Chapter 2 Phenomena Around Us
Changes are acts or processes through which something becomes different. In nature, various changes are taking place continuously.
Even if we do not notice, changes are occurring on a regular basis.
Changes in the weather, drying of clothes, cooking of rice and vegetables, formation of curd or paneer from milk, electric light and fan being switched on or off, burning of wood or kerosene oil, etc. are only a few instances. Some of the changes are taking place around us on their own, while some of the changes are occurring due to our activities.
For example, in our body, hairs and nails grow on their own. As we eat food, they are digested in our stomach. As you grow up, you slowly become taller and gain weight.
All these processes occur “naturally”. You cut a piece of paper with a pair of scissors or you draw a picture in your drawing book. Your mother cooks food in the kitchen.
All these changes occur because we “do” it.
So, you can very well understand that not all the changes are of the same type.
Read And Learn More: WBBSE Notes For Class 6 School Science
Let us systematically study these changes. As a first step, we need to group them. If we find similarities, then we can classify such changes in a particular group.
This will help us to understand the process changes better. Classification of changes into groups can be done in more than one way.
All changes can be categorized into one of the following ways
- Reversible and Irreversible changes
- Periodic and Non-periodic changes
- Desirable and undesirable changes
- Natural and Man-made changes
- Slow and Fast changes
- Physical and Chemical changes Let us illustrate changes in some detail.
Reversible And Irreversible Changes
Let us take some water in a plastic container. Keep it inside the freezer compartment of a refrigerator. Close the door of the refrigerator and wait for some time.
Now open the door and you will find that all the water has been transformed into ice. Now take out the plastic container containing ice, and keep it on the table in your room.
Again wait for some time. You will find that the ice has melted and has been transformed into water.
Let Us Consider Another Two Examples.
- Take some amount of wax on a steel spoon with a plastic handle. Now carefully heat it by keeping it over a flame.
- The solid wax will melt and form hot molten wax. Now keep the spoon away from the flame. The hot wax slowly cools down and the melted wax again solidifies.
- Take a balloon and blow it carefully so that it does not burst. The shape and size of the balloon change.
- Now allow the air to escape from the balloon. The deflated balloon regains its original size and shape.

The above three examples are such types of changes that are called Reversible Changes. But not all the changes can be reversed.
Let us take a balloon. Blow it hard. It grows bigger and bigger and ultimately it bursts. You know very well that there is no way you can get the balloon back to its original shape and size.
Consider Two More Examples :
- Take a candle. It is made of wax. If you light the candle, some wax will melt. As the candle continues to burn, its length goes on decreasing.
- After some time, almost all the wax will “disappear”. The portion of a candle which has been burnt can never be regained.
- Take a raw egg. Keep it in water and then boil it very well for some time. The egg is now boiled.
- Again you know that it is not at all possible to transform the boiled egg into a raw egg by any means.
Hence from the above examples, you can understand that there are some changes which
Changes cannot be reversed by any means. Such changes are called Irreversible Changes.
So you can say that these changes are permanent.
Once it occurs, the substances undergoing it can never go back to their original/initial conditions.
The changes that can be reversed whereby the substances undergoing them can return to their previous states after the changes are complete are termed Reversible Changes.
The changes which cannot be reversed whereby the substances undergoing them can not go back to the previous states after the changes are complete are termed Irreversible Changes.
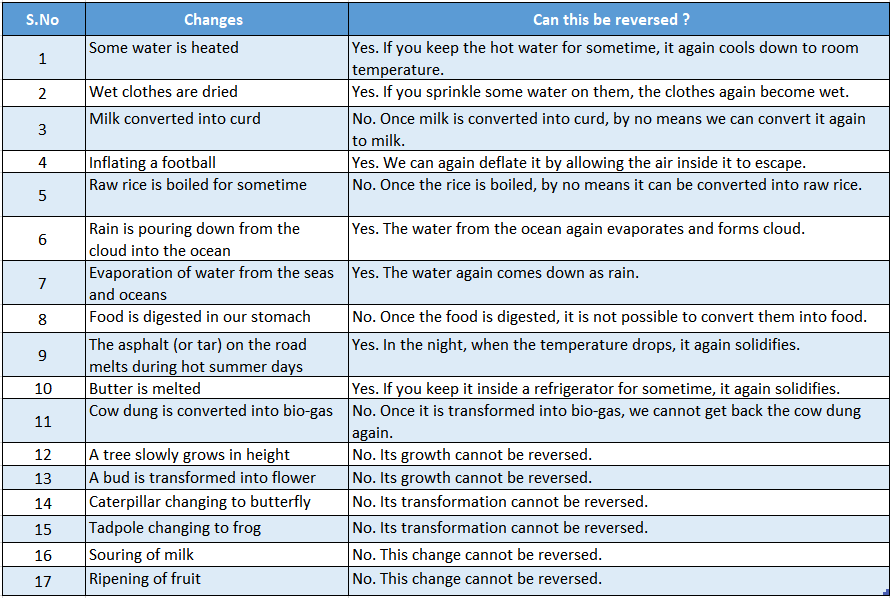
We have listed some common changes and categorized them as reversible or irreversible changes.
WBBSE Class 6 Changes Around Us Notes
Hence, you can see that all the changes, happening around us can be categorized either as a reversible process or as an irreversible process.
Think about some more examples and try to classify them as reversible/irreversible.
Periodic And Non-Periodic Changes
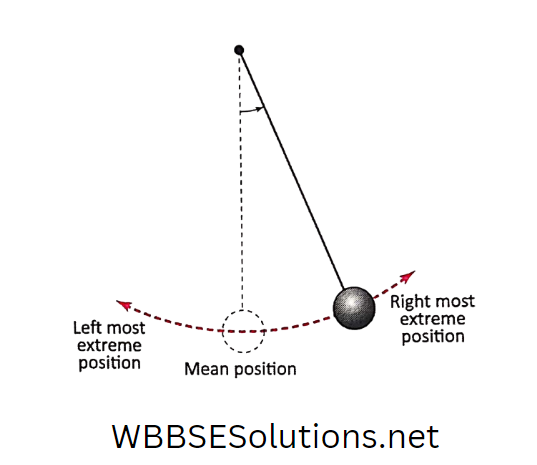
You must have seen a pendulum clock. It is basically a clock with a pendulum. A pendulum resembles a metal ball (called the bob) tied at the end of a thread.
If you hold the other end of the thread and push the ball slightly in any direction, the ball will swing up to a certain distance.
Then it will stop momentarily and then start swinging in the opposite. direction. Again, after traversing some distance it will momentarily stop and will reverse its direction of motion.
Unless stopped, this pendulum continues to move to and for some time.
Thus, after a certain period of time, the pendulum repeats its motion (between leftmost and rightmost extreme positions through mean position), again and again.
Every one of us has seen a clock. The hands of the clock rotate continuously. The bigger hand counting minutes comes back to the same position after every hour.
The smaller hand counting hours comes back to the same position after every 12 hours. Another hand, counting seconds, comes back to the same position after every 60 seconds.
We have all seen that day changes into night and night changes into day. The monsoon season or rainy season (or any other season) comes back after every year.
So all the above changes are repeated after a fixed time interval. These changes are called Periodic Changes.
But not all the changes are periodic. For example, during the rainy season sometimes there occurs flood in some places. But floods do not occur periodically.

This means floods do not occur regularly in the same place every year. In the year 2004, a tsunami struck some coastal cities of India like Chennai. But this type of natural disaster never occurs after a fixed interval of time.
The same is true for storms, cyclones or landslides mud-slides. All of these are examples of a type of change called non-periodic changes.
The changes which occur again and again after a fixed interval of time are called periodic changes.
The changes which do not take place after a regular or fixed interval of time are called non-periodic changes.
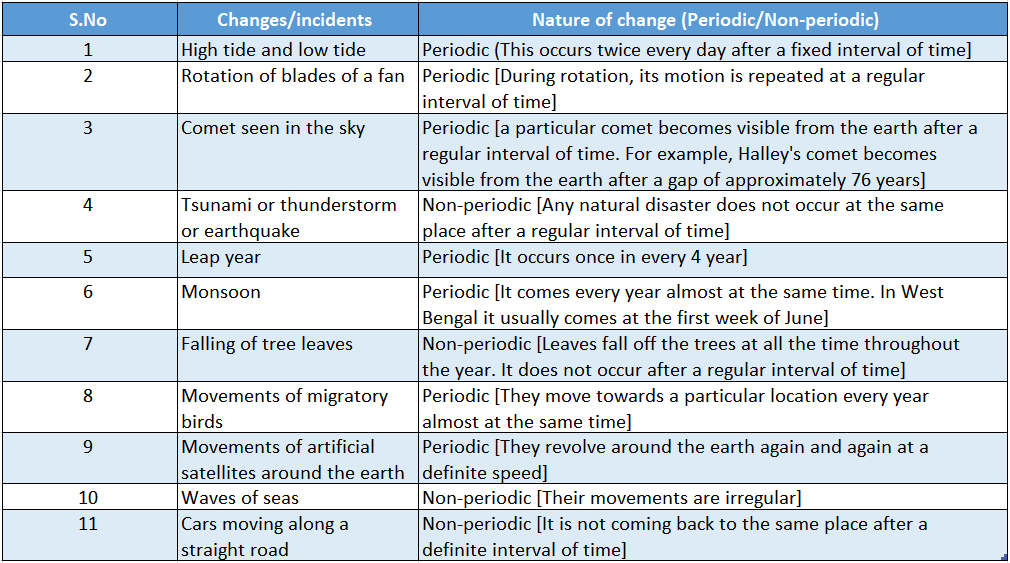
Below in we have listed some changes and categorized them as either periodic or non-periodic changes with short justification.

Desirable And Undesirable Changes
Changes occurring around us can also be classified as desirable changes and undesirable changes.
For example, if we cut trees unnecessarily, then it is an undesirable change. This is because this leads to deforestation, which can harm Mother Nature.
On the other hand, when a tree grows, then it is a desirable process. When flowers blossom then that also is a desirable change.
Let Us Study Some More Examples.
1. If we throw away the garbage and waste materials on the streets then it is definitely an undesirable change because this can create health hazards.
But if we throw the garbage and waste materials into the garbage bin or litter bin, then it is a desirable process, because this keeps the locality clean and beautiful.
2. If an automobile emits black smoke while moving, then it is an undesirable change. Black smoke creates air pollution.
3. During some functions or social gatherings if someone plays music very loudly using loudspeakers, then certainly that is an undesirable phenomenon.
This creates noise pollution. Playing music very loudly in a densely populated locality can harm old people and those people suffering from heart ailments.
It also disturbs the students from concentrating on their studies.
4. If cow dung is converted into bio-gas, then it is a desirable change. Biogas can be used as fuel and this fuel is environmentally friendly.
5. A thunderstorm or an earthquake is not at all desirable. These natural calamities bring devastation in the form of loss of lives and properties.
Hence, undesirable changes are those which harm nature or affect mankind adversely. Desirable changes are those which do not harm nature or mankind.
Natural And Man-Made Changes
Changes can also be classified as natural changes and man-made changes. For example, any natural calamity such as thunderstorms, earthquakes, floods, etc. are all natural changes.
We cannot control them. Those phenomena which occur on their own or “naturally” and are not controlled by human beings are called natural changes.
But we should also keep in mind that some of the above-mentioned natural processes can also occur due to severe and prolonged detrimental activities of man.
It is easily understood that activities or processes like agriculture, the industrial revolution, and progress in trade & commerce, all major forms of pollution are man-made phenomena.
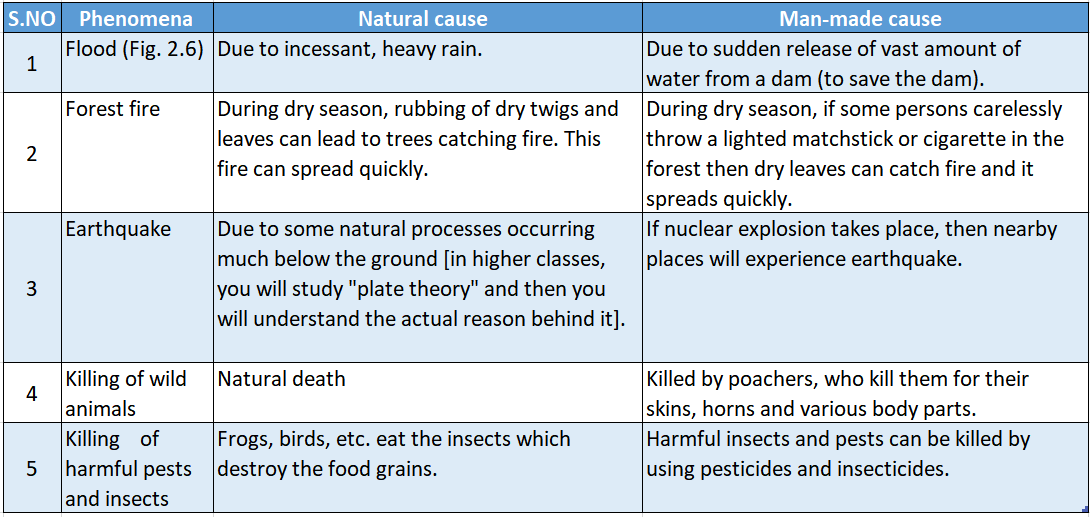
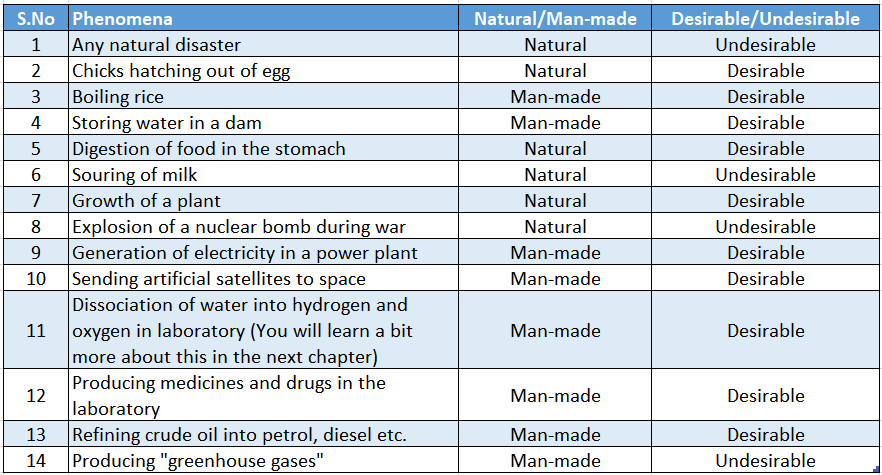
Below, in we have listed some phenomena which can occur both naturally and also by the activities of men.
Phenomena which occur due to the activities of men are called man-made phenomena. These phenomena are initiated by men.
Man-made activities and natural activities can be either desirable or undesirable.
Below, we have listed some phenomena and categorized them both as natural man-made and desirable undesirable changes.

Understanding Physical and Chemical Changes
In the above table, we have described processes which are natural but can be either desirable or undesirable. The same is true for man-made processes also.
We will now briefly discuss a man-made process which can also harm nature if not properly controlled.
Fertilizers, Pesticides And Insecticides
For the growth of plants, they need food and nutrients. These are absorbed by the plants from the soil.
But in various parts of the world, the amount of different nutrients present naturally in the soil varies.
Some are present in higher amounts while some may be present in smaller amounts.
So depending upon the nature of the soil, we have to apply such “food and nutrients” externally, into the soil for balanced growth of the plants. These are called fertilizers.
With the progress of time as the human population started increasing rapidly, they needed more food.
Cultivation of vast amounts of food grains within a short span of time was not possible naturally.
So scientists developed artificial fertilizers in the laboratory and applied them to the plants for enhancing their growth. That way the production of food grains was increased.
Some commonly used fertilizers are urea, ammonium nitrate, diammonium phosphate, ammonium sulphate, superphosphate (or calcium hydrogen phosphate) etc.
But another thing which has to be kept in mind is that fertilizers alone are not enough to guarantee the enhanced production of food grains.
There are several pests and insects which destroy the food grains.
Earlier farmers used to depend on other animals such as frogs and birds to get rid of such harmful pests and insects.
But a significant part of the food grains was lost due to this dependence.
With the advancement of science and technology, scientists developed some chemicals in the laboratory which are “poison” to those pests and insects.
These “poisons” are called “pesticides” (or pest-killers) and “insecticides” (or insect-killers) which need to be sprayed over the agricultural crops to kill harmful pests.
So it seems that preparing pesticides and insecticides are man-made phenomena and they are desirable. But these pesticides and insecticides have harmful impacts on nature also.
After all, these are poison. These are used to kill living beings. So these must be used carefully and judiciously.
Some common pesticides are BHC (benzene hexachloride), malathion, methyl parathion, heptachlor, etc.
The pesticides are sprayed on the crops. As a result pests and insects staying on those plants die.
Correct and scientific use of these chemicals demands that the pesticides and insecticides must be used in small amounts.
After spraying those chemicals, we are to wait for some days.
These poisons are made in such a way that during this time period, they are destroyed naturally in the presence of sunlight and are converted into harmless or much less harmful chemicals.
Only then do the crops become suitable for consumption.
It is a matter of concern that sometimes sections of farmers spray these poisons in large amounts without knowing the consequences.
Some of them also do not wait till the recommended period of time.
As a result, a substantial part of these poisons is not destroyed naturally and remains with the food grains.
If we eat such contaminated food grains, these pesticides and insecticides directly enter our bodies and remain stored in different human organs such as the liver, kidney, brain, etc. for a long time.
They, therefore, cause damage to such organs and result in diseases like cancer, asthma, mental depression etc.
Besides this, indiscriminate use of pesticides and insecticides can kill other small animals also apart from the pests.
If birds eat those “poisoned” food grains they may die prematurely due to toxicity.
The insecticides may dissolve in water and can ultimately reach the nearby ponds and water bodies.
As a result, the small fish living there may die. If we eat those fishes we indirectly experience the toxic effects of pesticides and insecticides.
Even some man-made fertilizers are also harmful to our bodies when present in large excess food grains.
In the past few decades, nature has been severely harmed by uncontrolled and indiscriminate use of fertilizers and insecticides.
Older people sometimes true that they don’t find small fishes used to be found in the agricultural land during the rainy season in their childhood.
A number of varieties of fish have just disappeared within the last few decades.
Scientists blame the uncontrolled use of pesticides, insecticides and fertilizers for this catastrophic phenomenon.
So we must consciously use man-made chemicals only after assessing the threats they pose to nature and wildlife.
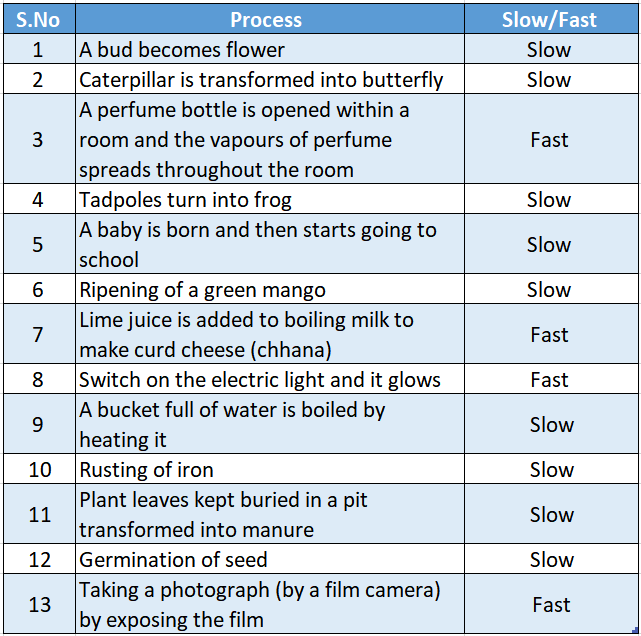
Slow And Fast Processes
We have so far classified different processes as reversible or irreversible, natural or man-made; periodic or non-periodic and desirable or undesirable.
But we have not considered the time it takes for a particular process to occur. If we consider this aspect then we can classify all the changes as either slow or fast processes.
Let Us Illustrate With A Few Examples.
- If you plant a sapling of the mango plant in your garden, then it will take years for the plant to grow up and get mangoes from it. So it is a slow process.
- If you pour some common salt in water and stir it with a spoon, it dissolves fairly quickly in water.
- When you light a candle, it takes a long time to burn completely. So we get light from the candle for a long time.
- If a bomb is exploded, within seconds everything around it is destroyed. So explosion is a very fast process.
- It takes time to dry wet clothes.
- It takes time to boil raw rice or egg.
- It takes time to digest the food materials we consume.
- It takes years to construct a multi-storied building or a bridge across a river.
School Science - If someone pours some acid slowly into the water with stirring, the solution immediately becomes hot.
- The process of making curd from milk is a slow process. During this process, a small amount of curd is added to warm milk. It is stirred well and is then set aside for a few hours at a warm place.
After that milk is converted into curd. So you can see that it is a slow, irreversible process.
Hence, depending on the time required for a process to occur, we can classify the processes as slow or fast processes.
A process occurring within a short time is termed a fast process, while a process taking a long time to occur is called a slow process.
A chemical reaction can also be classified as a slow reaction or a fast reaction. But in some cases, a slow reaction can be made faster by some means.
This can be illustrated by an example.
Activity 1: Take some clear lime water in a glass. Keep it in the open air for a few days. You will see that clear lime water has become milky.
This occurs because lime water reacts with the carbon dioxide gas present in the air and forms an insoluble, solid substance (known as calcium carbonate).
This solid, insoluble substance remains suspended within the solution. So, lime water turns milky.
As you can see that it takes a few days for the above chemical reaction to occur. Definitely, this reaction is a slow process.
It is slow because the concentration of carbon dioxide in the air is very small.
So it requires time to produce a sufficient amount of that insoluble substance to turn the clear lime water milky.
But if we can increase the concentration of carbon dioxide we can speed up this reaction.


Activity 2: Take some clear lime water in a glass. Now with a piece of straw, blow some air into it. Within a few minutes, the lime water turns milky.
This is because the concentration of carbon dioxide is higher in the air we breathe out. So the chemical reaction occurs more quickly.
A chemical reaction can be made faster by other means also.
Activity 3: Let us take the dilute solution of muriatic acid (which is used to clean bathrooms) in two separate glasses.
Now, in one glass drop a piece of marble and in another glass add the same amount (weight) of crushed marble. What will you observe?
In the case of the crushed marble added to the acid solution, a gas will immediately start evolving and the solid particles will dissolve very quickly.
But in the case of a piece of marble, it will take a much longer time to dissolve in the dilute acid solution.
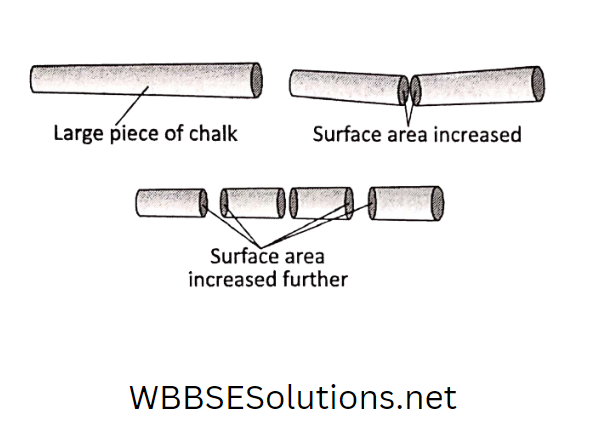
Why did that happen? When a piece of marble is crushed to powder, the total surface area of the marble increases manyfold.
This increased surface area helps the marble to interact quickly with the acid solution.
When we chew the food properly, they are converted into very small pieces.
Since digestion involves a series of complex chemical reactions, hence, within our stomach, they are digested quickly.
So we should chew the food properly. When vegetables are cut into smaller pieces, they can be boiled faster.
When we spray pesticides or fungicides on plants or when we apply body spray or increase in surface area of a solid substance when broken into pieces can be easily understood when you break a piece of chalk.
Each time you break it into pieces, a new surface is generated deodorant spray on our body, the liquid is converted into very fine droplets, possessing a much higher surface area. So they can act faster.
When chemicals are dissolved in a solvent, they become very fine particles and then they can react with one another at a much faster rate.
We have categorized some more processes as slow fast processes in Table.

Examples of Real-Life Physical Changes
Physical And Chemical Changes
Matters can undergo different changes. Let us now consider whether during such changes the matters remain the same or any other new substances are formed.
For example, when clear lime water is exposed to air for a few days, it turns milky. This is because a new substance is formed.
Again think of some water which is frozen to ice. In this case, no new substance has been formed. Only its state has been changed. Liquid happens.
Only the physical properties of a state have been transformed into a solid state. If we warm the ice, we will again get back water (i.e. the liquid state).
When the surface of iron is exposed to moist air for a prolonged period, rust is formed on the surface.
Laboratory analysis will tell you that “rust” is a different substance, different from pure iron. It is formed due to a reaction between iron, aerial oxygen and moisture.
Take a magnet near iron nails. The magnet will attract the nails. But here, no new substances are formed.
When you burn a paper, it is reduced to ashes. Ash is different from paper. It is formed due to a reaction between paper (cellulose) and oxygen at high temperature

When you dissolve some sugar in water, it “disappears” in the solution. But no new substances are formed.
If you can vapourize all the water (or most of it) you can recover the sugar.
So sugar has not been transformed into any new substances.
From this discussion, you can very well understand that in some cases new substances are formed while in other cases no such thing substance (such as physical state, shape, etc.) changes.
So on this basis, we can classify all changes as either physical changes or chemical changes.
The change which involves only a change in the physical properties of a substance and in which no new substances are formed is called Physical Change.
For example, dissolution of sugar in water, freezing of water, boiling of water etc.
The changes which involve the formation of one or more new substances having a completely different set of features or properties compared to the original substances are called Chemical Changes.
For example, rusting of iron, lime water turning milky when exposed to air, burning of paper or kerosene oil etc.
Let us give you a bit more detail about some of the physical and chemical processes.
Examples Of Physical Changes
- A piece of iron is magnetized. This is an example of a physical change. No new substances are formed during magnetization. When it is heated or dropped from a height, its magnetic property is lost. We get back the same material. So this physical change can be reversed.
- Change of state of matter is an example of physical change. For example, when water is frozen to ice or when water is boiled, only the physical
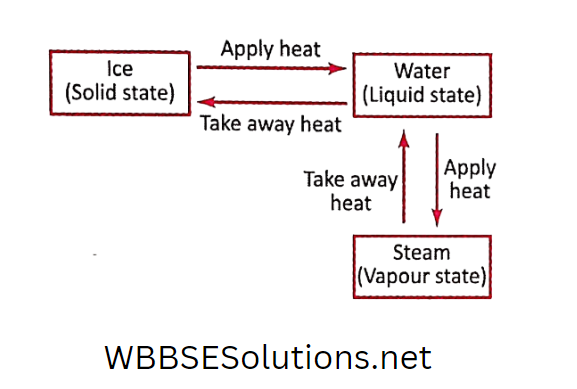
state of the matter is changed. No new substances are formed. When the ice is warmed, we get back to the water.
When hot steam is condensed on a cool metallic surface, we get back to the water.
So we can see that the change of state, which is a physical change, can be reversed by changing the temperature.
When a glass vessel is accidentally dropped, it breaks into pieces. It is a physical change. The glass vessel is broken into several small pieces, but no new substances are formed.
Though it is a physical change, of course, we cannot get back the glass vessel from so many broken pieces. So it is an irreversible physical change.

4. Tearing a sheet of paper into pieces is a physical change.
5. When you take an empty glass and breathe out on it, the moisture present in the air we breathe out is condensed on the glass in the form of very small water droplets.
When we keep some pieces of ice in a glass, the glass becomes cool and the moisture present in the air is condensed on the cool surface of the glass.
Examples Of Chemical Changes
- When rice is boiled in water, raw rice is converted into boiled rice. Some chemical changes occur during this boiling whereby new substances are formed. We cannot get back the original material (raw rice) by simple means.
- When iron is exposed to moist air for a prolonged period, rust is formed on its surface. Rust is a new substance. If we remove the rust slowly from the iron surface using a sand-paper and collect it, we will see that particles of “rust” are not attracted by a magnet. So properties of rust are different from that of pure iron. [Be careful while rubbing the rusted iron surface so that no pure iron should come out].
- When a piece of paper is set on fire, it quickly burns and reduces to ashes. A new substance is formed. It is not at all possible to get back the original piece of paper.
- Digestion of food inside our stomach involves chemical processes. New, simpler substances are formed from complex food materials.
- Ripening of mango involves chemical change, and this change cannot be reversed.
- Respiration also involves chemical changes.
Short Activities on Changes in Nature
Activity 4: Prepare a slurry of turmeric powder with water in one container and some amount of beetroot juice in another container.
Now take several strips of filter paper. Dip some of them in turmeric slurry and a few other strips in beetroot juice. Dry the strips.
Now dip those strips in two different solutions-limewater and lemon juice. Following changes in the colour of the strips are observed.
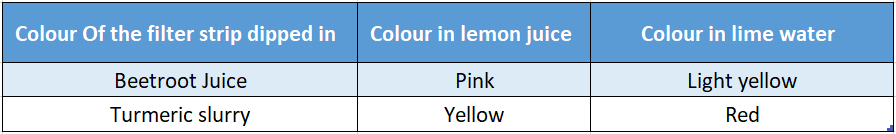
You can see that colour of the strips changed differently in different solutions. Chemical changes are responsible for this colour change.
This is an example of a chemical process accompanied by colour change.
Changes Involve Energy
We know that unless heated, rice is not boiled. If water is not heated it will not boil. To melt ice, it has to be warmed.
Solid naphthalene when heated directly, transforms into a gaseous state through a process called sublimation.
At room temperature also, it sublimes, but at a much slower rate. So, we see that heat energy is associated with all the above-mentioned processes.
It affects the rate of change. We have mentioned already that any change of state (which is a physical process) can occur only if heat is absorbed from a substance or is supplied to it.
Physical processes may or may not involve the absorption or liberation of heat. When dilute sulphuric acid is slowly added to water, the solution becomes hot.
So, heat energy is liberated during the mixing. When urea is dissolved in water, the solution becomes cold. So heat is absorbed during this physical mixing process.
When two gases are mixed together, no noticeable change in temperature is observed. So heat is neither liberated nor absorbed here.
Any change of state can be realized using heat energy. When heat is supplied, water boils. When heat is extracted, the vapour condenses.
This is the reason that on winter mornings, dew drops are formed on the leaves of grass.
At night, the moisture in the air cools down and condenses as droplets of water on the leaves of grass.

The chemical change also involves the absorption or evolution of heat. For example, when quicklime is dissolved in water, bubbles of gases are evolved and the solution becomes hot.
A chemical change is always associated with a change in energy- either in the form of heat energy or any other form of energy.
In some cases, a chemical process is initiated by heat energy. For example, when a piece of paper is held in flames, only then it catches fire and is reduced to ashes.
So this process occurs at a higher temperature. At elevated temperatures, the paper (cellulose) reacts with oxygen in the air to produce carbon dioxide and other substances.
When a firecracker is lit with a flame, it starts emitting light and sound. In fact, changes can be initiated with other forms of energy also.
In the laboratory, when the electric spark is passed through a gaseous mixture of hydrogen and oxygen, water is produced.
Again, when electricity is passed through water, it is dissociated to produce hydrogen. In this process, carbon dioxide and oxygen.
Lightning is also a kind of high-energy electric spark. When it strikes a tree, the tree catches fire. Every year, many people around the world die because of lightning.
If lightning strikes in nearby places, the electrical and electronic goods that are attached to the plug points are damaged. So during a thunderstorm, electrical equipment must be detached from the plug point.

You can explode caps either by pressing the trigger of a toy gun or by hitting it hard with a stone.
In this case, a chemical process is initiated by another form of energy known as mechanical energy.
Plants can prepare their “food” on their green leaves by a process called photosynthesis water combine in the presence of solar energy to form a new substance-glucose.
This chemical process can occur only in the presence of sunlight. That is why a plant dies when kept in the dark for a prolonged time.
So photosynthesis is an example of a chemical process being initiated by light energy.
When food matters are digested inside our stomach, heat is evolved.
This heat maintains the body temperature and provides energy for different processes occurring continuously within our body.
When you bring one finger close to your nose and breathe out deeply, you can feel that the air you are breathing out is warm.
This is because the inside of our body is warm. The warmth of the human body is the result of the metabolism of food.
Metabolism of food causes the release of energy in our cells which keeps our body warm and fit for work.

Some More Examples Of Physical And Chemical Processes
Let us provide you with some more examples of physical and chemical changes.
Important Definitions Related to Changes Around Us
Physical changes:
1. All of you must have seen tools which are used to dig the soil. In these tools, iron blades are fixed on a wooden handle.
But how is this done? The iron blade of these tools has a ring which is slightly smaller in size than the wooden handle.
As the iron blade is heated, the ring becomes slightly larger and then the handle easily fits into the ring. When the blade cools down, it again contracts and fits tightly on the handle.

This mechanism is also used to fix a metal ring on the wooden wheel of a cart.
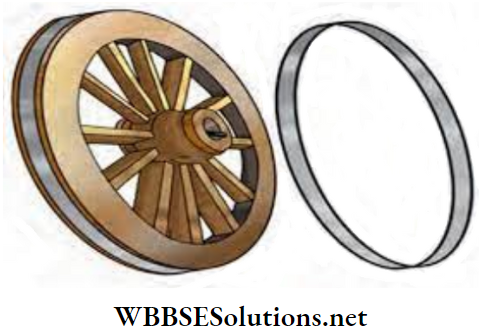
- A gap is left at regular intervals between the joints of railway lines. This is because, in summer, the rail lines become hot and expand in length.This is a physical change. If this gap is not present, the railway lines would bend.
- In winter our lips are cracked. This is because, in winter, water is lost more from our soft and exposed body parts such as lips, heels etc.As a result, the lips are cracked. To prevent this we apply cream or lip balm on them.This creates a protective layer on the lips and prevents the loss of water from the lips. Such loss of water is an example of a physical process.
- When vinegar is mixed in pickles, it absorbs water from the fruit and vegetable pieces.So those “dry” pieces of fruits and vegetables can be preserved 600 for a long time at room temperature.
- The melting of glaciers is an example of physical change which occurs naturally
Chemical changes:
When a banana is kept for a few days, black spots appear on its skin. This is due to some chemical changes.
When a piece of apple is cut and kept open in the air for some time, brown patches appear on the exposed surface. This happens due to some chemical reaction.

When leaves of eucalyptus trees fall on the ground, they are decomposed slowly and the chemicals present within these get mixed up with the soil.
Due to the presence of these chemicals in the soil, grasses cannot grow properly around these trees.
When a living animal dies and is kept in the open for some time, it is decomposed. Some microbes convert various body parts of the dead body into other substances.
So it is a chemical process.
In cities, chlorine water or halogen tablets are mixed with water to kill the germs present in them.
- Marble floors and tiles are often cleaned with “solid acid” (actually it is called oxalic acid). This cleaning process involves a chemical reaction.
- The weakening of our bones, the colour of our urine and faeces, the yellowing of teeth and cataracts in the eyes are all the results of some chemical processes.
- From the examples discussed so far about the physical and chemical changes, we can compare them as follows:
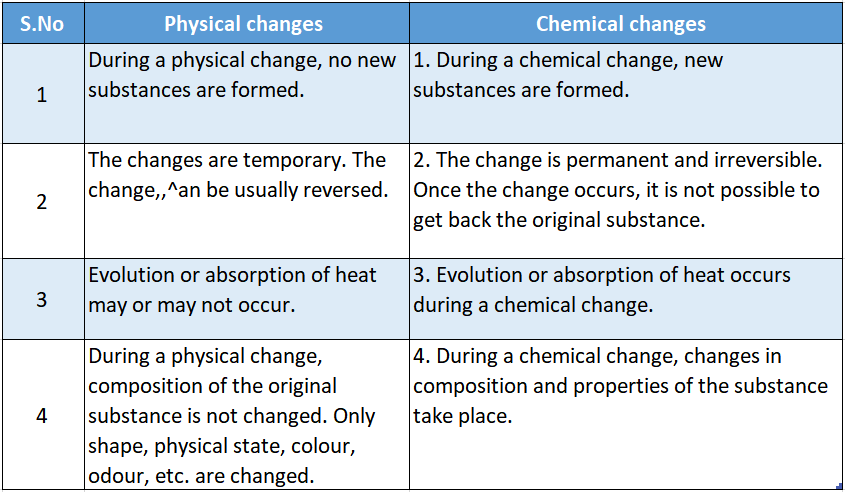
We conclude this chapter by classifying some common changes into different types.
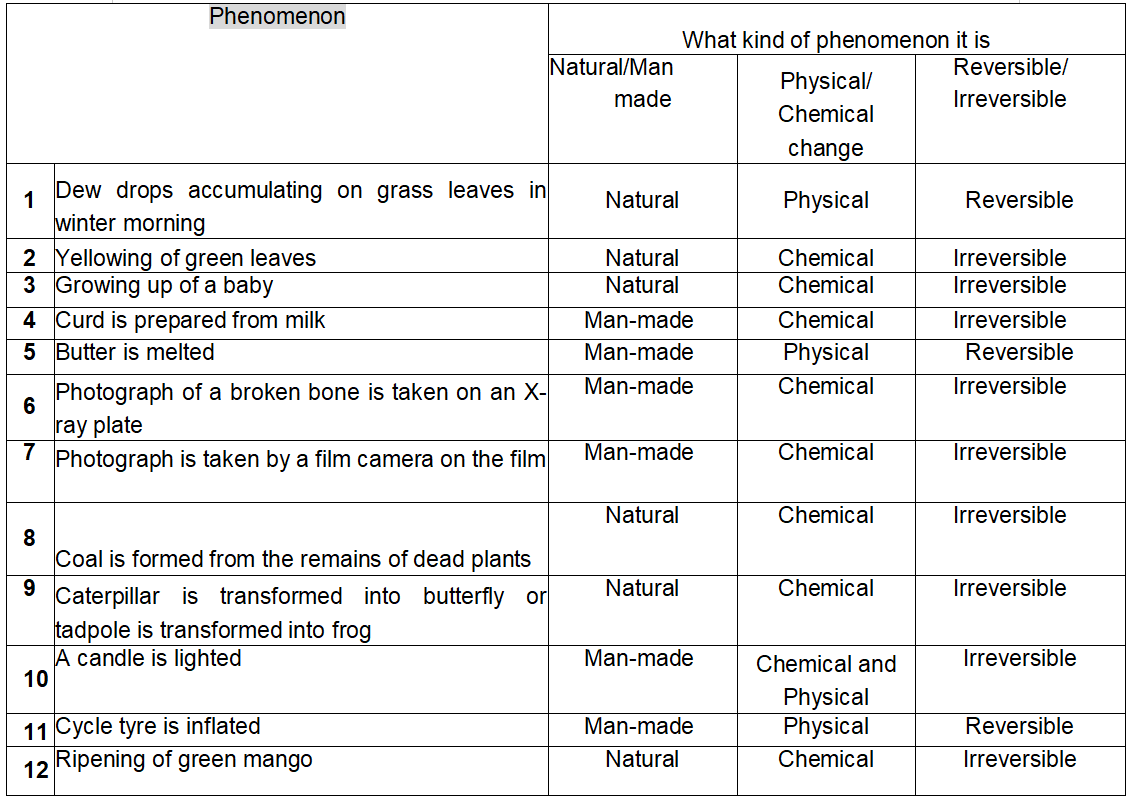
You can add some more examples to this list.


