WBBSE Class 6 Science Question Answer Chapter 2 Phenomena Around Us Review Questions Environment Review Questions MCQs
Question 1. Which one of the following is a physical change?
- Curd from milk
- Rusting of iron
- Melting of butter
- X-ray photography
Answer: 3. Melting of butter
Question 2. Which one of the following is a chemical change?
- Switching on a bulb
- Digestion of food
- Boiling of water
- Inflation of tyre
Answer: 2. Digestion of food
Read And Learn More: WBBSE Solutions For Class 6 School Science
Question 3. In cities, chlorine is mixed with water
- To kill the germs
- To colour the water
- To add a sweet odour to the water
- None of these
Answer: 1. To kill the germs
WBBSE Class 6 Phenomena MCQs
Question 4. The tyre of a cycle is inflated with air. This process is a
- Irreversible process
- Physical process
- Chemical process
- Natural process
Answer: 2. Physical process
Question 5. Melting of ice is an example of
- Man-made process
- Irreversible process
- Physical process
- Periodic process
Answer: 3. Physical process
Question 6. Identify the chemical change.
- Burning of magnesium filament in oxygen
- Mixing of hydrogen and oxygen
- Sulphuric acid is slowly poured into the water
- Heating of the metal rim of a cartwheel
Answer: 1. Burning of magnesium filament in oxygen
Question 7. Identify the irreversible change.
- Melting of wax
- Green leaves becoming yellow
- Bending of railway lines during hot summer days
- Formation of dew drops
Answer: 2. Green leaves becoming yellow
Question 8. Identify which of the following is a non-periodic change.
- Change of season
- Tide and ebb
- Flash flood
- Leap year
Answer: 3. Flash flood
Understanding Physical and Chemical Changes MCQ’s
Question 9. An example of a physical change is
- Making puffed rice (more) from rice
- Melting of wax
- Rusting
- Yellowing of teeth
Answer: 2. Melting of wax
Question 10. When a candle is lit
- Only physical changes occur
- Only chemical changes occur
- Physical and chemical changes occur simultaneously
- None of the changes occurs
Answer: 3. Physical and chemical changes occur simultaneously
Question 11. A piece of paper is burnt and reduced to ashes. This is
- An irreversible change
- Physical change
- Periodic change
- A change that does not need energy
Answer: 1. An irreversible change
Question 12. Which one is a physical change?
- Melting of glaciers
- Cataract in eyes
- Black stains on teeth
- Ripening of mango
Answer: 1. Melting of glaciers
Question 13. In which case heat energy is liberated?
- Urea is dissolved in water
- Sulphuric acid is dissolved in water
- Ammonium nitrate is dissolved in water
- Evaporation of spirit
Answer: 2. Sulphuric acid is dissolved in water
Question 14. Identify the process which is natural and irreversible.
- Ripening of fruit
- A candle is lit
- A football is inflated
- Curd is prepared from milk
Answer: 1. Ripening of fruit
Question 15. Identify the process which is a man-made and chemical process.
- Dissociation of water by passing electricity through it
- Growth of a cancer cell
- The magnetisation of a piece of iron
- Tadpole transforming into a frog
Answer: 1. Dissociation of water by passing electricity through it
Question 16. A chemical fertiliser among the following is
- Carbaryl
- Malathion
- Dap
- All of these
Answer: 2. Malathion
Question 17. Identify the desirable, periodic and natural change among the following:
- Growth of a plant
- Cleaning activities of the municipality
- Generation of electricity
- Rotation of earth
Answer: 4. Rotation of earth
Question 18. Which one among the following is an irreversible physical change?
- Heating of iron to red hot stage
- Dissolution of salt in water
- A glass pot breaking into pieces
- Water freezing into ice
Answer: 3. A glass pot breaking into pieces
WBBSE Class 6 Science Question Answer Chapter 2 Phenomena Around Us Fill In The Blanks
Question 1. When water is boiled, it is transformed into Vapour. This is a _________ change.
Answer: Physical
Question 2. Earthquake is a __________ and __________ change.
Answer: Natural; undesirable
Question 3. __________ is added to water to kill the germs.
Answer: Chlorine
Question 4. To and fro movement of a pendulum is a __________ change.
Answer: Periodic
Question 5. Freezing of water is a __________ change.
Answer: Physical
Question 6. Melting of glaciers is a __________ and __________ change.
Answer: Natural; physical
Question 7. Taking an X-ray photograph of a fractured bone on an X-ray plate is a __________ change.
Answer: Chemical
WBBSE Class 6 Science Question Answer
Question 8. When chemical substances are taken in a solution state, they react __________
Answer: Faster
Question 9. When an apple is cut and kept open in the air for sometime __________ stain appears on the exposed surface.
Answer: Brown
Question 10. When __________ is dissolved in water __________ is absorbed change.
Answer: Urea; heat
Question 11. When ammonium nitrate is dissolved in water, the solution becomes __________
Answer: Cold
Question 12. When iron is kept in moist air for a prolonged time, __________ is formed on the iron surface. It is a __________ change.
Answer: Rust; chemical
Question 13. Any sort of change (either physical or chemical) involves __________ to be initiated.
Answer: Energy
Question 14. BHC, DDT, malathion etc are commonly used __________
Answer: Pesticides
Question 15. The change which involves the formation of one or more new substances having different properties is called a __________ change.
Answer: Chemical
Class 6 WBBSE Science Question Answer Chapter 2 Phenomena Around Us Identify As True Or False
Question 1. Earthquake is an undesirable process.
Answer: True
Question 2. Flood is a periodic and natural change.
Answer: False
Question 3. Excessive use of insecticides is harmful to nature.
Answer: True
Question 4. When an apple is cut and kept open in the air, brown patches appear on the exposed surface. It is a physical process.
Answer: False
Question 5. Yellowing green leaves is a physical process.
Answer: False
Question 6. The preparation of curd from milk is a fast process.
Answer: False
Question 7. The growth of a plant is an irreversible process.
Answer: True
Question 8. Rusting involves the formation of a new compound.
Answer: True
Question 9. When ice cream melts, the heat lost by ice cream is gained by the air. change.
Answer: False
Question 10. Nuclear explosion for scientific purposes is a desired process, but its use in war is highly undesirable.
Answer: True
Question 11. All natural processes are desirable processes.
Answer: False
Class 6 WBBSE Science Question Answer
Question 12. Natural processes occur on their own.
Answer: True
Question 13. Physical processes are always reversible.
Answer: False
Question 14. Physical changes may or may not involve absorption or emission of heat.
Answer: True
Question 15. Chemical processes are always associated with changes in energy.
Answer: True
Question 16. Forest fire is a natural, undesirable, fast process.
Answer: True
Question 17. When a piece of chalk is successively broken into smaller pieces, the total surface area increases.
Answer: True
Question 18. Increased surface area may speed up a chemical change.
Answer: True
Question 19. Rusting of iron is a harmful chemical change.
Answer: True
Question 20. The weakening of our bones within the body is a chemical and physical change.
Answer: False
Chapter 2 Phenomena Around Us match The Column
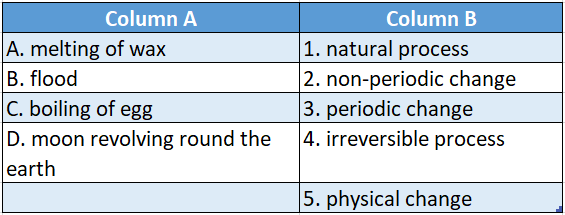
Answer: A-5,B-1,C-4,D-3
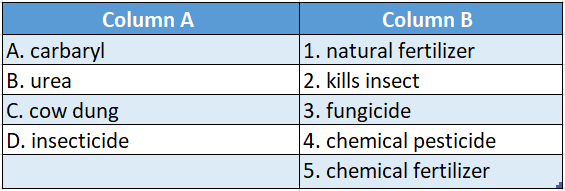
Answer: A-3,B-4,C-1,D-2
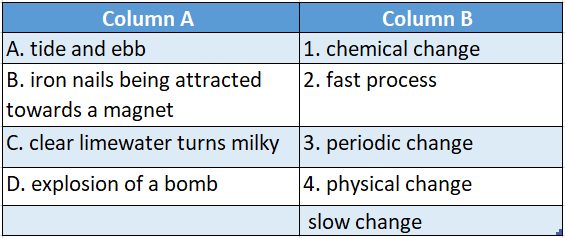
Answer: A-4,B-5,C-1,D-2
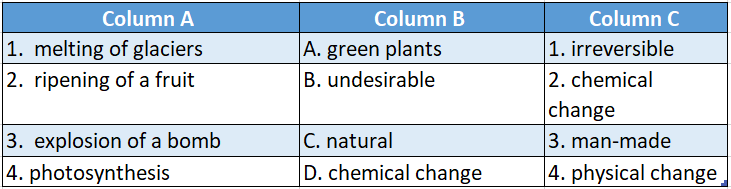
Answer: 1-C-4,2-D-1,3-B-3,4-A-2
Chapter 2 Phenomena Around Us Answer In Words Or A Sentence
Question 1. Plants prepare their own food. What kind of change is it?
Answer: This is a chemical change. In this case, glucose (a new compound) is prepared on the leaves from carbon dioxide and water in the presence of sunlight.
Question 2. Why chlorine is added to water?
Answer: Chlorine is mixed with water to kill the germs present in the water and to remove the smell of water.
Class 6 WBBSE Science Question Answer
Question 3. A mango is ripened. Is it a physical or chemical process?
Answer: It is a chemical process. During ripening new substances are formed within the fruit.
Question 4. Give an example of a process which is a natural, slow and chemical process.
Answer: Ripening of a fruit.
Question 5. Boiling raw rice to prepare boiled rice is a chemical process. -Justify.
Answer: It is a chemical process. During the boiling of raw rice, new substances are formed within it. Also, we cannot get back the raw rice by any means once it is boiled.
Question 6. Give an example of a fast and a slow man-made process.
Answer: The explosion of a bomb is an example of a fast process. Formation of curd from milk is a slow process.
Question 7. A piece of ice is slowly warmed. What type of change it is?
Answer: When a piece of ice is warmed, it melts. This is only a change of state, no new substances are formed. Hence it is a physical change.
Question 8. What do you mean by periodic changes?
Answer: The changes which occur again and again after a fixed interval of time are called periodic changes.
Question 9. Give an example of a natural process which involves a physical change.
Answer: The melting of glaciers is a natural process which involves a physical change.
Question 10. Give an example of a physical process which is reversible.
Answer: Inflating a football.
Question 11. Give an example where both physical and chemical changes occur simultaneously.
Answer: Burning of a candle.
Question 12. Choose the odd one Photosynthesis, rusting, condensation, electrolysis of water
Answer: Condensation: It is a physical change while others are chemical changes.
WBBSE Class 6 Science Question Answer Chapter 2 Phenomena Around Us Qshort Answer Type Questions
Question 1. Which process is called a physical process? Give an example.
Answer: Physical process is a process where no new substances are produced during the process, only the physical state of the matter is changed.
For example, freezing of water into ice is a physical process. Here no new substances are formed, only the state of the matter is changed from liquid state to solid state.
Question 2. When electricity is passed through salted water, which gases are produced? Is it a physical or chemical process?
Answer: When electricity is passed through salted water, hydrogen and oxygen gases are produced. This is a chemical change called the electrolysis of water.
Water is dissociated and two new gases are produced.
Short Aswers on Changes in Nature
Question 3. What will happen if sulphuric acid is slowly added to water? What type of change is it?
Answer: When sulphuric acid is slowly added to water, the solution will become hot. This is a physical process. Here, two liquids are just mixed, and no new substances are produced.
Question 4. Complete the table
Answer:
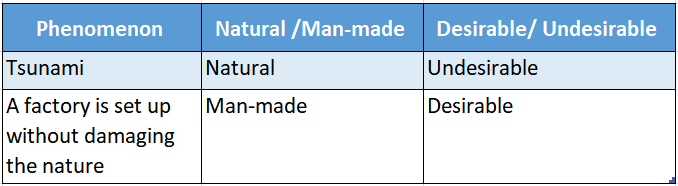
Question 5. Write four differences between a physical change and a chemical change.
Answer: See Table 2.6.
Question 6. What do you mean by periodic and non-periodic changes? Give example.
Answer: The changes which occur again and again after a fixed interval of time are called periodic changes.
The changes which do not take place after a regular or fixed interval of time are called non-periodic changes.
To and fro movement of a pendulum is an example of a periodic process. The movement of a car along a straight road is an example of a non-periodic process.
WBBSE Class 6 Science Question Answer
Question 7. Classify the following as chemical or physical change: plants preparing their own food; taking X-ray photographs of a broken bone; dissolving chlorine in water; melting of wax; ripening of fruit.
Answer: Plants preparing their own food: Chemical change;
Taking X-ray photograph of a broken bone: Chemical change;
Dissolving chlorine in water: Physical change; Melting of wax: Physical change;
Ripening of fruit: Chemical change.
Conceptual Questions on the Effects of Temperature on Changes
Question 8. Chemical fertilizers and insecticides may cause harm to nature. Why then should we use them?
Answer: Chemical fertilizers and insecticides are harmful to nature when they are used in excessive amounts.
When they are used in a recommended amount and all the guidelines are followed regarding their usage, they increase the productivity of the crops without causing pollution and health effects.
It helps to destroy the insects causing damage to crops in a short time. So farmers don’t have to depend on natural processes.
Hence the production of large amounts of food grains is possible to meet the growing demand for food worldwide.
Question 9. Give an example to justify that physical and chemical changes can occur simultaneously.
Answer: When a candle burns in the air, a part of the wax initially melts. This is only a change in the physical state of the matter.
When cooled, the molten part will again solidify. So it is a physical change. A major part of the candle (wax) is converted to carbon dioxide and water.
They are different substances. They are produced during the burning of the candle. So this is a chemical change.
Hence, when a candle burns both physical and chemical changes occur simultaneously.
Question 10. Write a short note on the chemical process.
Answer: The changes which involve the formation of one or more new substances having a completely different set of properties compared to the original substances are called chemical changes.
Rusting of iron, ripening of fruit, burning of a piece of paper etc. are all examples of chemical changes.
During these processes, in each case, new substances are formed. Chemical processes are usually irreversible.
Once the change occurs, it is not possible to get back the original substances. During a chemical change, either absorption or emission of heat must occur.
Question 11. When puffed rice (more) is prepared from rice, is it a physical or chemical change?
Answer: When puffed rice is prepared from rice, a chemical change occurs. New substances are formed during this and we cannot get back rice from the puffed rice.
Question 12. Rusting of iron is a chemical change. -Justify.
Answer: Rust is formed on the surface of iron when the iron surface is exposed to moist air for a prolonged time. It is a chemical process.
Real-Life Scenarios Involving Environmental Changes
Rust is a new substance called hydrated iron oxide formed due to the interaction between pure iron, water and oxygen.
Question 13. Write the names of two substances which are added to water to kill the germs.
Answer: Chlorine and halogen tablet are often added to water to kill the germs.
Question 14. Illustrate with an example how the speed of a chemical process can be made faster.
Answer: A glass of clear limewater is taken. If it is kept in the open air for a few days, it turns milky.
This occurs because lime water reacts with the carbon dioxide gas present in the air and forms an insoluble, solid substance (known as calcium carbonate).
This solid, insoluble substance remains suspended within the solution. So, limewater turns milky. It is a chemical change but a slow change.
It is slow because the concentration of carbon dioxide in the air is very small.
So it requires time to produce a sufficient amount of that insoluble substance to turn the clear limewater milky.
But if we can increase the concentration of carbon dioxide we can speed up this reaction.
This can be done by blowing air through a piece of straw into the clear lime water.
Within a few minutes, the lime water turns milky. This occurs because the concentration of carbon dioxide is higher in the air that we breathe out.
So the chemical reaction occurs more quickly.
Question 15. Give examples of two irreversible physical changes.
Answer: Irreversible physical changes:
- Glassware breaking into pieces
- Making of flour from wheat
Question 16. When a magnesium ribbon is burnt in the air it forms a white powder. What type of change is this? What is the chemical name of the white powder?
Answer: The burning of magnesium ribbon in the air is an example of chemical change. When magnesium metal burns it reacts with oxygen found in air to form a white powder which is chemically known as magnesium oxide.
Examples of Real-Life Applications of Chemical Changes
Question 17. Classify the following processes as desirable non- desirable and natural man-made processes: (1) Forest fire (2) explosion of the atomic bomb (3) tide and ebb (4) planned afforestation
Answer:
- Forest fire: non-desirable natural process the explosion of the atomic bomb: non-desirable man-made process
- Tide and ebb: desirable natural process planned afforestation: desirable man-made process
Question 18. Mention the names of a few factors which speed up the rate of a chemical change.
Answer: Factors that affect the rate of a chemical change include:
- the concentration of reactants which participate in the chemical change
- the surface area of reactants
- temperature
- physical states of the reactants presence of catalyst etc.
