Chapter 2 Element Compound And Chemical Reaction Section 2 Structure Of Matter
Constitution of matter
The constitution of matter that is how and with what matter it is composed has been the most important question, the answer to which humans are searching for the very ancient times.
The Indian Philosopher Kanada suggested the existence of atoms. In ancient Greece, Democritus and Leucippus and Roman philosopher Lucretius independently proposed the existence of a very small (invisible), indivisible and indestructible particle at around 400 B.C.
Democritus named the smallest particle of matter “atoms” meaning indivisible in Greek. The word “atom” originated from this Greek word.
Although the idea of the atom was fundamentally correct, they did not pursue it further to give the theory a scientific footing.
We can very well understand that in those ancient times, it was not possible to experimentally prove the existence of atoms. Naturally, the idea went into oblivion.
At the beginning of the 19th century, the English scientist John Dalton came forward with an elaborate theory, known as the Atomic Theory of Matter to solve the eternal problem related to *he constitution of matter Alton assumed the following in atomic theory.
Read And Learn More WBBSE Notes For Class 8 School Science
All elements are composed of very small, indestructible particles called atoms, which is indivisible.
Atoms can neither be created nor destroyed in chemical reactions.
Atoms of the same element are identical in mass and chemical properties. Ex: Element like oxygen may be obtained from different sources but the mass and properties of all the oxygen atoms are the same.
Atoms of different elements are different, having different masses and different chemical properties. Ex: Masses and properties of Hydrogen and Oxygen atoms are different.
During chemical reactions, atoms of different elements combine in simple numerical ratios (such as 1:1, 2:3, 2:1, etc.). Ex: Compound like NaCI is formed by combining one atom of sodium with one atom of chlorine.
The theory was first accepted as a hypothesis, as there was little experimental evidence in its support.
WBBSE Class 8 Structure of Matter notes
But during the next 50 years or so this theory was verified by several scientists the many experiments and the rectified theory created a strong foundation over which future research towards understanding the structure and constitution of matter could proceed.
Dalton’s original theory proposed that an atom was the smallest particle of all kinds of matter (elements and compounds).
The smallest particle of an element was termed a “simple atom” and the smallest particle of a compound was termed a “compound atom”.
A compound atom was right to be a combination of atoms of different ents in simple proportions.
At about the same time when Dalton proposed his atomic theory (1803), French cc “it’s. Gay Lussac (1808) put forward is volumes based on several experiments
The Law of Gaseous Volume estate orders the same condition of temperature and pressure the volumes of gases participating in a chemical reaction bear a simple ratio to one another- and also to the product if it is also gaseous.
Remember that Dalton’s theory suggests that atoms of different elements combine in a simple ratio during a chemical reaction.

Gay Lussac’s law suggests that gases combine in a simple ratio by volume. So it is obvious that there must be a simple relation between the volume of gases and the number of atoms contained in them.
Sweedish scientist Berzelius then proposed that an equal volume of gases under the same temperature and pressure contain a new number of atoms.
But soon it was clear through several experiments, that this contradicts the very essence of Dalton’s theory – the indivisibility of atoms.
It was the Italian scientist Amadeo Avogadro (1811), who distinguished between two kinds of ultimate particles of matter-atom and molecule.
He recognized that an atom is the smallest particle of an element, which participates in a chemical change, and a molecule is the smallest particle of a substance (element or compound) that can exist in a free state.
Read And Learn More WBBSE Notes For Class 8 School Science
Model of an Atom
Atoms are made up of three types of sub-atomic particles – neutron, proton, and electron. Both proton and neutron are sub-atomic particles with unit weight, but a proton has a unit positive charge and a neutron is electrically neutral (i.e., it is uncharged).
Electrons are relatively much lighter – about 1/1836 the weight of a proton. Each electron is negatively charged.
Electron was the first sub-atomic particle that was discovered.
By studying the deflection of cathode rays in an electric and magnetic field, Sir J. J. Thomson (1897) realized that cathode rays are nothing but a stream of negatively charged particles.
American scientist George Stoney termed these particles electrons. Since an atom is uncharged, the discovery of negatively charged particles within an atom prompted the search for positively charged sub-atomic particles which balance the negative charges due to electrons.
Rutherford in the year 1913 established experimentally the presence of positively charged particles within an atom. Later (in 1920) he named these particles protons.
A proton has a charge which is equal in magnitude but opposite in sign to that of an electron. The neutron – another important sub-atomic particle was discovered in 1932 by James Chadwick (during an experiment where a beryllium target was bombarded with an alpha particle).
Elements and Compounds Class 8 summary
Neutron has almost the same mass as that of a proton (It is slightly heavier than a proton) and it is an uncharged particle present within the atom.
But the question is how these sub-atomic particles are arranged within an atom. In the early years of the twentieth century, attempts were made to obtain a physical picture of the atom by several scientists.
To get an idea about the structure of an atom, New Zealand-born physicist Ernest Rutherford did some experiments (for example alpha-particle scattering experiment from thin gold foil) and on the basis of this he arrived at the following conclusions:
1. Most of the space occupied by an atom is practically empty
2. Nearly all mass of the atom is concentrated in a very small, dense, centrally located portion within it. Rutherford named this central part of the atom the nucleus.
3. The nucleus contains the entire positive charge of an atom.
4. The electrons revolve around the nucleus in circular orbits at various distances at very high speeds in order to counterbalance the electrostatic force of attraction between a positive charge and electrons.
5. The structure of an atom as proposed by Rutherford is known as Rutherford’s Atomic Model, also called the planetary model of the atom.
This model was later modified by Niels Bohr (1885 – 1962) [Nobel Prize winner in Physics in 1922], the Danish physicist, who is regarded as one of the foremost physicists of the 20th century.
[It is worth mentioning that Niels Bohr did his post-doctoral research in England with Rutherford at the University of Manchester].
Several scientists also contributed to the understanding of the structure of an atom. To summarize all these findings,
We can conclude that the nucleus, consisting of neutrons and protons, makes up most of the weight of an atom and carries all the positive charges of the atom.
These positive charges are exactly balanced by negatively charged electrons moving around the centrally located nucleus in different orbits like planets moving around the sun. Protons and neutrons are collectively named nucleons.
We have learned that “like” charges repel each other. If that is the case then positively charged protons within the nucleus must repel each other with great repulsive force and this can destabilize the structure of the nucleus.
But a strong attractive force exists within the nucleus, called nuclear force, which strongly holds neutrons and protons together within the nucleus.
This nuclear force overcomes the repulsive forces existing between the positively charged protons. This force has such a great magnitude that to separate the nucleons (i.e., protons and neutrons) very high energy would be required.
According to the Bohr – Rutherford model of an atom, the electrons revolve around the nucleus in different orbits, just like the planets moving around the sun.
The major difference is that each orbit can accommodate more than one electron. The negatively charged electrons in an atom do not fall to the nucleus containing positively charged protons.
The first orbit (nearest from the nucleus) can accommodate at most 2 electrons. The second orbit can accommodate at most 8 electrons and the third orbit can accommodate at most 18 electrons, and so on.
Chemical reactions and structure of matter for Class 8
The first orbit (nearest to the nucleus) is denoted as K shell, the second orbit is called L shell, the third orbit is called M shell, and so on. The maximum number of electrons that can be accommodated in each shell is 2n2.
Here, “n” represents a number; for the first orbit, n = 1, for the second orbit n = 2, and so on. n is called Principal Quantum Number.
The energy of different orbits increases with increasing Principal quantum number. The electron in each shell has a definite energy.
The energy of an electron remains constant as long as it revolves in a particular shell. An electron moves from a lower energy level to a higher energy level when it absorbs energy from an external source. An electron gives out energy while jumping from a high energy level to a low energy level
Shell Maximum number of electrons that can be accommodated in the shell
- K-shell (n = 1) 2n² = 2×12 = 2
- L-shell (n = 2) 2n²= 2×22 = 8
- M —shell (n = 3) 2n² = 2 x 3² = 18
Of course, a shell may contain less number of electrons than that shown here. Also, the outermost shell will never contain more than 8 electrons.
We have already mentioned that the nucleus occupies a very small part of an atom. In fact, Rutherford’s experiments revealed that only a very small part of the atom is occupied by the nucleus which contains almost the whole mass of an atom.
If we could magnify a nucleus to the level of a marble of diameter 1 cm, the atom has to be enlarged to a sphere of diameter 1 kilometer.
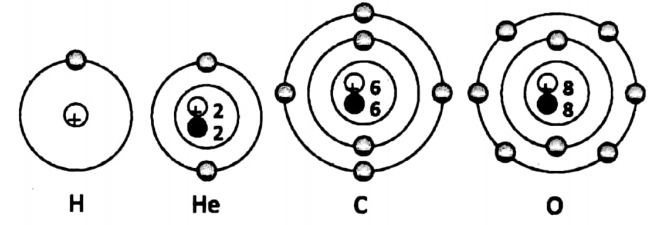
With these things in mind, we are now in a position to diagrammatically represent the following four atoms – a hydrogen atom, a helium atom, a carbon atom, and an oxygen atom.
The hydrogen atom is unique in the sense that it is the only atom that has no neutron in its nucleus. It has one proton in the nucleus. One electron is revolving around the nucleus.
(K-shell has 1 electron). Helium atom has one proton and one neutron in the nucleus. Two electrons are present in the first orbit and are revolving around the nucleus. (K-shell has two electrons).
Understanding structure of matter for Class 8
The carbon atom has six protons and six neutrons in the nucleus. A total of six electrons are revolving around the nucleus.
Among them, two electrons are present in the first orbit (i.e. K-shell) and four electrons are present in the second orbit (i.e. L-shell).
The oxygen atom has eight protons and eight neutrons in the nucleus. A total of eight electrons are revolving around the nucleus.
Among them, two electrons are present in the first orbit (i.e. K- shell) and six electrons are present in the second orbit (i.e. L-shell).
The total number of protons in the nucleus of an atom is called the atomic number of the atom. The atomic number is represented by the letter Z.
The total number of protons and neutrons in the nucleus of an atom is called its mass number. The mass number is represented by the letter A.
Since an atom is electrically neutral as a whole, it is clear that the total number of protons in an atom is equal to the total number of electrons present in that atom.
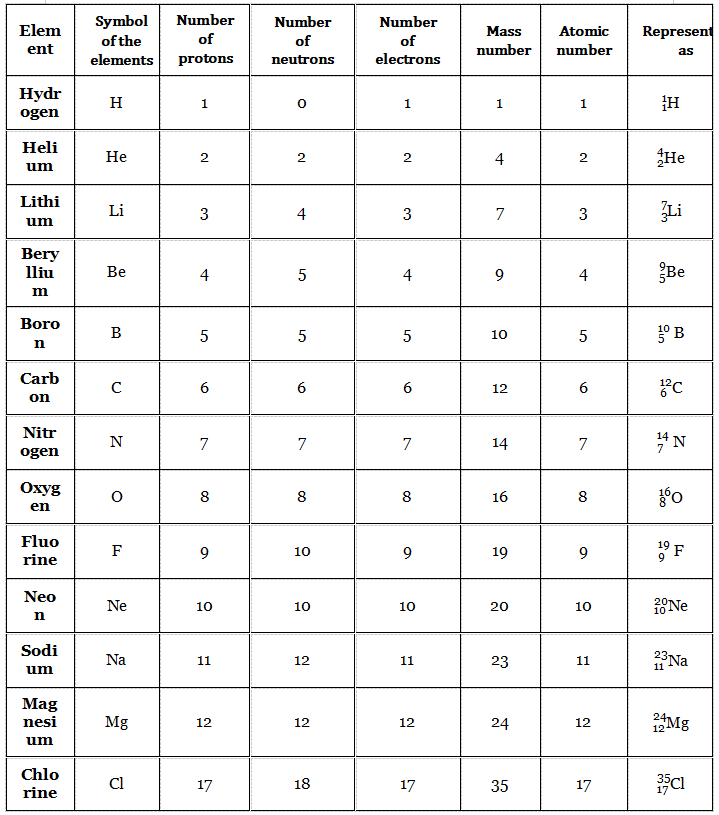
Each different element is represented by a symbol. For example, the symbol of hydrogen is “H”, that of helium is “He”, the symbol of carbon is “C”, and the symbol of oxygen is “O” and so on.
Let the symbol of an element be “X” (Remember that there is no such element represented by “X”, it is just a hypothetical assumption). The element is represented as follows.
Mass. number x Atomic. number (Z)
In this representation, with respect to the symbol of the element, its mass number is written as left superscript and its atomic number is written as a left subscript.
For example, a carbon atom with 6 protons and 6 neutrons (which means mass number = 6 + 6 = 12; atomic number = 6) is represented as.
More Examples Are Given In The Following Table.
Different Types Of Atom
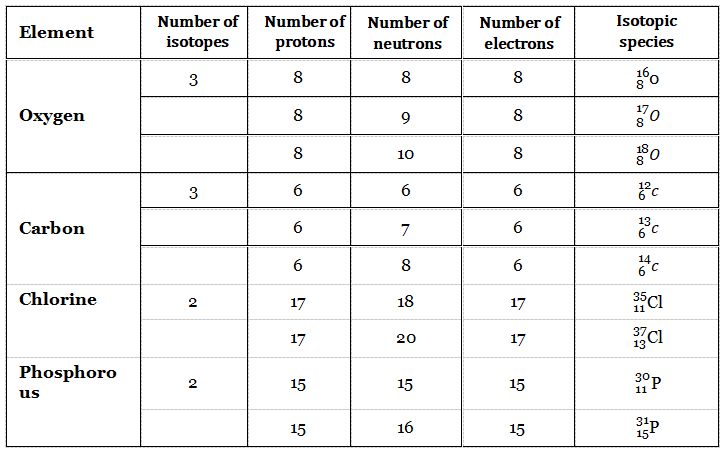
Atoms of the same elements (having the same atomic number) having different numbers of neutrons (i.e., having different mass numbers) are called isotopes.
So two isotopes have the same number of protons (i.e., the same atomic number) but different numbers of neutrons. For example, hydrogen ( \H ), deuterium ( \H ), and tritium ( \H ) are isotopes.
Apart from hydrogen, oxygen, carbon, and chlorine have isotopes.
Properties of matter for Class 8 students
The chemical properties of an element are regulated by the electronic configuration of the atom, particularly by the number of extranuclear electrons (in the outermost shell). So isotopes of an element have similar chemical properties.
Isobars
Atoms of different elements that have the same mass number but a different atomic number are called isobars. This means that in the case of isobars, the number of protons and the number of neutrons are different, but the total number of neutrons and protons is the same. Examples of isobars are latex-
Isotone
Atoms of different elements having the same number of neutrons in the nucleus are called isotones. In the case of isotones, the number of protons in the nucleus or the atomic number is different. They also have different mass numbers. Examples of isotones are In all three atoms the number of neutrons is 16.
Structure of Matter
All the substances or matter that we see around us are nothing but the aggregation of atoms and molecules. A very minute mass of a substance is composed of a very large number of atoms and molecules. For example, 1 milligram of gold contains about 3 x 1018 atoms.
We know that the various kinds of substances can be divided mainly into three categories – gas, liquid, and solid. These are called the three states of matter.
All substances are made up of atoms or molecules. Then one may wonder how some of the substances are gaseous, while some are liquids and others are solids.
So, one can guess that there must be differences in the arrangement of atoms or molecules in three different states of matter.
1. Gaseous state
In a gas, the molecules move at a very large speed and move randomly. The gas molecules possess translatory, rotatory, and vibratory motions in all directions.
During this random, chaotic movement, they collide with each other and also with the walls of the container in which they are kept. The average distance between the molecules is quite large compared to liquids and solids.
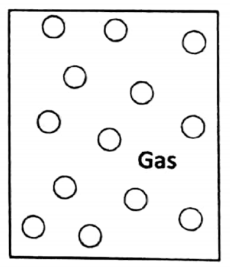
The force of attraction between the atoms or molecules in a gas is very small. Due to this random movement of molecules, gas tends to fill up completely any available space. So they have no finite volume (or shape).
By applying pressure, the volume of a gas can be easily reduced. So gases are easily compressible. The density of a gas is generally the lowest among the three states.
The random movement of molecules in gas can be easily understood from the following experiment. If we open a small bottle of perfume in one corner of the room, we can smell it from the other side of the room almost immediately.
This is because perfumes are generally volatile liquids. Once the bottle is opened, some of the liquid is vaporized (i.e., converted to a gaseous state).
The molecules in the gaseous state move randomly with great speed. So almost immediately after opening the bottle of perfume, we can smell it from any corner of the room.
2. Liquid state
Compared to a gaseous state, in a liquid state, the molecules are closer to one another- The force attraction between the molecules is greater compared to that in the gaseous state.
The molecules can still move but their movements are somewhat restricted. They can rotate, vibrate and move over a small distance.
The translatory motion is either sideways or downwards. Consequently, liquids also do not have definite shapes, but due to greater interaction between the molecules, they have definite volumes. Liquids are much dense and much less compressible compared to gases.
3. Solid state
The attraction between the atoms or molecules is very high compared to liquids and gases. As a result, the position of atoms or molecules remains fixed with respect to one another.
So atoms or molecules remain in a relatively ordered state. The atoms or molecules within a solid have no mobility (i.e., cannot move from one place to another) and cannot rotate.
Each atom or molecule can only vibrate about its mean position. Solids, in general, have definite shapes and volumes and can have any number of free surfaces. The density of a solid is the highest among the three states of matter.
Solids can be further classified into two categories – amorphous and crystalline. In crystalline solids, atoms or molecules remain in a highly ordered fashion and they are characterized by sharp melting points. But amorphous solids lack such highly ordered arrangement and melts over a range of temperature.
We have observed that when heated, ice melts to form liquid water and if water is heated it starts boiling and forms vapor.
Similarly, on cooling the vapor condenses to liquid, and on further cooling the liquid freezes to solid.
This observation suggests that change of state can be effected by supplying (or extracting) heat (i.e., by increasing or decreasing temperature).
So we may wonder how a change in temperature can bring about a change in state. Actually, in a solid, the force of attraction between the atoms or molecules is very great.
Each of them remains fixed at a particular position. They can neither rotate nor move from one place to another. They can only vibrate about their mean positions.
As the temperature is increased due to the supply of heat energy, the kinetic energy of vibration gradually increases and beyond a certain temperature, the vibration of molecules becomes so energetic that the force of attraction between the atoms or molecules significantly decreases.
The atoms or molecules become free from their fixed positions and the solid is transformed into a liquid state. As more and more heat energy is supplied to a liquid, its temperature increases gradually and the mobility of the atoms or molecules further increases.
The average distance between the atoms or molecules also increases and beyond a certain temperature, the mobility becomes so high that the average distance between the atoms or molecules becomes very high and the atoms or molecules become completely free. Then the liquid is transformed into a gaseous state.
Plasma
We have mentioned that generally there are three states of matter. Plasma is the fourth fundamental state of matter. The term “plasma” was first coined by Irving Langmuir.
It has properties unlike those of the other states. Plasma may be produced by heating a gas to an extremely high temperature.
Due to the heating of gas at very high temperatures, vigorous collisions between atoms and molecules take place as a result of which electrons are ripped off yielding electrons and ions.
So, plasma is an electrically conducting medium. Like the gaseous state, plasma does not have a definite shape or a definite
volume.
Plasma is the most abundant form of matter in the universe, most of which is present in intergalactic regions and in stars including the sun.
The center or core of the sun is extremely hot (approximately 107 °C). At this extremely high temperature, matter can exist only in the form of plasma.

Ionic Compounds And Covalent Compounds
So far we have mentioned that substances are made up of atoms and molecules. But there are many substances that are actually aggregates of ions.
Common salt (sodium chloride, NaCI), ammonium chloride, potassium chloride, etc., are only a few examples. Ions are produced from atoms when it accepts or rejects electron(s).
The electrons in the distant shells away from the nucleus are attracted by the protons of the nucleus with a weaker force than that acting on the electrons in the shells closer to the nucleus.
So, a certain small amount of energy may detach one or more electrons from the outer shells and in that case, the atom which was initially electrically neutral will be positively charged or it will be a positive ion or cation.
Again if somehow an atom captures one or more electrons it will be a negative ion or anion. Thus cations are formed due to the loss of electrons and anions are formed due to the gain of electrons.
Substances, made up of ions, are called ionic compounds. But there is another kind of substance – known as covalent compounds. In such cases, different atoms share electron(s) with another atom (s) to form a compound.
Ionic Compounds
Sodium chloride (NaCI) is an example of an ionic compound. It is made up of Na+ ions and Cl” ions. The biggest experimental proof that the solid NaCI contains ions is that fused or molten NaCI conducts electricity.
To conduct electricity, there must be some charged particles in the molten or fused state. In a metal, which is a good conductor of electricity, electrons are charged particles. But experiments have proved that in the case of NaCI, it is the ions (i.e., positively charged Na+ ions and negatively charged Cl” ions)
which are responsible for the conduction of electricity. The aqueous solution of NaCI also is a good conductor and here also Na+ and Cl” are the carriers of electricity.
NaCI can be formed by the reaction between solid sodium metal and chlorine gas. \(2 \mathrm{Na} \text { (solid) }+\mathrm{Cl}_2 \text { (gas) } \rightarrow 2 \mathrm{NaCl} \text { (solid) }\)
1. Alternatively, we can think of the formation of NaCI, consisting of the following steps
Na atom loses one electron to form Na+ cations; the Cl atom (formed from the Cl2 molecule) accepts one electron to form Cl” anions.
An equal number of Na+ ions and Cl” ions then combine to form solid sodium chloride. In NaCI crystal, Na+, and Cl” ions exist at every alternate position (along any direction), as
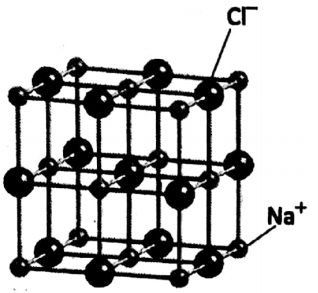
Na has 11 protons, 12 neutrons and 11 electrons. It can lose one electron to produce a positively charged Na+ ion or cation (containing 10 electrons).
Cl atom has 17 protons, 18 neutrons and 17 electrons. It can accept one electron to form a negatively charged Cl” ion or anion (containing 18 electrons).
Two ions are strongly held together by strong electrostatic force. Solid NaCI is a solid compound consisting of a large number of cations and anions. For every Na+, there is one Cl’ ion.
\(\begin{aligned}& \mathrm{Na}-\mathrm{e} \rightarrow \mathrm{Na}^{+} \\
& \mathrm{Cl}+\mathrm{e} \rightarrow \mathrm{Cl}^{-}
\end{aligned} \longrightarrow \mathrm{Na}^{+} \mathrm{Cl}^{-} \text {or } \mathrm{NaCl}\)
So, overall NaCI crystal has no net charge. In fact, in all ionic compounds, the total number of positive charges on cations must be equal to the total negative charge on the anions. Thus all the ionic compounds are charge neutral (i.e., no net charge).
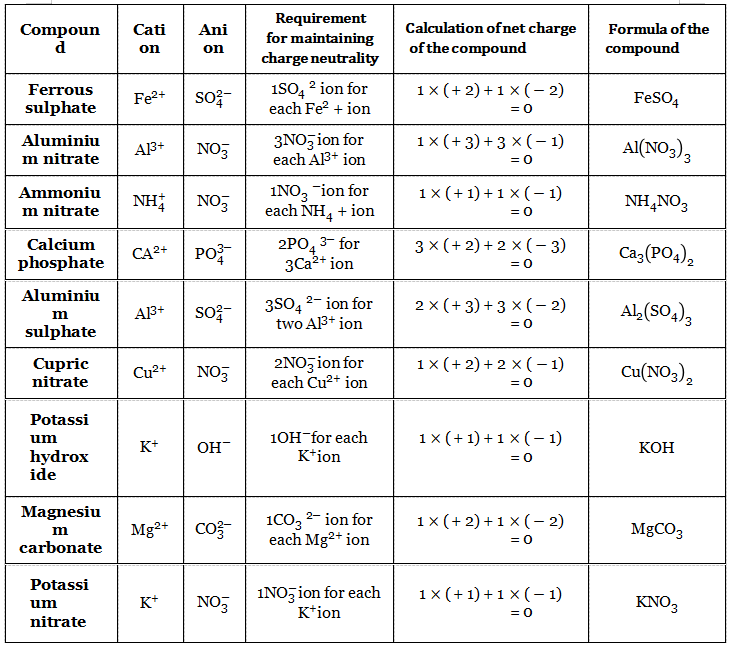
2. We can give some more examples in the following table
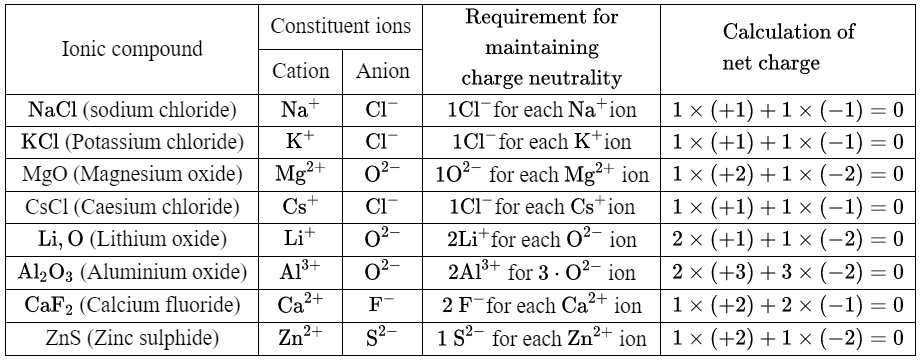
In the above table, there are some metal atoms that can lose only one electron to form a cation (such as Na, K, Cs, Li) and there are some metal atoms that can lose more than one electron to form a cation {such as Mg (loses 2 electrons), Zn (loses 2 electrons), Al (loses 3 electrons), Ca (loses 2 electrons)}.
These metals can form only one type of cation. The metal atom gives up the electron(s) to have 8 electrons in its outermost shell. The number of electrons (s) given up by a metal atom is called its valency. So the valency of U is 1, Na is 1, K is 1, Zn is 2, etc.
3. But there are some metal atoms, each of which can form more than one type of cation by losing a different number of electrons For example, a Fe atom can lose two electrons to form Fe2+ ions, called ferrous ions.
When the same Fe atom loses three electrons, it forms a Fe3+ion called a ferric ion. Among these two ions, the ion with lower charge is represented with the “ous” suffix (for example, the Fe2+ ion is called a ferrous ion), and the ion with higher charge is represented with ic” suffix (for example, Fe3+ is called “ferric” ion). The element Fe is said to have variable valency.
Some more examples of elements having variable valency are given below.

Similarly, a non-metal usually accepts electron(s) to form an anion. By gaining the requisite number of electron(s) an atom attains a structure where 8 electrons (or 2 electrons in the case of H only) exist in the outermost shell.
For example, the Cl atom gains one electron to form Cl” ion. So its valency is 1. Similarly, the valency of F, Cl, Br, I is 1.
Now, let us get back to the compound NaCI. We have mentioned that solid sodium chloride consists of Na+ and Cl- ions. The question one may ask is why the sodium atom is losing one electron and the chlorine atom is gaining an electron.
Actually, during the formation of a compound, each atom attains the electronic configuration of inert gas. Helium, neon, argon, etc., are examples of inert gases.
Helium (atomic number 2) has 2 electrons in its outermost shell. All other gases have 8 electrons in their outermost shells.
Structure of matter examples for Class 8
Neon (atomic number 10) has 2 electrons in K-shell and 8 electrons in L-shell. Argon (atomic number 18) has 2 electrons in K-shell and 8 electrons in L-shell and 8 electrons in M-shell.
The electronic configuration observed in inert gases is stable, and during the formation of compounds or during a chemical reaction, atoms try to attain this stable configuration.
When the Na atom (atomic number 11) loses an electron, 10 electrons are left – 2 in K-shell and 8 in L-shell. This is the electronic configuration of neon (an inert gas).
Again, when the chlorine atom (atomic number 17) gains an electron, it has 18 electrons – 2 in K-shell, 8 in L-shell, and 8 in M- shell.
This is the electronic configuration of argon. So, in sodium chloride, the electronic configuration of both the cation and the anion is similar to that of an inert gas. Na+ and Cl are held together strongly by electrostatic force.
4. The general characteristics of an ionic compound can be summarized as follows :
- In the case of ionic compounds, molecules do not exist. In solid-state, cations and anions are properly arranged in a definite pattern.
- In ionic compounds, the total charges on cations will be the same as the total charges on anions.
- Ionic compounds are generally soluble in water.
- An aqueous solution of ionic compounds which are soluble in water conducts electricity.
- The melting point and boiling point of ionic compounds are totally high.
Radicals
Sometimes, a group of atoms consisting of different elements collectively behave as a single entity during a chemical reaction. Such a group of atoms is known as a radical.
Since a radical behaves like a single entity during a chemical reaction, it must have a definite valency. For example, ammonium ion (NH4+) is formed by one nitrogen atom and four hydrogen atoms.
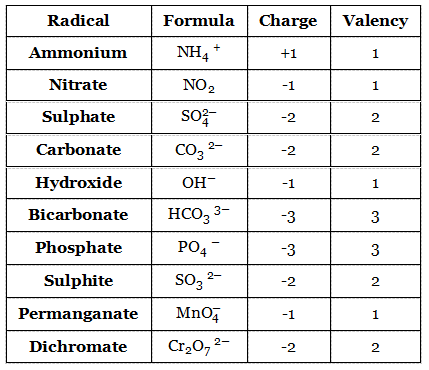
The net charge of this radical is +1. As an approximation, the charge of a radical may be taken as its valency. This means the valency of NH4+ is 1. Examples of some other radicals are given below:
Atoms of elements combine to form Let us construct the formula of some molecules. The number of atoms of each element compound using the above information, present in a particular molecule is definite. let us construct a formula for some compounds using the above information.
We should mention here that in ionic compounds no molecules exist. For example, in solid sodium chloride, there are only properly arranged Na+ and Cl’ ions; no entity like “NaCI” exists in it.
When dissolved in water or in a molten state, these ions come apart readily and move chloride. So, basically what we are writing is the number of cations per anion and in this way, we are representing the ionic compound.
Covalent Compound
Ionic compounds are made up of ions. Molecules do not exist there. But there is another class of compounds which are known as covalent compounds.
Unlike ionic compounds, here the presence of molecules has been experimentally established. Water is the most common example of a covalent compound. Some elemental gases such as H2, Cl2, etc., are also covalent.
Let us consider a diatomic covalent compound such as H2, Cl2, etc. Here, two atoms unite to form a molecule. No electron transfer from one atom to the other occurs here.
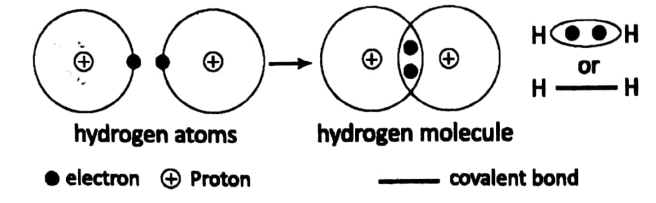
But atoms share one electron each to form a pair of electrons. This pair of electrons becomes joint property, so as to say, of the two atoms.
For example, H2 is a diatomic molecule formed by two H-atoms. Each atom has 1 electron. The valency of the H-atom is 1. Each H-atom shares its only electron with the other atom.
Thus an electron pair is formed. This pair of electrons holds the two H-atom by a chemical force – which is called a covalent bond.
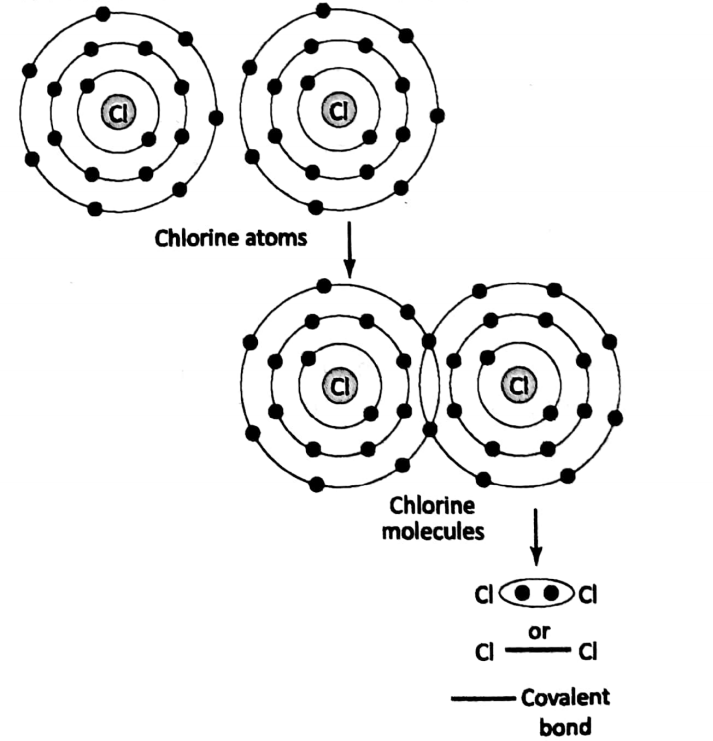
The covalent bond is usually represented by “• •” or Let us consider the case of Cl2. Each chlorine atom has a total of 17 electrons.
Nature of matter in chemistry for Class 8
out of these 17 electrons, 7 electrons are in the outermost shell. The valence of chlorine is 1. Each chlorine atom shares one of its electrons from its outermost shell with the other chlorine atom, thus forming a covalent bond.
Thus chlorine molecule is formed. When more than two atoms of two or more elements combine to form a covalent compound, then the atom of the element having the highest valency is usually considered a “central atom”.
It is surrounded by other atoms. The valency of the surrounding atoms is not the same. More than one covalent bond is formed, one each for each pair of electrons shared between the central atom and another surrounding atom.
\(\mathrm{H}_2 \mathrm{O}, \mathrm{H}_2 \mathrm{~S}, \mathrm{NH}_3, \mathrm{CH}_4, \mathrm{PCl}_3,\) etc., are all covalent compounds.
Let us consider the case of H,0 molecules. The valency of oxygen is 2 and the valency of hydrogen is 1. So, oxygen having the highest valency is considered a central atom in water molecules.
The oxygen atom has a total of 8 electrons, out of which 6 electrons are at the outermost shell. 2 electrons are shared with two electrons of two hydrogen atoms (one each from each hydrogen atom).
Thus two covalent bonds are formed. (In the H20 molecule, oxygen has effectively 8 electrons in its outermost shell).
Some more examples are given below in tabular form.
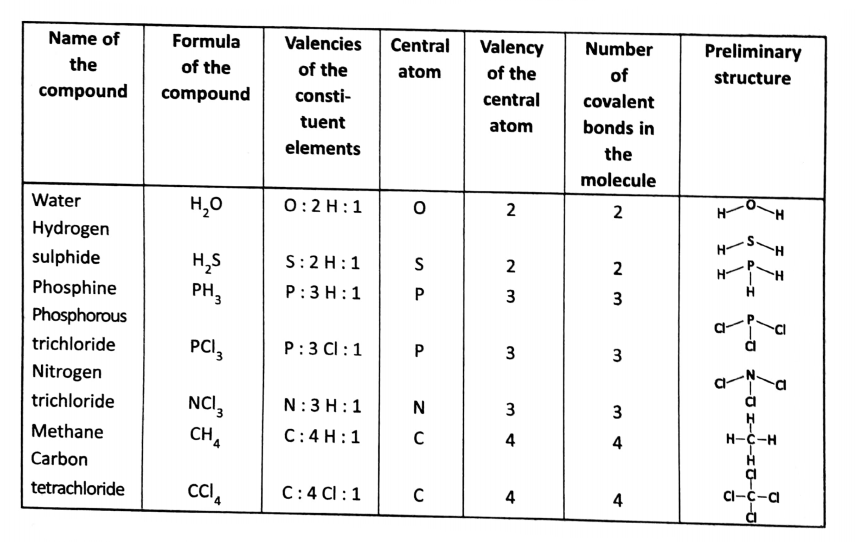
[We must mention here that using this concept, only the preliminary structure of a molecule can be predicted. But for the actual three-dimensional structure of a molecule, we need more advanced scientific theory.]
- The General Characteristics Of Covalent Compounds Can Be Summarized As Follows:
- In covalent compounds, molecules exist.
- The melting point and boiling point of covalent compounds are usually low.
- Covalent compounds are generally soluble in organic solvents and usually insoluble in water.
- These compounds usually exist as gas or liquid under normal temperatures and pressure.
