Thermodynamic Processes
Question 1. A thermodynamic system is taken from state A to state B along ACB and brought back to A along BDA as shown in the adjoining p-V diagram. The net work done during the complete cycle is given by the area
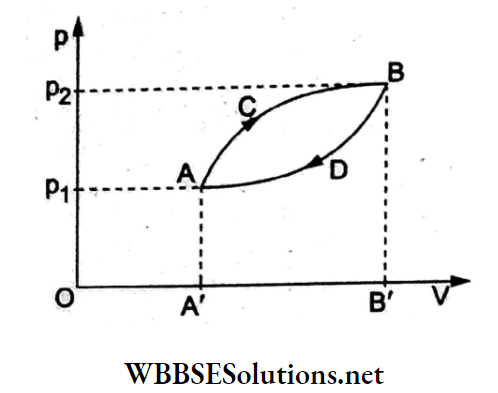
- p1ACBp2p1
- ACBB’A’A
- ACBDA
- ADBB’A’A
Answer: 3. ACBDA
Work done in a cyclic process is the area enclosed by the cycle on the p-V diagram.
Thus, W = area ACBDA. Note that work done is positive for a clockwise cycle and negative for an anticlockwise process in the p-V diagram.
Question 2. An ideal gas undergoing an adiabatic process has a pressure-temperature relationship
- pγ-1Tγ = constant
- pγTγ-1 = constant
- pγT1-γ = constant
- p1-γTγ = constant
Answer: 4. p1-γTγ = constant
For an adiabatic process, the relation between p and V is pVγ = constant. From the equation of state pV = RT, we get
⇒ \(V=\frac{R T}{p}\)
or, \(p\left(\frac{R T}{p}\right)^\gamma=\text { constant or } p^{1-\gamma} T^\gamma=\text { constant. }\)
“thermodynamics multiple choice questions “
Question 3. An ideal gas at 27 °C is compressed adiabatically to 8/27 of its original volume. The rise in temperature is (take y = 5/3)
- 475°C
- 402°C
- 275°C
- 375°C
Answer: 4. 375°C
For adiabatic compression,
⇒ \(T_1 V_1^{\gamma-1}=T_2 V_2^{\gamma-1}\)
∴ \((27+273) V^{\frac{5}{3}-1}=T_2\left(\frac{8}{27} V\right)^{\frac{5}{3}-1}\)
∴ \(300 V^{\frac{2}{3}}=\left(T_2\right)\left(\frac{2}{3}\right)^2 V^{\frac{2}{3}}\)
9(300) = 4T2
∴ \(T_2=\frac{9 \times 300}{4}\)
= 675K.
Hence, the rise in temperature is T2 – T1 = 675 – 300
= 375°C.
Question 4. The molar heat capacity at constant pressure of an ideal gas is \(\frac{7}{2}\) R. The ratio of the molar heat capacity at constant pressure to that at constant volume is
- \(\frac{7}{5}\)
- \(\frac{8}{7}\)
- \(\frac{5}{7}\)
- \(\frac{9}{7}\)
Answer: 1. \(\frac{7}{5}\)
Given that,
⇒ \(C_p=\frac{7}{2} R, \text { but } C_p-C_V=R \text { or } C_V=\frac{7}{2} R-R=\frac{5}{2} R\)
∴ \(\frac{C_p}{C_V}=\frac{\frac{7 R}{2}}{\frac{5 R}{2}}=\frac{7}{5}\)
Question 5. A thermodynamic system is taken through a cyclic process ABCDA as shown in the figure. Heat expelled by the gas during the cycle is
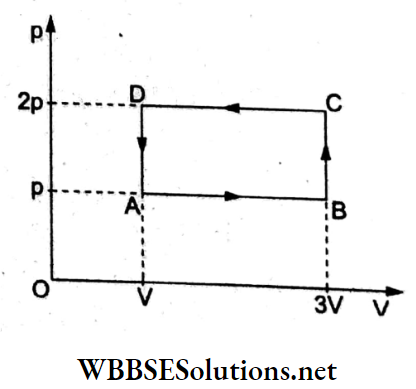
- 2pV
- 4pV
- \(\frac{1}{2}\) pV
- pV
Answer: 1. 2pV
From the first law of thermodynamics, dQ = dU + dW, and the change in internal energy in a cyclic process is dU = 0.
dQ = dW = area of cyclic process = (2p-p)(3V- V)
= 2pV.
Since the cycle is anticlockwise, work done is negative; hence heat expelled is 2pV
Read And Learn Also NEET Physics Multiple Choice Question and Answers
thermodynamics questions
Question 6. A gas is taken through the cycle ABCA as shown in the figure. What is the net work done by the gas?
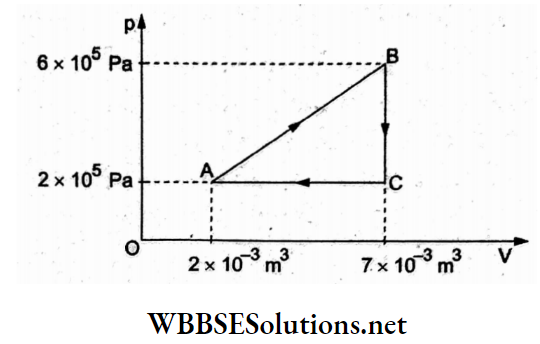
- 2000 J
- 1000 J
- -200 J
- Zero
Answer: 2. 1000 J
Net work done is the area enclosed by the cycle in the p-V diagram.
Hence,
⇒ \(W=\frac{1}{2}(A C)(B C)=\frac{1}{2}\left(5 \times 10^{-3} \mathrm{~m}^3\right)\left(4 \times 10^5 \frac{\mathrm{N}}{\mathrm{m}^2}\right)\)
= 1000J.
Question 7. The molar heat capacities of an ideal gas at constant pressure and constant volume are Cp and Cv respectively. If \(\gamma=\frac{C_p}{C_V}\) and R is the universal gas constant then Cv is equal to
- \(\frac{1+\gamma}{1-\gamma}\)
- \(\frac{R}{\gamma-1}\)
- \(\frac{\gamma-1}{R}\)
- yR
Answer: 2. \(\frac{R}{\gamma-1}\)
For a gas, Cp -Cv = R.
Dividing throughout by Cv,
⇒ \(\frac{C_p}{C_V}-1=\frac{R}{C_V}\)
⇒ \(\gamma-1=\frac{R}{C_V}\)
⇒ \(C_V=\frac{R}{\gamma-1}\)
“thermodynamics mcq class 11 “
Question 8. In the given (V-T) diagram, what is the relation between pressures p1 and p2?
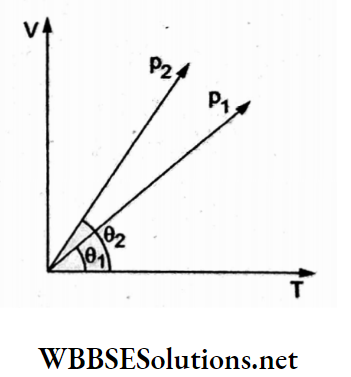
- p2 = p1
- p2 > p1
- p2 < P1
- Cannot be predicted
Answer: 3. p2 < P1
From the gas laws, pV = nRT or V = \(V=\left(\frac{n R}{p}\right) T\)
Hence, the slope of the V-Tline is inversely proportional to the pressure
⇒ \(\left(\text { slope } \propto \frac{1}{p}\right)\),
Hence p2 < P1.
Question 9. During an adiabatic process, the pressure of a gas is found to be proportional to the cube of its temperature. The ratio Cp/Cv for the gas is
- \(\frac{4}{3}\)
- 2
- \(\frac{5}{3}\)
- \(\frac{3}{2}\)
Answer: 4. \(\frac{3}{2}\)
For an adiabatic process,
p1-γTγ = constant
⇒ \(p T^{\frac{\gamma}{1-\gamma}}=\text { constant }\)
⇒ \(\frac{p}{T^{\frac{\gamma}{\gamma-1}}}=\text { constant }\)
⇒ \(p \propto T^{\frac{\gamma}{\gamma-1}}\)…..(1)
Comparing (1) with the given condition p ∝ T3, we conclude that
⇒ \(\frac{\gamma}{\gamma-1}=3, \text { thus } \gamma=\frac{3}{2}\)
Question 10. One mole of an ideal diatomic gas undergoes a transition from A to B along the straight path AB as shown in the figure. The change in internal energy of the gas during the transition is
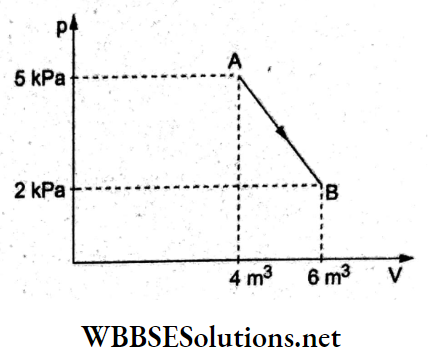
- 20 kJ
- -20 kJ
- 20 J
- -12 kJ
Answer: 2. -20 kJ
For a diatomic gas, Cv = \(\frac{5}{2}\) R, and change in internal energy is
⇒ \(\Delta U=n C_V \Delta T=\frac{5}{2} R\left(T_2-T_1\right)\) [∵ n = l].
From the gas laws, pV = RT, hence T = \(\frac{pV}{R}\)
∴ \(\Delta U=\frac{5}{2} R \frac{\left(p_2 V_2-p_1 V_1\right)}{R}=\frac{5}{2}\left(p_2 V_2-p_1 V_1\right)\)
Substituting the values from the given graph,
“thermodynamics practice problems “
⇒ \(\Delta U=\frac{5}{2}\left[\left(2 \times 10^3 \mathrm{~Pa}\right)\left(6 \mathrm{~m}^3\right)-\left(5 \times 10^3 \mathrm{~Pa}\right)\left(4 \mathrm{~m}^3\right)\right]\)
= \(\frac{5}{2}(-8 \mathrm{~kJ})\)
= -20 kJ.
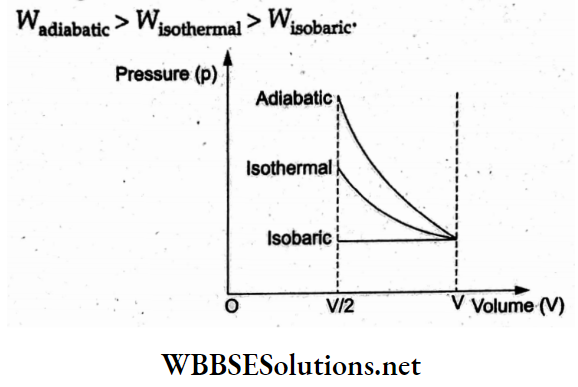
Question 11. An ideal gas is compressed to half its initial volume by means of several processes. Which of the processes results in the work done on the gas?
- Isochoric
- Adiabatic
- Isobaric
- Isothermal
Answer: 2. Adiabatic
For the gas undergoing an isochoric process (volume constant), no work is done by the gas. The work done on the gas is the area under the p-V diagram. Hence, according to the given figures,
Question 12. A thermodynamic system undergoes a cyclic process ABCDA as shown in the figure. The work done by the system in one cycle is
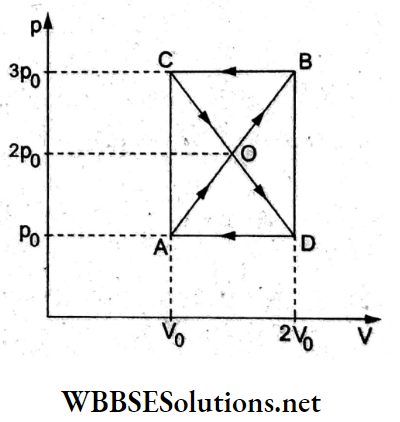
- p0V0
- 2p0V0
- \(\frac{p_0 V_0}{2}\)
- Zero
Answer: 4. Zero
For a cyclic process, the net work done by the system is equal to the area enclosed in the p-V diagram. The given process comprises two closed areas, AODA and COBC. The first is clockwise, so the work done is +ve while that for the second is -ve (being anticlockwise).
∴ Wnet = area AOD- area COB
⇒ \(\frac{1}{2}\left(2 V_0-V_0\right)\left(2 p_0-p_0\right)-\frac{1}{2}\left(2 V_0-V_0\right)\left(3 p_0-2 p_0\right)\)
⇒ \(\frac{1}{2} p_0 V_0-\frac{1}{2} p_0 V_0\)
= 0.
Question 13. One mole of an ideal monatomic gas undergoes a process described by the equation (pV³ = constant). The heat capacity of the gas during this process is
- \(\frac{3}{2}\) R
- \(\frac{5}{2}\) R
- 2R
- R
Answer: 4. R
For a polytropic process (pVn = constant), the molar heat capacity is
⇒ \(C=C_V+\frac{R}{1-n}\)
In the given process, pV³ = constant, we have n = 3
“thermodynamics practice problems “
∴ \(C=C_V+\frac{R}{1-3}=\frac{3}{2} R-\frac{R}{2}=R\) [∵ \(C_V=\frac{3}{2} R\)]
Question 14. One mole of an ideal gas undergoes a process from an initial state A to the final state B via two processes. It first undergoes isothermal expansion from volume V to 3V and then its volume is reduced from 3V to V at constant pressure. The correct p-V diagram representing the two processes is
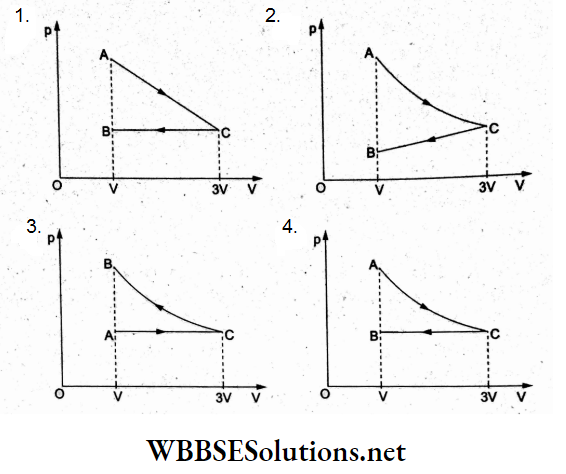
Answer: 4.
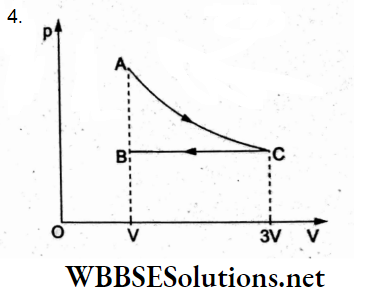
The isothermal expansion from state A (volume V) to state C (volume 3V) is represented by the rectangular hyperbola AC in (4).
In the second process, the isobaric compression from state C to final state B is shown by the line CB in (4).
Hence, the correct p-V is (4).
Question 15. The ratio of the molar heat capacities \(\frac{C_p}{C_V}=\gamma\) in terms of degrees of freedom (f) is given by
- 1 + \(\frac{1}{f}\)
- 1 + \(\frac{f}{3}\)
- 1 + \(\frac{2}{f}\)
- 1 + \(\frac{f}{2}\)
Answer: 3. 1 + \(\frac{2}{f}\)
If the total number of degrees of freedom of a polyatomic gas molecule is f, the internal energy per mole of the gas will be
U = \(\frac{f}{2}\) RT.
∴ \(C_V=\frac{d U}{d T}=\frac{1}{2} f R, \text { hence } C_p=C_V+R=\left(\frac{f}{2}+1\right) R\)
∴ \(\frac{C_p}{C_V}=\frac{\left(\frac{f}{2}+1\right) R}{\frac{f_2}{2} R}=1+\frac{2}{f}\)
Question 16. A gas undergoes two processes to reach the final state C from the initial state A as shown in the p-V diagram. In the process, AB, 400 J of heat is absorbed by the gas, and in the process BC, 100 J of heat is absorbed. The heat absorbed by the system in the process of AC will be
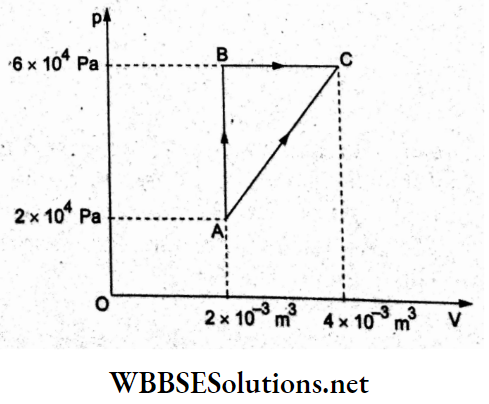
- 380 J
- 500 J
- 460 J
- 300 J
Answer: 3. 460 J
Since the initial state (A) and the final state (C) are the same for the two processes, the change in internal energy AU will be the same.
For the process ABC,
ΔQABC = WAB + WBC + AU
=> 400J +100J = 0(isochoric) + 6 x 20J + AU
=> 500 J = 120 J + AU……(1)
Similarly, for the process AC,
ΔQAC = AU + AW = AU + \(\frac{1}{2}\)(8 x 20)J
⇒ ΔQAC = 80J + AU…..(2)
Subtracting (2) from (1),
500J – ΔQAC = 40J
ΔQAC = 500 J- 40 J
= 460 J.
Question 17. A monatomic gas at pressure p and volume Vexpands isothermally to a volume 2V and then adiabatically to a volume of 16V. The final pressure of the gas is (take y = 5/3)
- 64p
- 32p
- \(\frac{p}{34}\)
- 16p
Answer: 3. \(\frac{p}{34}\)
For the isothermal process, pV = \(\frac{p}{2}\)(2V).
“thermodynamics practice problems “
For the adiabatic process, pVγ = constant.
∴ \(\frac{p}{2}(2 V)^{5 / 3}=p^{\prime}(16 V)^{5 / 3} \Rightarrow p \cdot 2^{2 / 3}=p^{\prime} \cdot 2^{20 / 3}\)
∴ final pressure = \(\frac{p}{2^6}=\frac{p}{64}\)
Question 18. If All and AW represent the increase in internal energy and work done by the system respectively in a thermodynamical process, which of the following is true?
- ΔU = -ΔW in an adiabatic process
- ΔU = ΔW in an isothermal process
- ΔU = ΔW in an adiabatic process
- ΔU = -ΔW in an isothermal process
Answer: 1. ΔU = -ΔW in an adiabatic process
For an isothermal process, ΔU = 0,
So, options (2) and (4) are incorrect.
For an adiabatic process,
ΔQ = ΔU + ΔW
⇒ ΔU + ΔW = 0
⇒ ΔU = – ΔW is correct.
Question 19. In a thermodynamic process, which of the following statements is not true?
- In an adiabatic process, the system is insulated from the surroundings.
- In an isochoric process, pressure remains constant.
- In an isothermal process, the temperature remains constant.
- In an adiabatic process, pVγ = constant.
Answer: 2. In an isochoric process, pressure remains constant.
For an adiabatic process, there is no exchange of heat between the body and the surroundings and the process follows the law pVγ = constant.
Hence, (1) and (4) are true.
For an isothermal process, temperature remains constant, so (3) is true.
Finally, for an isochoric process, the volume remains constant and not the pressure.
So, option (4) is incorrect.
Question 20. The internal energy change in a system that has absorbed 2 kcal of heat and done 500 J of work is
- 8900 J
- 6400 J
- 5400 J
- 7900 J
Answer: 4. 7900 J
From the first law of thermodynamics, ΔQ = ΔU + ΔW.
Given, ΔQ = 2000 cal
= 2000 x 4.2J
= 8400 J and ΔW
= 500 J.
∴ ΔU = ΔQ – ΔW
= 8400 J- 500 J
= 7900 J
Question 21. In the cyclic process shown in the V-p diagram, the magnitude of work done is
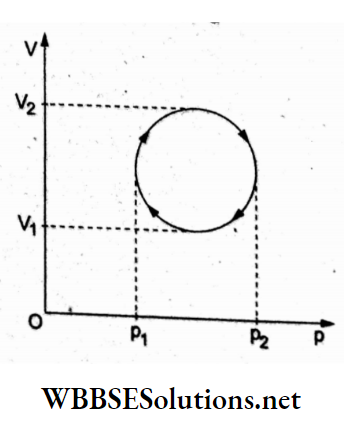
- \(\frac{\pi}{4}\left(p_2-p_1\right)^2\)
- \(\frac{\pi}{4}\left(V_2-V_1\right)^2\)
- \(\frac{\pi}{4}\left(p_2-p_1\right)\left(V_2-V_1\right)\)
- \(\pi\left(p_2 V_2-p_1 V_1\right)\)
Answer: 3. \(\frac{\pi}{4}\left(p_2-p_1\right)\left(V_2-V_1\right)\)
For a cyclic process plotted in the p-V diagram, the work done = area inside the closed curve. We can treat the circle as an ellipse ot
semimajor axis \(\frac{1}{2}\)(P2 – P1) semimmor axis \(\frac{1}{2}\)(V2 – V1).
∴ work done \(\pi \frac{1}{2}\left(p_2-p_1\right) \frac{1}{2}\left(V_2-V_1\right)=\frac{\pi}{4}\left(p_2-p_1\right)\left(V_2-V_1\right)\)
Question 22. A gas with \(\frac{C_p}{C_V}=\gamma\) goes from an initial state (p1, V1, T1) to a final state (P2, V2, T2) Enough an adiabatic process. The work done by the gas is
- \(\frac{p_1 V_1-p_2 V_2}{\gamma-1}\)
- \(\frac{p_1 V_1+p_2 V_2}{\gamma+1}\)
- \(\frac{n R\left(T_1+T_2\right)}{\gamma-1}\)
- nyR(T1 – T2)
Answer: 1. \(\frac{p_1 V_1-p_2 V_2}{\gamma-1}\)
Work done during the adiabatic expansion from state A (p1, V1, T1) to state B (p2, V2, T2) is given by
⇒ \(W=\frac{n R}{\gamma-1}\left(T_1-T_2\right)=\frac{1}{\gamma-1}\left(n R T_1-n R T_2\right)\)
⇒ \(\frac{1}{\gamma-1}\left(p_1 V_1-p_2 V_2\right)\) [∵ pV = nRT]
| Class 11 Physics | Class 12 Maths | Class 11 Chemistry |
| NEET Foundation | Class 12 Physics | NEET Physics |
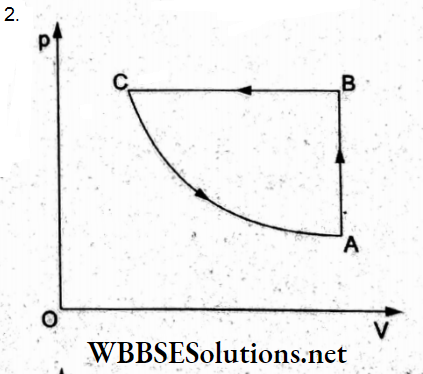
Question 23. A cyclic process ABCA is shown in the p-T diagram. Which of the following diagrams shows the same process in the p-V diagram?
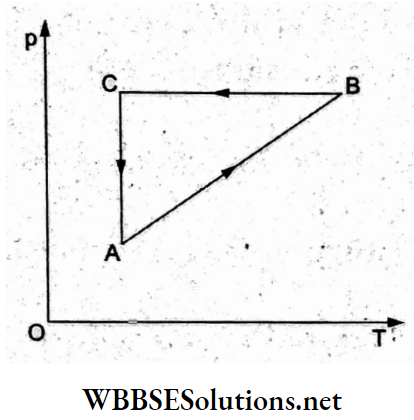
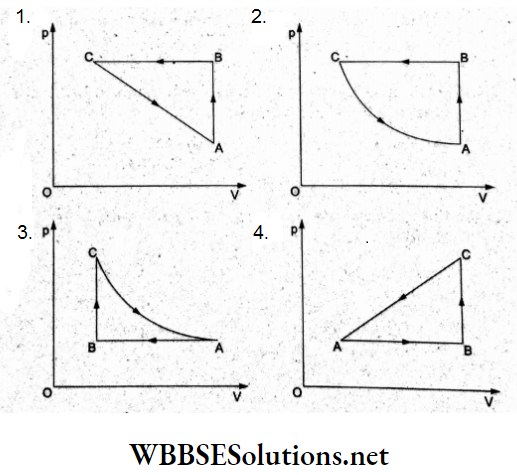
Answer: 2.
In the given cyclic process, consider the three processes individually.
(1) Process AB: p oc T(for the straight line) The process is isochoric (volume constant) as in options (1) and (2).
(2) Process BC: Isobaric compression as in options (a) and (b).
(3) Process GA: Isothermal process (T – constant), so according to Boyle’s law (pV = constant) which gives a rectangular hyperbola, option (2) is true.
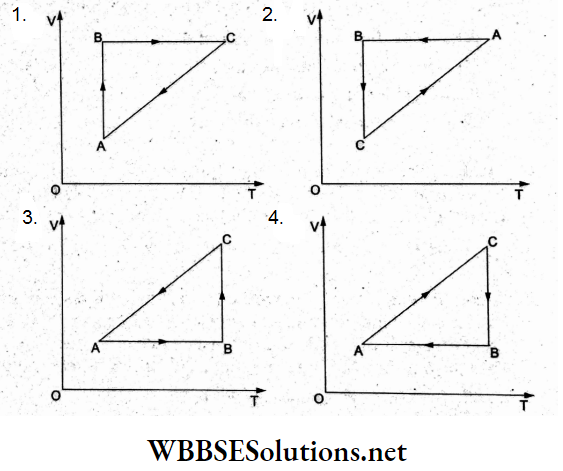
Question 24. A cyclic process ABC is shown in the p-T diagram. Which of the following curves shows the same process in a V-T diagram?
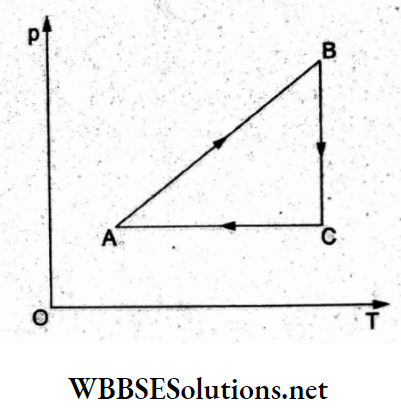
Answer: 3.
Process AB: pecT, hence V = constant with T increasing: option (3).
Process BC: Isothermal expansion (T=constant), T constant and volume increasing: option (3).
Process CA: Isobaric (p = constant), volume decreases linearly with temperature as in option (3).
“thermodynamics practice problems “
Hence (3) is the correct representation.
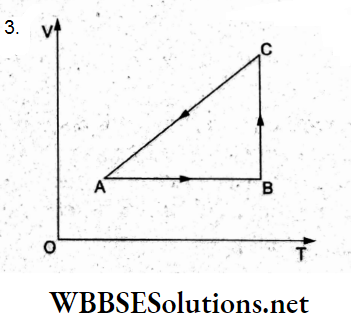
Question 25. A sample of n moles of a gas undergoes isothermal expansion from volume V1 to V2 at temperature T. The work done by the gas is
- \(n R T \ln \left(\frac{V_2}{V_1}+1\right)\)
- \(n R T \ln \left(\frac{V_2}{V_1}-1\right)\)
- \(n R T \ln \frac{V_2}{V_1}\)
- \(n R T\left(\frac{V_2}{V_1}\right)\)
Answer: 4. \(n R T\left(\frac{V_2}{V_1}\right)\)
Work done during the isothermal expansion is
⇒ \(W=n R T \ln \frac{V_2}{V_1}\)
Question 26. A system is taken from state A to state B along two different paths, 1 and 2. The heat absorbed and work done by the system along these two paths are Q1 and Q2, and W1 and W2 respectively. Which of the following is true?
- Q1– Q2
- W1 = W2
- Q1-Q2 = W1-W2
- Q1 + W1 = Q2 + W2
Answer: 3. Q1-Q2 = W1-W2
The internal energy function is a state function, which depends only on the initial and final states.
For A: Q1 = W2 + ΔU.
For B: Q2 = W2 + ΔU, where ΔU= UB-UA.
∴ Q1 – W1 = Q2 – W2
Q1 – Q2 = W1 – W2
Question 27. If the ratio of the specific heat capacity of a gas at constant pressure to that at constant volume is y then the change in internal energy of a given mass of the gas when its volume changes from V to 2V at constant pressure p is
- \(\frac{R}{\gamma-1}\)
- pV
- \(\frac{pV}{\gamma-1}\)
- \(\frac{\gamma p V}{\gamma-1}\)
Answer: 3. \(\frac{pV}{\gamma-1}\)
Change in internal energy is
⇒ \(\Delta U=n C_V \Delta T=n\left(\frac{R}{\gamma-1}\right)\left(T_2-T_1\right)\)
⇒ \(\frac{1}{\gamma-1}\left(n R T_2-n R T_1\right)=\frac{1}{\gamma-1}\left(p_2 V_2-p_1 V_1\right)\)
Given that p1 = p2 = p, V2 = 2V, V1 = V, so
⇒ \(\Delta U=\frac{p}{\gamma-1}(2 V-V)=\frac{p V}{\gamma-1}\)
Question 28. An ideal gas A and a real gas B have their volumes increased from V to 2V under isothermal conditions. The increase in internal energy
- Will be the same in both A and B
- There will be zero in both gases
- Of B will be more than that of A
- Of A will be more than that of B
Answer: 2. Will be zero in both the gases
Under isothermal conditions temperature T remains constant, so dT = 0. The change in internal energy = AU = nCvdT = 0. Hence, the increase in internal energy in both A and B is zero.
Question 29. The temperature inside a refrigerator is maintained at 4°C and the temperature of the atmosphere is 15°C. If the gas enclosed undergoes the Carnot process in its working, find the Carnot efficiency.
- 0.028
- 0.038
- 0.072
- 0.054
Answer: 2. 0.038
During the Carnot cyclic process, efficiency
⇒ \(\eta=1-\frac{T_2}{T_1}=1-\frac{(273+4) \mathrm{K}}{(273+15) \mathrm{K}}\)
⇒ \(1-\frac{277}{288}=\frac{11}{288}\)
= 0.038.
Question 30. A conducting, closed container of capacity 100 litres contains an ideal gas at a high-pressure p0. Using an exhaust pump the gas from the container is pumped out at a constant rate of 5 litres per second. Find the time in which the pressure inside the container is reduced to \(\frac{p_0}{100}\) (Assume isothermal conditions.)
- 92 s
- 110 s
- 45 s
- 150 s
Answer: 1. 92 s
For an isothermal process,
pV = constant
pdV + Vdp = 0
⇒ \(\frac{d p}{p}=-\frac{d V}{V}\)……(1)
Given that V = 100 L = 100 x 10-3 m3, initial pressure = p0,
⇒ \(\frac{d V}{d t}=5 \mathrm{~L} \mathrm{~s}^{-1}\)
= \(5 \times 10^{-3} \mathrm{~m}^3 \mathrm{~s}^{-1}\)
⇒ \(d V=\left(5 \times 10^{-3} d t\right) \mathrm{m}^3 \mathrm{~s}^{-1}\)
Final pressure = \(\frac{p_0}{100}\)
Integrating (1),
⇒ \([\ln p]_{p_0}^{p_0 / 100}=-\int_0^t \frac{5 \times 10^{-3} d t}{100 \times 10^{-3}}\)
⇒ \(\ln p_0-\ln \frac{p_0}{100}=5 \times 10^{-2} t\)
∴ time \(t=\frac{1}{5 \times 10^{-2}}(2 \ln 10)\)
= 0.4 x 100 x 2.3
= 92 s.
“thermodynamics practice problems “
Question 31. The given p-V indicator diagram p shows four processes: isochoric, isobaric, isothermal, and adiabatic. The correct assignment of the processes in the same order is given
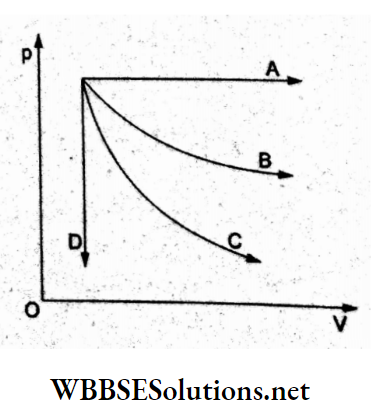
- ADCB
- ADBC
- DABC
- DBCA
Answer: 3. DABC
Process A: Isobaric (p constant)
Process B: Isothermal (T constant)
Process C: Adiabatic (greater slope, ΔQ = 0)
Process D: Isochoric (V constant)
Hence, option (3)
Question 32. In an isobaric process, work done by a diatomic gas is 10 J. The heat given to the gas will be
- 35 J
- 25 J
- 45 J
- 30 J
Answer: 1. 35 J
For an isobaric process (p constant),
pV =nRT
=> pdV = nRdT.
Work done = dW = pdV = nRdT……(1)
Heat given to the gas is
⇒ \(d Q=n C_p d T=n\left(\frac{7}{2} R\right) d T\) [∵ \(C_p=\frac{7}{2} R\)]
⇒ \(\frac{7}{2}(n R d T)=\frac{7}{2}(d W)\) [from (1)]
= \(\frac{7}{2}\) (10J)
= 35J.
Question 33. An ideal gas initially at a pressure of 1 bar is compressed from 30 m³ to 10 m³ and its temperature decreases from 320 K to 280K. The final pressure will be
- 3.4 bar
- 1.25 bar
- 2.625 bar
- 4.36 bar
Answer: 3. 2.625 bar
From the gas laws, \(\frac{p_1 V_1}{T_1}=\frac{p_2 V_2}{T_2}\)
⇒ \(\frac{(1 \mathrm{bar})\left(30 \mathrm{~m}^3\right)}{(320 \mathrm{~K})}=\frac{p_2\left(10 \mathrm{~m}^3\right)}{(280 \mathrm{~K})}\)
final pressure = p2 = 2.625 bar.
Question 34. One mole of nitrogen is heated isobarically from 300 K to 600 K. The change in its entropy is
- 20 J K-1
- 30 JK-1
- 40 JK-1
- 50 JK-1
Answer: 1. 20 J K-1
Change in entropy \(\Delta S=\int \frac{d Q}{T}\)
But \(d Q=n C_p d T=1\left(\frac{7}{2} R\right) d T\)
⇒ \(\Delta S=\frac{7}{2} R \int_{300 \mathrm{~K}}^{600 \mathrm{~K}} \frac{d T}{T}\)
= \(\frac{7 R}{2} \ln 2\)
= \(\frac{7}{2}(8.3)(0.693) \mathrm{JK}^{-1}\)
= 20JK-1
“thermodynamics practice problems “
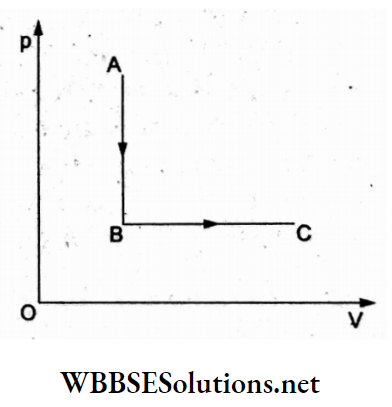
Question 35. One mole of an ideal gas undergoes the process A → B → C. Given p A that TA = 40 K, Tc = 400 K, and \(\frac{p_{\mathrm{B}}}{p_{\mathrm{A}}}=\frac{1}{5}\). The heat supplied to the gas is
- 2059.2 J
- 2659.2 J
- 3659.2 J
- 2259.2 J
Answer: 2. 2659.2 J
For isochoric compression A → B, heat expelled = nCvdT = nCv(TB– TA)
and for isobaric expansion B → C, heat absorbed = nCpdT = nCp(TC– TB).
∴ net heat absorbed is
ΔQ = nCpdT + nCvdT
= nCp(TC – TB) + nCv(TB– TA).
Given that
⇒ \(\frac{p_{\mathrm{B}}}{p_{\mathrm{A}}}=\frac{1}{5}=\frac{T_{\mathrm{B}}}{T_{\mathrm{A}}}\)
⇒ \(T_{\mathrm{B}}=\frac{T_{\mathrm{A}}}{5}=\frac{400 \mathrm{~K}}{5}\)
∴ ΔQ = (TC – TB)Cp-Cv(TC-TB) [∵ TA = TC (given)]
⇒ \(\left(400-\frac{400}{5}\right) R\)
= \(\frac{4}{5}(400)(8.31) \mathrm{J}\)
= 2659.2J
Question 36. Cp/Cv for a mixture of 11 g of CO2 and 14 g of N2 is
- \(\frac{7}{5}\)
- \(\frac{11}{5}\)
- \(\frac{4}{3}\)
- \(\frac{11}{8}\)
Answer: 4. \(\frac{11}{8}\)
For CO2: \(n=\frac{1}{4}, C_V=3 R, C_p=4 R\)
For N2: \(n=\frac{1}{2}, C_V=\frac{5}{2} R, C_p=\frac{7}{2} R\)
∴ \(\gamma_{\text {mixture }}=\frac{n_1 C_{p_1}+n_2 C_{p_2}}{n_1 C_{V_1}+n_2 C_{V_2}}=\frac{\frac{1}{4} \times 4 R+\frac{1}{2} \times \frac{7}{2} R}{\frac{1}{4} \times 3 R+\frac{1}{2} \times \frac{5}{2} R}=\frac{11}{8}\)
“thermodynamics practice problems “
Question 37. For the given cyclic process CABC for a gas shown in the p-V indicator diagram, the work done by the gas is
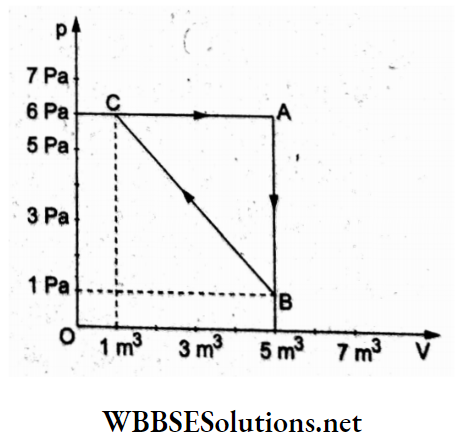
- 5 J
- 1 J
- 30 J
- 10 J
Answer: 4. 10 J
Work done by the gas during the process
C → A (expansion) = area under CA
WCA = (6 Pa)(5 m3-1 m3) = 24J.
WAB = zero, for the isochoric process.
WBC = area under BC.(compression)
= –\(\frac{1}{2}\)(6 Pa +1 Pa)(5 m3-1 m3)
= -14J.
Net work done during one complete cycle is
W = 24J + (-14) J
= 10J.
“thermodynamics practice problems “
Question 38. When heat Q is supplied to a diatomic gas of rigid molecules at constant volume, its temperature increases by AT. The heat required to produce the same change in temperature at a constant pressure is
- \(\frac{7Q}{5}\)
- \(\frac{5Q}{3}\)
- \(\frac{3Q}{2}\)
- \(\frac{2Q}{3}\)
Answer: 1. \(\frac{7Q}{5}\)
For a diatomic gas, \(C_V=\frac{5}{2} R, C_p=\frac{7}{2} R\)
At constant volume, \(Q=n C_V \Delta T=n \frac{5}{2} R \Delta T\)
At constant pressure, \(Q^{\prime}=n C_p \Delta T=n \cdot \frac{7}{2} R \Delta T\)
∴ \(\frac{Q^{\prime}}{Q}=\frac{7}{5}, \text { hence } Q^{\prime}=\frac{7}{5} Q\)
Question 39. A diatomic gas with rigid molecules does 10 J of work when expanded at constant pressure. The amount of heat absorbed during the process will be
- 40 J
- 35 J
- 30 J
- 25J
Answer: 2. 35 J
Work done at constant pressure is
dW= pdV= nRdT = 10J …..(1)
Heat absorbed = dQ = dU + dW.
For a diatomic gas, C = \(\frac{5}{2}\)R.
Hence, change in internal energy is
⇒ \(d U=n C_V d t=\frac{5}{2} R n d T=\frac{5}{2}(10 \mathrm{~J})\) [from (1)]
= 25J.
Substituting in (2), heat absorbed is
dQ = 25J +10J
= 35J.
Question 40. A cylinder with a fixed capacity of 67.2 L contains helium gas at the stop. The amount of heat needed to raise the temperature of the gas by 20°C is (given that R = 8.31 J mol-1 K-1)
- 748 J
- 700 J
- 374 J
- 350 J
Answer: 1. 748 J
Helium is monatomic, for which Cv = \(\frac{3}{2}\)R.
Number of moles = \(\frac{67.2 \mathrm{~L}}{22.4 \mathrm{~L}}\) = 3.
The amount of heat required is
⇒ \(\Delta Q=n C_V \Delta T=3 \mathrm{~mol}\left(\frac{3}{2} R\right) \Delta T\)
= \(\frac{9}{2}\) mol (8.31 J mol-1 K-1)(20 K)
= 747.9 J ≈ 748 J.
Question 41. A gas is taken from state A to state P B via two different paths, ACB and c ADB. When path ACB is used, 60 J of heat flows into the system and the system does 30 J of work. If the path ADB is used, work done by the system is 10 J. The heat which flows A into the system for the path ADB is
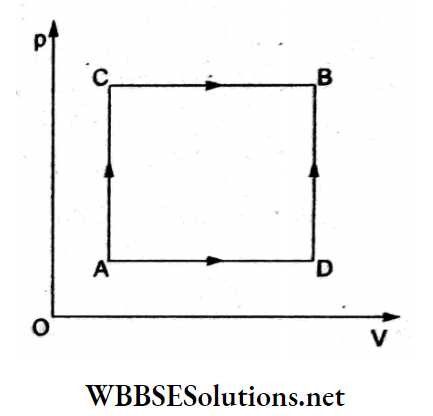
- 80 J
- 40 J
- 100 J
- 20 J
Answer: 2. 40 J
Internal energy is a state function and depends only on the initial and final states—it is path-independent.
From the first law of thermodynamics,
ΔQ = ΔU + ΔW
⇒ ΔU = ΔQ – ΔW.
For process ACB, ΔU = 60 J-30 J
= 30 J.
For process ADB, ΔU = ΔQ-10 J.
Since AU is the same for both,
ΔQ – 10J = 30J
⇒ ΔQ = 40J.
Question 42. A sample of an ideal gas is taken through a cyclic process abca as p shown in the figure. The change in the internal energy of the gas along the path ca is -180 J. The gas absorbs 250 J of heat along the path ab and 60 J along the path be. The work done by the gas along the 0L path abc is
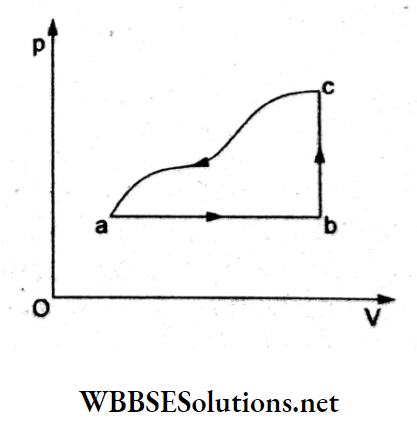
- 120 J
- 140 J
- 100 J
- 130 J
Answer: 4. 130 J
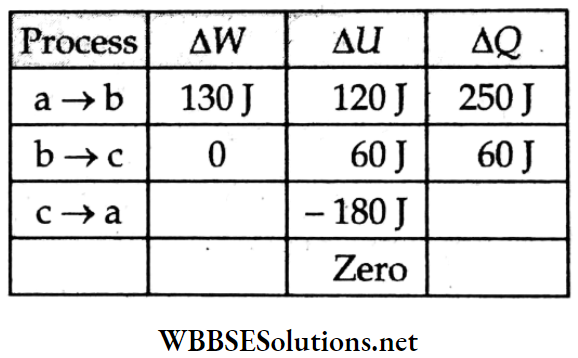
Consider the values of ΔQ, ΔU, and ΔW given in the following table.
For the process b → c (isochoric),
ΔW = 0,
so, (ΔU)bc = (dQ)bc = 60J.
In the total cyclic process, ΔU = 0.
So,(ΔU)ab + (ΔU)bc + (ΔU)ca = 0
=> (ΔU)ab + 60J – 180J = 0
=> (ΔU)ab = 120J.
For a → b, ΔW = ΔQ – ΔU
= 250 J-120 J
= 130 J.
∴ Wabc = Wab + Wbc
= 130 J + 0
= 130 J.
Question 43. Two moles of helium gas is mixed with three moles of hydrogen molecules. The molar heat capacity of the mixture at constant volume is (given that = 8.3 J KT-1 mol-1)
- 21.6 J mol-1 K-1
- 15.7 J mol-1 K-1
- 19.7 J mol-1 K-1
- 17.4 J mol-1 K-1
Answer: 4. 17.4 J mol-1 K-1
Helium → monatomic \(\left(C_V=\frac{3}{2} R\right)\)
Hydrogen → diatomic \(\left(C_V=\frac{5}{2} R\right)\)
∴ \(\left(C_V\right)_{\text {mixture }}=\frac{n_1 C_{V_1}+n_2 C_{V_2}}{n_1+n_2}\)
⇒ \(\frac{2\left(\frac{3}{2} R\right)+3\left(\frac{5}{2} R\right)}{2+3}=\frac{21}{10} R\)
= \(\frac{21}{10}\)(8.3Jmol-1K-1)
= 17.43 J mol-1 K-1.
Question 44. A sample of n moles of an ideal gas with heat capacity at constant volume Cv undergoes an isobaric expansion by a certain volume. The ratio of the work done in the process to the heat supplied is
- \(\frac{4 n R}{C_V+n R}\)
- \(\frac{n R}{C_V+n R}\)
- \(\frac{n R}{C_V-n R}\)
- \(\frac{4 n R}{C_V-n R}\)
Answer: 2. \(\frac{n R}{C_V+n R}\)
Heat capacity of n moles of a gas at constant volume = nCv-Cv (given).
Work done = ΔW = pΔV = nRΔT,
Heat absorbed = nCpΔT = n(Cv + R)ΔT
⇒ (nCv + nR)ΔT = (C2 + nR)ΔT.
∴ ratio = \(\frac{\Delta W}{\Delta Q}=\frac{n R \Delta T}{\left(C_V+n R\right) \Delta T}=\frac{n R}{C_V+n R}\)
Question 45. Half a mole of an ideal monatomic gas is heated at a constant pressure of 1 atm from 20°C to 90°C. The work done by the gas is close to (take R = 8.31 J mol-1 K-1)
- 291 J
- 581 J
- 146 J
- 73 J
Answer: 1. 291 J
Work done by a gas is ΔW = pΔV = nRΔT.
Substituting the given values,
AW = (\(\frac{1}{2}\) mol) (8.31 J mol-1K-1)(70 K)
= 291 J
Question 46. A diatomic ideal gas undergoes an adiabatic process at room temperature. The relation between temperature and volume for this process is TVn = constant. The value of n is
- \(\frac{2}{5}\)
- \(\frac{3}{5}\)
- \(\frac{5}{3}\)
- \(\frac{2}{3}\)
Answer: 1. \(\frac{2}{5}\)
For an adiabatic process, TVγ-1 = constant.
For a diatomic gas, \(C_V=\frac{5 R}{2}, C_p=\frac{7 R}{2}\)
∴ y = \(\frac{7}{5}\)
Given that TVn = constant.
∴ \(n=\gamma-1=\frac{7}{5}-1=\frac{2}{5}\)
Question 47. Three moles’ of oxygen is mixed with 5 moles of argon, both at temperature T. The total internal energy of the mixture is
- 15RT
- 12RT
- 19RT
- 10RT
Answer: 1. 15RT
Oxygen is diatomic, meaning that f = 5, and argon is monatomic, implying f = 3.
Total internal energy \(U=\left(\frac{1}{2} k T\right)(3 N) 5+\left(\frac{1}{2} k T\right)(5 N) 3\)
= 15NkT
= 15JRT.
Question 48. The internal energy of 1 mol of a nonlinear triatomic molecule at temperature T is
- \(\frac{9}{2}\) RT
- \(\frac{3}{2}\) RT
- 3RT
- \(\frac{5}{2}\) RT
Answer: 3. 3RT
For a nonlinear triatomic molecule,f = 6.
∴ \(U=\left(\frac{1}{2} k T\right)(6 N)\)
= 3 RT
Question 49. 0.1 mol of a gas at 200 K is mixed with 0.05 mol of the same gas at 400 K. If the final temperature of the mixture is 10T0, the value of T0 is (in kelvin)
- 20.44
- 26.66
- 22.44
- 25.15
Answer: 2. 26.66
Conserving internal energy,
⇒ \(n_1 C_V T_1+n_2 C_V T_2=\left(n_1+n_2\right) C_V T_0\)
⇒ (0.1)(200 K) + (0.05)(400 K) = (0.15)(10T0)
⇒ \(T_0=\frac{20 \mathrm{~K}+20 \mathrm{~K}}{0.15}\)
= 26.66 K
Question 50. The absorption of 160 J of heat by an ideal gas at constant pressure increases its temperature by50°C. For the same gas, the temperature rises by 100°C when 240 J of heat is absorbed at constant volume. The number of degrees of freedom of each of its molecules is
- 5
- 3
- 6
- 7
Answer: 3. 6
At constant pressure, ΔQ = nCpΔT
⇒ 160 J = nCp 50.
At constant volume, 240 J = nCv .100
∴ \(\frac{C_p}{C_V}=\gamma\)
= \(\frac{160}{240} \times \frac{100}{50}\)
= \(\frac{4}{3}\)
But y =1 + \(\frac{2}{f}\)
⇒ f = 6.
Question 51. An ideal diatomic gas undergoes an adiabatic process in which its density is increased to 32 times its original value. If the pressure increases to n times its original value, the value of n will be
- 4
- 8
- 64
- 128
Answer: 4. 128
For an adiabatic process, pVγ = p’V’γ
⇒ \(p\left(\frac{M}{\rho}\right)^\gamma=p^{\prime}\left(\frac{M}{\rho^{\prime}}\right)^\gamma\)
⇒ \(p\left(\frac{1}{\rho}\right)^\gamma=(n p)\left(\frac{1}{32 \rho}\right)^\gamma\)
=> n = 32γ
= (25)7/5
= 128 [ ∵ y =\(\frac{7}{5}\)]
Question 52. A bullet of 5 g moving at 210 m s-1 strikes a fixed wooden target. Half of its kinetic energy is converted into heat in the bullet while the remaining half is absorbed by wood. What is the rise in temperature of the bullet? (Given that the specific heat capacity of the bullet’s metal = 0.03 cal g-1 °C-1 and 1 cal = 4.2 J)
- 83.3°C
- 38.4°C
- 87.5 °C
- 110°C
Answer: 3. 87.5 °C
Kinetic energy, E = \(\frac{1}{2}\)mv²;
Heat absorbed = H = \({E}{2}\) = \(\frac{1}{4}\)mv²
= mcΔθ.
Hence, rise in temperature,
⇒ \(\Delta \theta=\frac{v^2}{4 c}\)
= \(\frac{\left(210 \mathrm{~m} \mathrm{~s}^{-1}\right)^2}{4\left(0.03 \times 4200 \mathrm{~J} \mathrm{~kg}^{-1}{ }^{\circ} \mathrm{C}^{-1}\right)}\)
= 87.5°C.
Question 53. A helium-filled balloon at 32°C and 1.7 atm suddenly burst. Immediately after it bursts, the expansion of helium can be considered as
- Irreversible isothermal
- Irreversible adiabatic
- Reversible adiabatic
- Reversible isothermal
Answer: 2. Irreversible adiabatic
An isothermal process is slow but bursting is fast and sudden. Hence, the bursting of the balloon is an irreversible adiabatic process.
