_2 \times 76}{273}\)
or, \(V_2=\frac{273 \times 74.26}{3 \times 76}=88.92 \mathrm{~cm}^3\).
∴ At STP, the volume of dry gas will be 88.92 cm³.
Factors Affecting Volume and Pressure Coefficients of Gases
Example 9. A person measures the pressure of his car tyre to be 2 x 105 Pa. At that time the temperature and pressure of the atmosphere are 27°C and 1 x 105 Pa respectively. Then he travels to another city where the temperature and pressure of the atmosphere are 12°C and 6.7 x 104 Pa respectively. Then what will be the pressure of his car tyre at that time? Assume the volume of the tyre is the same in both cases.
Solution:
Given
A person measures the pressure of his car tyre to be 2 x 105 Pa. At that time the temperature and pressure of the atmosphere are 27°C and 1 x 105 Pa respectively. Then he travels to another city where the temperature and pressure of the atmosphere are 12°C and 6.7 x 104 Pa respectively.
The pressure in a tyre is a gauge pressure, which is the difference between the pressure in the tyre and atmospheric pressure.
Hence, the absolute pressure in the tyre = gauge pressure + atmospheric pressure.
So in 1st case, absolute pressure, p1 = 2 x 105 + 105 = 3 x 105 Pa
and temperature, T1 = 273 + 27 = 300 K
Let in the 2nd case the measured pressure (gauge pressure) = x Pa.
So the absolute pressure, p2 = (x+ 6.7 x 104) Pa and temperature, T2 = 273 + 12 = 285 K
Since the volume of the tyre is constant, \(\frac{p_1}{T_1}=\frac{p_2}{T_2}\)
or, \(\frac{3 \times 10^5}{300}=\frac{x+6.7 \times 10^4}{285} \text { or, } x=2.18 \times 10^5\)
So the measured pressure is 2.18 x 105 Pa.
