Periodic Trends In Properties Of The Elements
WBBSE Class 11 Periodic Trends Overview
The properties of elements can be divided into two categories:
Properties of individual atoms: The properties such as atomic and ionic radii, ionisation energy, electron affinity, electro negativity and valency are the properties of the individual atoms & are directly related to their electronic configurations.
Properties of groups of atoms: The properties such as melting point, boiling point, the heat of fusion, heat of vaporisation, atomic volume, density etc. are the bulk properties i.e., the properties of a collection of atoms and are related to their electronic configurations indirectly.
Periodic Table Of Elements Trends
All these properties which are directly or indirectly related to the electronic configurations of the elements are called atomic properties.
Since electronic configurations of the elements are periodic functions of their atomic numbers, these atomic properties are also periodic functions of atomic numbers of the elements. Thus, atomic properties are also called periodic properties.
Read and Learn More WBCHSE Class 11 Chemistry
Periodic Trends
Remember that, when we descend a group, the chemical properties ofthe elements remain more or less the same due to the same valence shell configuration, but there is a gradual change in physical properties due to a gradual change in the size ofthe atoms owing to the addition of new electronic shells.

Understanding Atomic Radius Trends for Class 11
Atomic size or atomic radius
If an atom is assumed to be a sphere, the atomic size is given by the radius of the sphere, called atomic radius.
Atomic size or atomic radius Definition: The distance from the centre of the nucleus to the outermost shell containing the electrons is called the atomic radius
Difficulties in precise measurement of atomic radius:
It is not possible to isolate a single atom for the measurement of its radius.
The electron cloud surrounding the nucleus does not have a sharp boundary as the probability of finding an electron can never be zero even at a large distance from the nucleus.
The magnitude of atomic radius changes from one bonded state to another.
Types of atomic radius: As already mentioned, the size of an atom varies from one environment to another.
Therefore, several kinds of atomic radii have been defined. These are—
- Covalent radius
- Metallic radius
- van der Waals radius.
Covalent radius: It is defined as one-half of the distance between the centres of two atoms of the same element bonded by a single covalent bond.
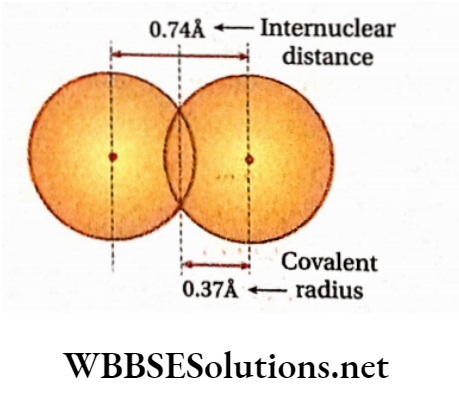
Thus for homonuclear diatomic molecules, covalentradius\((r)=\frac{1}{2} x\)
internuclear distance.
Example: In the H2 molecule, the internuclear distance is = 0.74 A =74pm. So, covalent radius ofhydrogen atom=\(=\frac{1}{2} \times 0.74\) = 0.37 A= 37pm. lA = 10-10m, 1 pm = 10-12m.
For the heteronuclear diatomic molecule A—B, internuclear distance, rAB = rA + rB.
Therefore rA = rAB-rB rB = rAB-rA
[rA and rB represent radii ofthe atoms, A & B respectively]
Example: For HC1 molecule, internuclear distance, rHCl = L36A & covalent radius of hydrogen atom (rH) = 0.37 A
Therefore Covalent radius ofchlorine atom (rcl) = rHC1- rH = 1.36-0.37 = 0.99 A
Metallic radius: It is defined as one-half of the internuclear distance between two adjacent atoms in a metallic lattice.
Example: The distance between two adjacent copper atoms in solid copper is 2.56 A (determined by X-ray diffraction). Hence, the metallic radius ofcopperis1.28
A. Similarly, the metallic radii of sodium and potassium have been determined as 1.86 A and 2.31 respectively.
Note—covalent radii of Na and K are 1.54 A and 2.03 A.
Metallic radius is always greater than the covalent radius van der Waals radius: It is defined as one-half of the distance between the nuclei of two non-bonded neighbouring atoms belonging to two adjacent molecules of an element in the solid state.
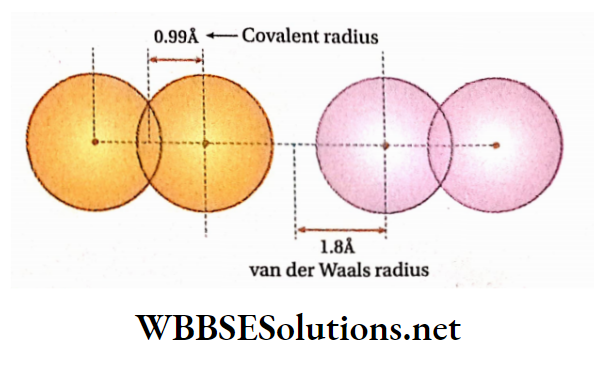
Example: The distance between nuclei of two adjacent Cl -atoms of two adjacent chlorine molecules in a solid state is 3.6 A.
Ionization Energy Trends in the Periodic Table
Ionization Radius
So, the van der Waals radius of Cl-atom is \(\frac{3.6}{2}=1.8 \AA\) Since the 2 van der Waals force of attraction is weak even in the solid state, the internuclear distances between the atoms of two adjacent molecules held by van der Waals forces are much larger than those between covalently bonded atoms (which involve mutual overlap of atomic orbitals). Van der Waals radii are always greater than covalent radii. For example, the van der Waals radius ofchlorine is1.80 A while its covalent radius is 0.99 A.
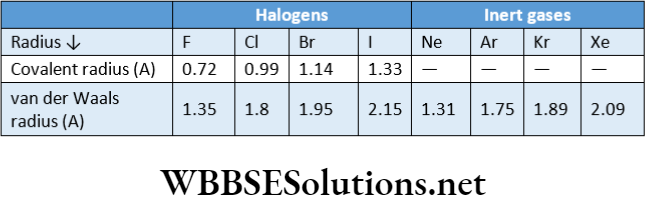
The sequence of three types of atomic radii: van der Waals radius >Metallic radius > Covalentradius.
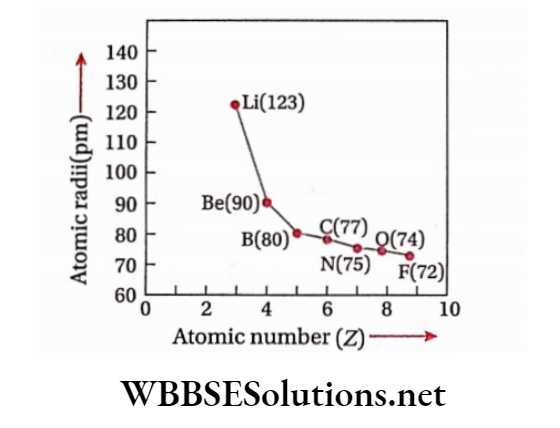
Variation of atomic radii or sizes in the periodic table: While moving from left to right across a period in the periodic table, atomic radii or atomic sizes progressively decrease.
Explanation: The principal quantum shell remains unchanged in the same period. So, the differentiating electrons enter the same shell but due to an increase in the number ofprotons, the positive charge of nucleus also increases.
So, the attractive force of the nucleus for electrons in the outermost shell also increases.
Hence, the atomic sizes, rather than the atomic radii of elements in the same period, gradually decrease with an increase in atomic number. In any period, the atomic size of the element of group LA is maximum and that of the halogen of group VILA is minimum.
Variation of atomic [covalent] radii of the elements of the third period (n = 3]
![Class 11 Chemistry Classification Of Elements And Periodicity in Properties Variatioon of atmoic [Covalent] radii of the elements of thirs period [n=3]](https://wbbsesolutions.net/wp-content/uploads/2023/12/Class-11-Chemistry-Classification-Of-Elements-And-Periodicity-in-Properties-Variatioon-of-atmoic-Covalent-radii-of-the-elements-of-thirs-period-n3.png)
Variation of atomic radii or sizes down a group: On moving down along any group of the periodic table, the atomic sizes rather the atomic radii of the elements increase remarkably.
Explanation: On moving down a group, a new electronic shell is added to each succeeding element, though the number of electrons in the outermost shell remains the same. This tends to increase the atomic size.
At the same time, there is an increase in nuclear charge with an increase in atomic number. This tends to decrease the size.
However, the effect of the increased nuclear charge is partly reduced by the shielding effect of the inner electronic shells. In practice, it is found that the effect of the addition of a new electronic shell is so large that it outweighs the contractive effect of the increased nuclear charge.
Hence, there occurs a gradual increase in atomic radii on moving down a group in the periodic table.
Variation of size in a group for heavier elements: On moving down a group, the relative rate of increase of atomic radii slow for heavier elements.
So, the differences in size for heavier species such as Cs (6s1) and Fr(7s1) of group-1 or Ba(6s2) and Ra(7s2) of group-2 are very small.
This is due to the presence of electrons in the inner d and f-orbitals having poor screening effect.
D and f-electrons do not screen the outer electrons effectively from the pull of the nucleus. There is only a small increase in size despite addition of a new electronic shell. Example— Na(l.54 A), K(2.03 A), Rb(2.16 A), Cs(2.35 A) etc.
In the case of transition elements, such a decrease in the atomic size is relatively less. Here the differentiating electron instead of entering the outermost orbit, goes to the penultimate (n- 1 )d -subshell which is closer to the nucleus.
However, due to the poor shielding effect of the additional d -electrons, there is a small increase in effective nuclear charge and hence, the decrease in the size with an increase in the atomic number is relatively small.
In the case of lanthanide elements, the differentiating electrons enter the (n – 2)/-orbital having a very poor shielding effect.
| Class 11 Physics | Class 12 Maths | Class 11 Chemistry |
| NEET Foundation | Class 12 Physics | NEET Physics |
So, In such cases, the increase in the effective nuclear charge is greater than that in the case of transition elements.
Thus, the decrease in atomic sizes of the lanthanides is much more regular and distinct as compared to the transition elements.
So, the difference in the extent of the decreased atomic sizes ofthese elements is due to their relative inability to screen the outermost electrons from attraction ofthe nucleus.
![Class 11 Chemistry Classification Of Elements And Periodicity in Properties Covalent radiii[pm] of representative element](https://wbbsesolutions.net/wp-content/uploads/2023/12/Class-11-Chemistry-Classification-Of-Elements-And-Periodicity-in-Properties-Covalent-radiiipm-of-representative-element.png)
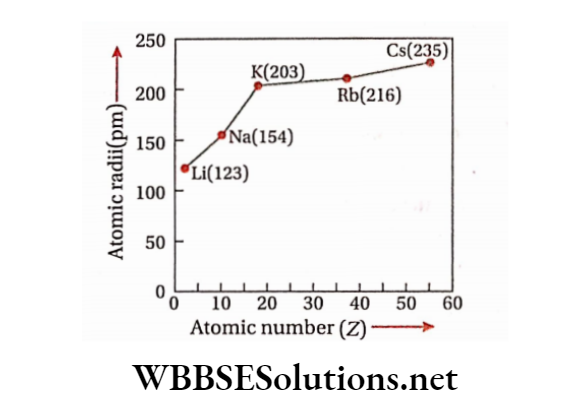
Electronegativity Trends Explained for Class 11
Screening effect or shielding effect: in multi-electron atoms, the electrons present in the inner shells shield the electrons in the valence shell from the pull of the nucleus.
It means that the electrons of the inner shells act as a screen between the nucleus and the electrons in the valence shell. This is known as the screening effect shielding effect.
Screening effect or shielding effect Definition: The ability of the inner electronic shells to shield the outer electrons from the attraction of the nucleus is called the screening effect or shielding effect.
The magnitude of the screening effect of electrons belonging to different subshells follows the sequence: s>p> d>f.
Important points regarding atomic (covalent) radius:
The alkali metals occupying positions at the extreme left side ofthe periodic table have the largest size in each period.
The halogens occupying positions at the right side of the periodic table have the smallest size in each period.
The noble gases present at the extreme right of the periodic table have larger atomic radii than those of the preceding halogens because van der Waals radii (but not covalent radii) are taken into consideration for noble gases.
Atomic Size Trend
In transition series {d -block elements), there is only a small decrease in size with successive increases in atomic number because the differentiating electrons enter into (n-l)d subshell, which partially shields the increased nuclear charge acting on the valence electrons. This is known as d contraction.
For the inner-transition series ( f-block elements), the decrease in atomic radii and wide increase in atomic number is relatively greater and more regular as compared to the transition series elements.
This is so because the differentiating electrons enter into (n- 2)f -subshell havingverypoor shielding effect.
In a group of representative elements, there is a continuous increase in atomic radii with an increase in atomic number.
On going down a group of transition elements, there is an increase in size from first member to second member as expected, but for higher members, the increase in size is quite small. This is due to lanthanide contraction. For example, Cu(1.28A), Ag(1.44A), Au (1.44A)etc.
Ionic radii
Ionic radii refers to the radii of the ions in the ionic crystals. Ionic radius may be defined as the effective distance from the centre of the nucleus of an ion to the point upto which it exerts its influence on the electron cloud.
Theoretically, the electron cloud may extend itself to a very large distance from the nucleus. So, it is not possible to measure the ionic radius directly.
It is measured indirectly as follows. The equilibrium distance between nuclei of adjacent cation and anion in an ionic crystal can be determined by X-ray analysis.
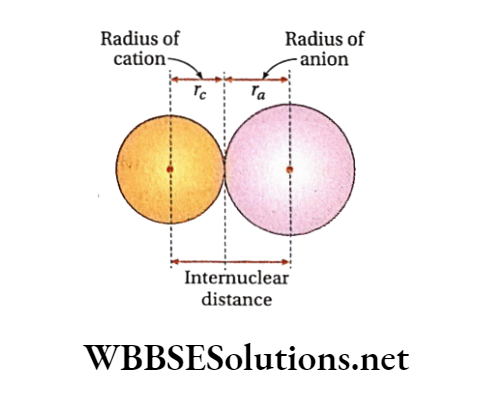
Regarding ions as spheres, the internuclear distance may be taken as the sum of the radii of the adjacent ions. Knowing the radius of one, that ofthe other can be calculated. Several methods have been developed to fix the absolute value of at least one ion.
Pauling’s method is the most widely accepted and the values given here are based on this method.
For example, based on Pauling’s method, the radius ofthe Na+ion has been determined to be 0.95 A.
Again, the intemuclear distance between Na+ and Cl- ions in NaCl crystal is 2.76 A (from X-ray studies). So, (2.76-0.95) = 1.81 A.
Some characteristics of ionic radii:
The radius ofthe cation is always smaller than that of the neutral atom: A cation is formed loss of one or more electrons from the neutral atom (e.g., Na-e→Na+; Mg- 2e→Mg2+ ).
With the removal of electron (s) from an atom, the magnitude of nuclear charge remains the same while the number of electrons decreases. That means the same nuclear charge now acts on a decreased number of electrons.
As a result, effect of nuclear charge per electron increases and the electrons are more strongly attracted towards the nucleus. This causes a contraction in size ofthe cation.
In most cases, all the electrons from the outermost shell of the atom are completely removed so that the penultimate shell of the atom now becomes the outermost shell ofthe cation.
As a result, the size ofthe cation becomes smaller than that of the parent atom from which it is formed.
\(\begin{array}{cc}\mathrm{Na}\left(1 s^2 2 s^2 2 p^6 3 s^1\right) \stackrel{-e}{\longrightarrow} & \mathrm{Na}^{+}\left(1 s^2 2 s^2 2 p^6\right) \\
r_{\mathrm{Na}}=1.54 \AA & r_{\mathrm{Na}^{+}}=0.95 \AA
\end{array}\)
When an element forms more than one type of cation, then the cationwithhigher chargewillbe smallerin size: The cations (e.g., Fe2+ and Fe3+) derived from a specific element contain same number of protons but different number of electrons.
The cation with a higher charge (e.g., Fe3+) contains fewer electrons and the effective nuclear charge per electron is greater, thereby causing a contraction in size.
Example: Ionic radius of Fe2+ = 0.75 A but ionic radius of Fe3+ = 0.60 A
The radius ofthe anionic is always greater than that ofthe neutral atom: An anion is formed by the gain of one or more electrons by a neutral atom (e.g., Cl+e→Cl-; O+2e→O2-)
This increases the number of electrons in the anion but the nuclear charge remains the same as that in the parent atom.
That means the same nuclear charge now acts on an increased number of electrons. As a result, effective nuclear charge per electron decreases and the electrons are less tightly held by the nucleus. This causes an increase in the size of the anion.
Furthermore, the addition of one or more electrons would result in increased repulsion among the electrons, thereby causing the expansion of the outermost electronic shell. This also causes an increase in the size of the anion
Chemical Reactivity of Elements: Periodic Trends
Example-1
\(\begin{array}{cc}\mathrm{Cl}(17 p+17 e) \stackrel{+e}{\longrightarrow} \mathrm{Cl}^{-}(17 p+18 e) \\
r_{\mathrm{Cl}}=0.99 \AA & r_{\mathrm{Cl}^{-}}=1.81 \AA
\end{array}\)
Variation of ionic radii within a group:
For cations: Like covalent radii of atoms, the radii of cations also increase on moving down a group. This is primarily due to the addition of a new electronic shell at each succeeding member on moving down a group. Thus,
\(r_{\mathrm{Li}^{+}}<r_{\mathrm{Na}^{+}}<r_{\mathrm{K}^{+}}<r_{\mathrm{Rb}^{+}}\)For anions: Like cationic radii, the radii of anions also increase on moving down a group. This is primarily due to the addition of a new electronic shell at each succeeding member on moving down a group. Thus, \(r_{\mathrm{F}^{-}}<r_{\mathrm{Cl}^{-}}<r_{\mathrm{Br}^{-}}<r_{1^{-}}\)
![Class 11 Chemistry Classification Of Elements And Periodicity in Properties Variation of ionic radii of cations of Gr-1[1A] and anions of group- 17](https://wbbsesolutions.net/wp-content/uploads/2023/12/Class-11-Chemistry-Classification-Of-Elements-And-Periodicity-in-Properties-Variation-of-ionic-radii-of-cations-of-Gr-11A-and-anions-of-group-17.png)
Variation of ionic radii along a period:
For cations: On moving from left to right along a period, the radii of isoelectronic cations (cations having the same number of electrons) decrease progressively due to an increase in the magnitude of nuclear charge (i.e., an increase in the number of protons).
Thus, rNa+ > rMg2+ > rAl3+.
For anions: Onmovingfromlefttorightalong period, the radii of isoelectronic anions (anions having the same number of electrons) decrease progressively due to increase in the magnitude of nuclear charge [i.e., an increase in the number of protons). Thus, rN3¯ > rQ2‾ > rp¯
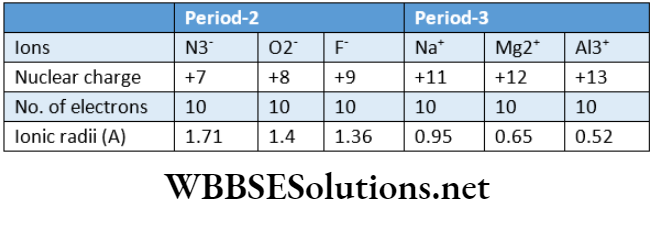
Variation of ionic radii of isoelectronic ions (cations or anions) belonging to the same or different periods:
Isoelectronic species Cations or anions or neutral atoms having the same number of electrons but different magnitude of nuclear charge [i.e., different number of protons) are called isoelectronic species.
A set of isoelectronic ions (belonging to periods 2 and 3) are shown in the following table. All the ions have an equal number (10) of extranuclear electrons but a different number of protons.
As we move from one member to another, the nuclear charge increases. Consequently, the electrons are pulled more and more strongly, thereby causing a gradual decrease in size.
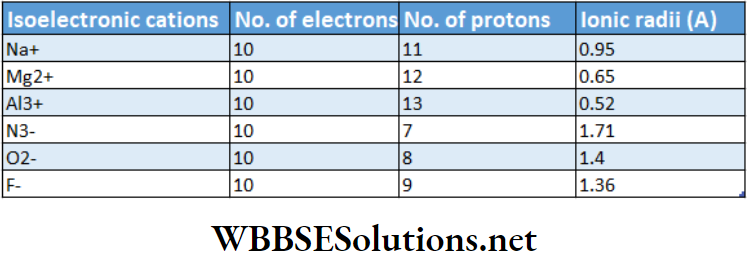
Note that the cation with a greater positive charge has a smaller radius while the anion with a greater negative charge has a greater radius.
For any atom or ion, (Z/e) oc(1f): where Z- atomic number or nuclear charge, e = number of electrons and r = atomic or ionic radius.
Since the quantity, Z -e varies inversely with ionic radius (r), so increase in the magnitude of Z/e brings about a decrease in the value of ionic radius, and a decrease in the magnitude of Zfe results in an increase in ionic radius.
Forinstance,in case of O -atoms,(Z/e) = 1 and for O2¯ ion, (Z/e) = (8/10) = 0.8. So, the ionic radius of O2¯ ion (1.40 A) is greater than the atomic radius of O-atom (0.66A).
Similarly for K-atom, (Z/e) = 1 and for K+ ion, (Z/e) = (19/18) = 1.05. Hence, the ionic radius of the K+ ion (1.33 A) is less than the atomic radius of the K -atom (2.27 A)
Important points to remember about ionic radii:
- The radius of the cationic is always smaller than that of the parent atom.
- Theradius of anion is always greater than that of the parent atom.
- The ionic radii (both cationic and anionic) in any group increase on moving down the group.
- The radii of isoelectronic cations decrease with increasing atomicnumber across a period.
- The radii of isoelectronic anions decrease with increasing atomicnumber across a period.
- The radii of isoelectronic cations or anions (of the same or different period) decrease with increasing atomic number.
- The sizes of cations (of the same element) decrease with increasing positive charge (e.g., rpe2+ > rFe3).
Atomic volume Definition: It is the volume occupied by the gram-atom (or mole atom) of an element at its melting point in the solid state.
Measurement: The gram-atomic mass of an element, when divided by its density, gives the atomic volume ofthe element.
Atomic masses. He plotted atomic volumes of different elements against the corresponding atomic masses and the curve thus obtained looked like a wave with several sharp peaks and broad minima.
The curve reveals that the atomic volumes of elements having similar properties are periodic functions of their atomic masses. For instance, l] Each peak ofthe curve is occupied by the first member of a period.
That means the reactive and light alkali metals, Li, Na, K, Rb, Cs and Fr occursuccessivelyat at the peaks. They have the largest atomic volumes.
The less reactive transition elements (e.g., Cr, Mn, Fe, Co, Ni )which are heavy and possess high melting points occupy the bottom portions ofthe curve.
Electronegative elements (e.g., F, Cl, Br, I) occupy the ascending portions of the curve (i.e., the left side of each peak) and their non-metallic character increases while moving towards the peak.
Each of the descending portions (i.e., the right side of each peak) of the curve is occupied by electropositive metals (e.g., Mg, Ca, Sr, Ba). v] Elements occupying similar positions on the curve are placed in the same group of the periodic table.
\(\text { Atomic volume }=\frac{\text { Gram-atomic mass }}{\text { Density }\left(\text { in } \mathrm{g} \cdot \mathrm{cm}^{-3}\right)}\)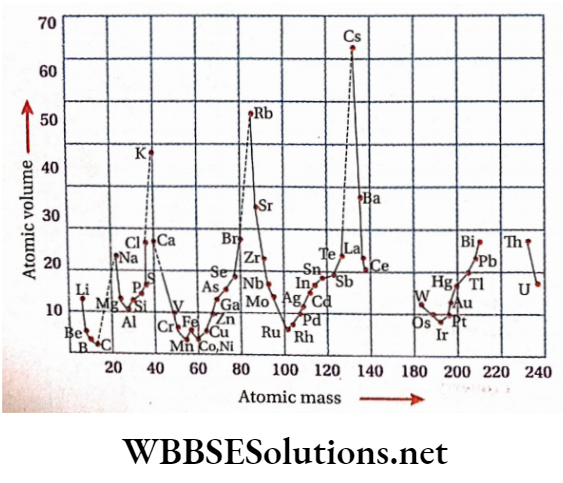
Electron Affinity Trends Across the Periodic Table
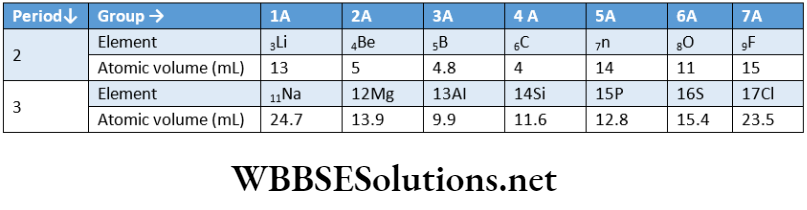
Variation of atomic volume along s period: On moving from left to right along a period, the atomic volume of the elements gradually decreases to a minimum value and then increases successively and attains a maximum value the first member of the next period.
Variation of atomic volumes of elements belonging to the second and third periods
Variation of atomic volume down a group: On moving down a group, (i.e., with increasing atomic mass) the atomic volume of elements increases almost regularly due to successive addition of new principal quantum shells.
Metallic and non-metallic character
The metallic property or electropositive character of an element is measured as the tendency of its atom to form a cation with loss of electron (s). The more the tendency ofthe atom of an element to lose electrons, the more will be its metallic property.
Variation of metallic and non-metallic character across a period: In any period, as we move from left to right, the atomic size ofthe elements progressively decreases with a consequent steady increase in nuclear attractive force for the valence electrons; i.e., the valence electrons lose their freedom.
As a result, metallic property gradually decreases which is reflected in the fall of thermal and electrical conductivity of the elements. On the other hand, the non-metallic character ofthe elements increases along a period from left to right.
Explanation: Because of the large size of the metallic atoms, electrons in their valence shell are loosely held. So, they exhibit both thermal and electrical conductivity.
On the contrary, atoms of non-metals are smaller in size and hence their valence shell electrons are relatively strongly held therefore, they are thermally and electrically non-conductive. Moreover, non-metals have a strong tendency to form anions by gaining electrons. So, they are electronegative.
Metalloids like the nonmetals, are characterised by their small atomic sizes and their valence-electrons are more or less strongly confined.
However, at high temperatures, the confined electrons become mobile and contribute to electrical conductivity.
Example: Elements belonging to groups 1 and., Na and Mg are good conductors of of heat and electricity, and A1 in group 13 has moderate conductivity. Other elements of this period are either or conductors on-conductors.
Variation of the metallic and non-metallic character down a group: In the periodic table, as we move down a group, the metallic character gradually increases and consequently, the non-metallic character gradually decreases.
Explanation: Due to the introduction of new electronic shells, the hold of the nucleus on the valence electrons gradually decreases thereby making these electrons more and more mobile on moving down a group.
Consequently, metallic character increases with increasing atomic number. Thus in group 15, N and P are typical non-metals, As and Sb are metalloids and Bi is a typical metal. C in group-14 is a typical non-metal, Ge possesses metallic character while Pb and Sn are typical metals.
All transition elements as well as the elements oflanthanoid and actinoid series are typical metals. Transition elements are good conductors of heat and electricity. Coinage or noble metals (Cu, Ag, Au and Pt) have maximum thermal and electrical conductivity.
Variation of the metallic and non-metallic character of elements from the nature of their oxides: On moving across a period from left to right, metallic character decreases and within a group, the metallic character increases from top to bottom.
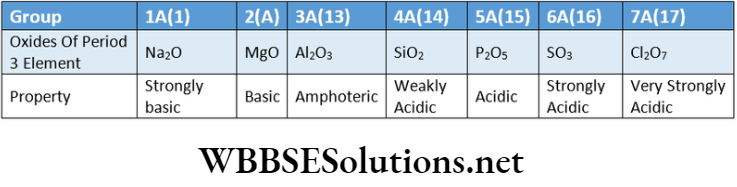
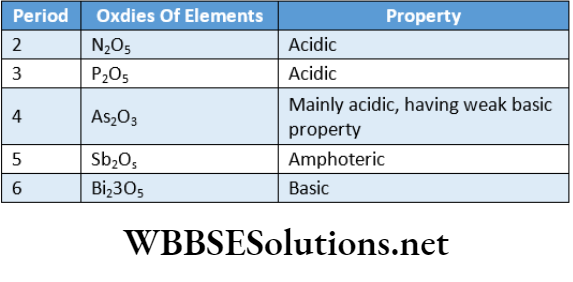
Generally, oxides of strongly electropositive elements are basic while oxides of strongly negative elements exhibit acidic character.
Example:
Oxides of the representative elements ofthe third period show that their basic character i.e., the metallic property gradually decreases while their acidic character i.e., non-metallic character gradually increases from left to right across the period.
Oxides of elements of group VA reveal that the acidic character progressively decreases while the basic character increases down a group. Hence, the non-metallic character gradually decreases and the metallic character gradually increases down a group.
Oxidising and reducing properties
An oxidising agent can gain electrons while a reducing agent can donate electrons. So strong oxidising agents have a pronounced tendency to accept electrons and strong reducing agents give up electrons easily.
Variation of oxidising & reducing properties across a period:
On moving from left to right a period, nuclear charge increases by one unit and at the same time one electron is added to the same outermost shell in each succeeding element.
So nuclear pull on the electrons of the outermost shell increases with increasing atomic number.
Consequently, the tendency of an atom to give up electrons decreases and the reverse tendency i.e., the tendency to accept electrons increases on increasing atomic numbers over a period.
In other words, the elements on the right side of a period [Le., gr.-VA(15), VIA(16) and VIIA(17) elements] are good oxidising agents because they are good acceptors of electrons.
Trends in Metallic and Non-Metallic Character
Their oxidising power increases as: VA < VIA < VIIA. On the other hand, the elements on the left side of a period [le., gr-IA(l), IIA(2)] are good-reducing agents because they are good donors of electron. Their reducing power decreases as 1A > 2A > 3A
Example: Oxidisingpower: Na < Mg < A1 < Si < P < S < Cl
Reducing power: Na > Mg > Al > Si > P > S > Cl
Variation of oxidising and reducing properties down a group:
On moving down a group, a new electronic shell is added at each succeeding element. This tends to decrease the nuclear pull on the outer electrons.
But at the same time, there is an increase in nuclear charge with an increase in atomicnumber This tends to increase the nuclear pull on the outer electrons. In practice, it is found that the effect of the addition of a new electronic shell is greater than the effect of increased nuclear charge.
This means that, on moving down a group, nuclear pull on the outer electrons gradually decreases.
Consequently, reducing power gradually increases while the oxidising power gradually decreases on moving down a group in the periodic table.
Example: The oxidising power of halogens follows the order: of F(+2.87) > Cl(+1.36) > Br(+1.06) >I(+0.53). The reducing power of group-IIA metals follows the order:
Be(-1.85) < Mg(—2.37) < Ca(-2.87) < Sr(-2.89) < Ba(-2.90). where the quantities within the bracket indicate the standard reduction potential, £°ed(volt) at 25°C for the indicated (12X2 or M2+/M ).
