Thermal Expansion and Calorimetry
Question 1. A Celsius thermometer and a Fahrenheit thermometer are dipped in boiling water. The water temperature is lowered until the Fahrenheit thermometer registers 140 °F. What is the fall in temperature as registered by the Celsius thermometer?
- 80°C
- 30 °C
- 60°C
- 40 °C
Answer: 4. 40 °C
Given that F = 140°.
Putting this value of F in the relation
⇒ \(\frac{F-32}{9}=\frac{C}{5}\), we get
⇒ \(\frac{140-32}{9}=\frac{C}{5}\)
or, \(C=60^{\circ} \mathrm{C}\)
The fall in temperature in the Celsius scale is
AC = 100°C – 60 °C
= 40°C

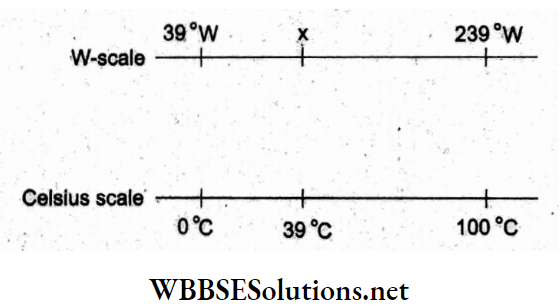
Question 2. On a new scale of temperature (which is linear), called the W-scale, the freezing and boiling points of water are 39 °W and 239°W respectively. What will be the temperature on the new W-scale, corresponding to a temperature of 39 °C on the Celsius scale?
- 200°W
- 117°W
- 78 °W
- 139°W
Answer: 2. 117°W
Comparing the two scales, we get
“mcqs on heat “
⇒ \(\frac{x-39}{239-39}=\frac{39-0}{100-0}\)
x = 117°W
Question 3. The coefficients of linear expansion of a brass rod and a steel rod are 04 and their lengths are l1 and l2 respectively. If (l2– l1) is maintained the same at all temperatures, which of the following relations holds good?
- \(\alpha_1^2 l_2=\alpha_2{ }^2 l_1\)
- \(\alpha_1 l_1=\alpha_2 l_2\)
- \(\alpha_1 l_2=\alpha_2 l_1\)
- \(\alpha_1 l_2^2=\alpha_2 l_1^2\)
Answer: 2. \(\alpha_1 l_1=\alpha_2 l_2\)
Coefficient of linear expansion \(\alpha=\frac{l_2-l_1}{l_1 \Delta T}\)
Increase in length = (l2– l1) = l1αΔT.
Read And Learn Also NEET Physics Multiple Choice Question and Answers
Let the initial lengths of brass and steel be l1 and l2 and on increasing the temperature by AT let their lengths be l’1 and l’2.
So, (l’1 – l1)= α1ΔT and (l2 —l2) = l2α2ΔT.
Subtracting, (l’1 – l2) – (l1 – l2) = (l1α1 -l2α2)AT.
Since the change in length (l2– l1) is maintained the same,
l1α1 – l2α2 = 0
α1l1 = α2l2
Question 4. The coefficient of volume expansion of glycerine is 5 x 10-4 K-1. The fractional change in the density of glycerine for a rise of 40°C in its temperature is
- 0.025
- 0.015
- 0.010
- 0.020
Answer: 4. 0.020
Since the mass remains constant,
Vθpθ = Vθp0
or, \(\frac{\rho_\theta}{\rho_0}=\frac{V_0}{V_\theta}=\frac{V_0}{V_0(1+\gamma \theta)}\)
= \((1-\gamma \theta)\)
heat mcqs
or, \(\frac{\rho_0-\rho_\theta}{\rho_0}=\gamma \theta\)
∴ the fractional change in density is
⇒ \(\frac{\Delta \rho}{\rho}=\frac{\rho_0-\rho_\theta}{\rho_0}=\gamma \theta\)
= \(\left(5 \times 10^{-4} \mathrm{~K}^{-1}\right)(40 \mathrm{~K})\)
= 0.020.
Question 5. The density of water at 20°C is 998 kg m-3 and at 40°C it is 992 kg m-3. The coefficient of volume expansion of water is
- 3 x 10-4 °C-1
- 2 x 10-4 °C-1
- 6 x 10-4 °C-1
- 10-4 °C -1
Answer: 1. 3 x 10-4 °C-1
Since the fractional change in the density of a liquid is
⇒ \(\frac{\Delta \rho}{\rho}=\gamma \theta \Rightarrow \frac{\rho_{20^{\circ} \mathrm{C}}-\rho_{40^{\circ} \mathrm{C}}}{\rho_{20^{\circ} \mathrm{C}}}\)
= \(\gamma\left(40^{\circ} \mathrm{C}-20^{\circ} \mathrm{C}\right)\)
\(\frac{998 \mathrm{~kg} \mathrm{~m}^{-3}-992 \mathrm{~kg} \mathrm{~m}^{-3}}{998 \mathrm{~kg} \mathrm{~m}^{-3}}\)= \(\gamma \times 20^{\circ} \mathrm{C}\)
Hence, the coefficient of volume expansion of water is
⇒ \(\gamma=\frac{6}{20^{\circ} \mathrm{C}(998)}=\frac{3}{10(998)^{\circ} \mathrm{C}} \approx 3 \times 10^{-4}{ }^{\circ} \mathrm{C}^{-1}\)
Question 6. 4.0 g of a gas occupies 22.4 L at stp. The molar heat capacity of the gas at constant volume is 5.0 J mol-1 K-1. If the speed of sound in this gas at NTP is 952 m s-1 then the molar heat capacity of that gas at constant pressure is (take R = 8.3 J K-1 mol-1)
- 8.0 J K-1 mol-1
- 8.5 J K-1 mol-1
- 7.0 J K-1 mol-1
- 7.5 J K-1 mol-1
Answer: 1. 8.0 J K-1 mol-1
Given that mass m- 4 g; volume V = 22.4 L; Cv = 5 J 10-1 mol-1; velocity
of sound v = 952 ms-1.
∵ velocity of sound, v = \(v=\sqrt{\frac{\gamma p}{\rho}}=\sqrt{\frac{\gamma p V}{M}}\)
∴ \(\frac{C_p}{C_V}=\gamma=\frac{M}{p V} v^2\)
⇒ \(C_p=C_V \frac{M}{p V} v^2=\left(5 \mathrm{~J} \mathrm{~mol}^{-1} \mathrm{~K}^{-1}\right) \frac{\left(4 \times 10^{-3} \mathrm{~kg}\right)\left(952 \mathrm{~m} \mathrm{~s}^{-1}\right)^2}{\left(10^5 \mathrm{~Pa}\right)\left(\left(22.4 \times 10^{-3} \mathrm{~m}^3\right)\right.}\)
= 8.09 J mol-1K-1
Question 7. 1.0 g of ice at its melting point is mixed with 1.0 g of steam. At thermal equilibrium, the resultant temperature of the mixture is
- 240°C
- 100°C
- 120°C
- 200 °C
Answer: 2. 100°C
Heat required to convert 1.0 g of ice (at 0°C) to water (at 100°C) is
H = mLice + mCwater (100°C – θ)
= (1.0 g)(80 cal g-1) + (1.0 g) (1 cal g-1 °C-1)(100°C)
= 80 cal + 100 cal
= 180 cal.
The heat released by 1.0 g of steam to convert itself into water at 100°C is 540 cal, which is greater than 180 cal. Hence, the temperature of the mixture will be100°C.
Question 8. If 10 g of ice is added to 40 g of water at 15 °C then the temperature of the mixture is (Cwater = 4.2 x 103 J kg-1 K-1, Lice = 3.36 x 10s J kg-1)
- 10°C
- 12°C
- 0°C
- 15°C
Answer: 3. 0°C
The heat required to melt 10 g of ice (at 0°C) to water (at 0°C) is
H1 = mLice =(10 x 10-3 kg)(3.36 x 105 J kg-1)
= 3360J.
heat mcqs
Heatlostby 40 g of water to cool down from 15°C to 0°C is
H2 = mCwater Δθ
= (40 x 10-3 kg) (4.2 x 10³ J kg-1 K-1)(15 °C – 0°C)
=2520J.
Since H2 < H1 heat lost by water is not sufficient to melt all the ice, the the temperature of the mixture, containing some ice and water will be 0°C.
| Class 11 Physics | Class 12 Maths | Class 11 Chemistry |
| NEET Foundation | Class 12 Physics | NEET Physics |
Question 9. A body initially at 80°C cools down to 64°C in 5 min and 52°C in 10 min. The temperature of the surroundings is
- 16 °C
- 36 °C
- 40 °C
- 26 °C
Answer: 1. 16 °C
According to Newton’s law of cooling, the rate of cooling = the difference in temperature between the body and the surroundings
⇒ \(\frac{\theta_1-\theta_2}{t}=k\left(\frac{\theta_1+\theta_2}{2}-\theta_0\right)\)
⇒ \(\frac{80^{\circ} \mathrm{C}-64^{\circ} \mathrm{C}}{5}=k\left(\frac{80+64}{2}-\theta_0\right)\)
⇒ \(\frac{16}{5}=k\left(72-\theta_0\right)\)….(1)
Similarly, \(\frac{64-52}{5}=k\left(58-\theta_0\right)\)
⇒ \(\frac{12}{5}=k\left(58-\theta_0\right)\)….(2)
Taking the ratio of (1) and (2),
⇒ \(\frac{16}{5} \times \frac{5}{12}=\frac{72-\theta_0}{58-\theta_0}\)
⇒ \(\frac{4}{3}=\frac{72-\theta_0}{58-\theta_0}\)
θ0 =16 °C
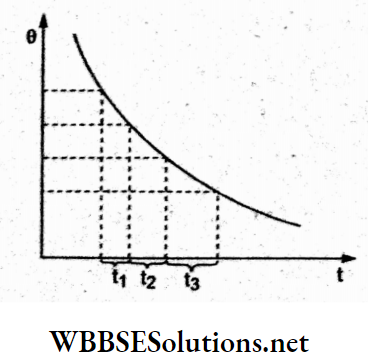
Question 10. A beaker full of hot water is kept in a room. If it cools from 80 °C to i 75 °C in t1 min, from 75°C to 70°C in t2 min and from 70 °C to 65 °C in t3 min then
t1 = t2 = t3
t1< t2 = t3
t1<t2<t3
t1>t2> t3
Answer: 3. t1<t2<t3
The cooling curve (temperature 0 vs time t) is shown in the figure. The slope of this curve \(\left(\frac{d \theta}{d t}\right)\) gives the instantaneous rate of cooling, which decreases as time increases. So, for the same fall in temperature successively more time is required. Hence, t1<t2< t3.
Question 11. Water cools from 70 °C to 60°C in the first 5 min and to 54°C in the next 5 min. The temperature of the surroundings is
- 45 °C
- 10 °C
- 20 °C
- 42°C
Answer: 1. 45 °C
According to the law of cooling,
⇒ \(\frac{70-60}{5}=k\left(\frac{70+60}{2}-\theta_0\right)\)
or 2 = k(65 – θ0),…..(1)
Similarly,
heat mcqs
⇒ \(\frac{60-54}{5}=k\left(\frac{60+54}{2}-\theta_0\right)\)
or, \(\frac{6}{5}=k\left(57-\theta_0\right)\)…..(2)
Dividing (1) by (2),
⇒ \(\frac{5}{3}=\frac{65-\theta_0}{57-\theta_0}\)
θ0 = 45C.
Question 12. A body cools from temperature 3T to 2T in 10 min. The room temperature is T. Assuming that Newton’s law of cooling is applicable, the temperature of the body at the end of the next 10 minutes will be
- \(\frac{7}{4}\)T
- \(\frac{3}{2}\)T
- \(\frac{4}{3}\)T
- T
Answer: 2. \(\frac{3}{2}\)T
From the law of cooling, \(\frac{3 T-2 T}{10 \min }=k(1.5 \mathrm{~T})\)
\(k=\left(\frac{2}{30 \min }\right)\)After the next 10 min, let x be the temperature of the body. So,
⇒ \(\frac{2 T-x}{10 \min }=k\left(\frac{2 T+x}{2}-T\right)=\left(\frac{2}{30 \min }\right)\left(\frac{x}{2}\right)\)
⇒ \(2 T-x=\frac{x}{3} \Rightarrow x=\frac{3 T}{2}\)
Question 13. A piece of ice falls from a height h so that it melts completely. Only one quarter of the heat produced is absorbed by the ice and all the energy of the ice gets converted into heat during its fall. The value of ft is (Lice = 3.4 X 105 J kg-1, g = 10 N kg-1)
- 34 km
- 68 km
- 136 km
- 544 km
Answer: 3. 136 km
Loss in potential energy mgh is spent in the form of heat, i.e., H = mgh.
Since one-quarter of the heat is used in melting the ice,
⇒ \(\frac{1}{4}\)mgh = mLice.
height = \(h=\frac{4\left(L_{\text {ice }}\right)}{g}=\frac{4\left(3.4 \times 10^5 \mathrm{~J} \mathrm{~kg}^{-1}\right)}{\left(10 \mathrm{~N} \mathrm{~kg}^{-1}\right)}\)
= 136 x 10³ m
= 136 km.
Question 14. Steam at 100°C is passed into 20 g of water at10°C. When the water acquires a temperature of 80°C, the mass of the water present will be (Cwater =1 cal g-1 °C-1, Lsteam– 540 cal g )
- 24 g
- 31.5 g
- 5g
- 22.5 g
Answer: 4. 22.5 g
Let m be the mass of steam (at 100°C), which is condensed into water at a temperature of 80°C.
∴ heat lost by steam is
⇒ \(H_1=m L_{\text {steam }}+m C_{\text {water }} \Delta \theta\)
= m(540 cal g-1) + m(1 cal g-1 °C-1)(100°C – 80°C)
= 560m cal g-1
Heat gained by water to increase its temperature is
heat mcqs
H2 = mwater Cwater A0 = (20 g)(l cal g-1 °C-1)(80°C-10 °C)
= 1400 cal.
Since heat gained- heat lost, H1 = H2.
∴ 560m cal g-1 = 1400 cal
m = 2.5 g.
∴ total mass of water present = 22.5 g.
Question 15. Ice cubes of mass 10 g are released at 0°C in a calorimeter (water equivalent = 55 g) at 40°C. Assuming no loss of heat to the surroundings, the temperature of water in the calorimeter becomes (Lice = 80 cal g-1)
- 30 °C
- 20°C
- 22°C
- 15°C
Answer: 3. 22°C
Let the final temperature of the system be 0.
Heat lost by ice is
H1 = miceLice + mice Cwater (θ – 0°C)
= (10 g)(80 cal g-1) + (10 g)(1 cal g-1 °C-1)0
= 800 cal + 100 cal.
Heat lost by calorimeter is
H2 = (water equivalent)(l cal g-1 °C-1)(40 °C – θ)
= (55 g)(l cal g-1 °C-1)(40°C- θ).
Equating H1 and H2,
800 + 10θ = 55(40-θ)
0 = 21.54°C ≈ 22°C
Question 16. The amount of heat energy required to raise the temperature of 1g of helium at stp from T1 K to T2 K is
- \(\frac{3}{8} N_{\mathrm{A}} k_{\mathrm{B}}\left(T_2-T_1\right)\)
- \(\frac{3}{2} N_{\mathrm{A}} k_{\mathrm{B}}\left(T_2-T_1\right)\)
- \(\frac{3}{4} N_{\mathrm{A}} k_{\mathrm{B}}\left(T_2-T_1\right)\)
- \(\frac{3}{4} N_{\mathrm{A}} k_{\mathrm{B}} \frac{T_2}{T_1}\)
Answer: 1. \(\frac{3}{8} N_{\mathrm{A}} k_{\mathrm{B}}\left(T_2-T_1\right)\)
Helium is monatomic and has three degrees of freedom for translational motion.
Further,
⇒ \(1 \mathrm{~g} \text { of } \mathrm{He}=\frac{1}{4} \mathrm{~mol}\)
⇒ \(U_{\mathrm{i}}=\left(\frac{1}{2} k_{\mathrm{B}} T_1\right) \frac{3}{4} N_{\mathrm{A}} \text { and } U_{\mathrm{f}}\)
= \(\left(\frac{1}{2} k_{\mathrm{B}} T_2\right) \frac{3}{4} N_{\mathrm{A}}\)
energy required = \(\Delta U=U_{\mathrm{f}}-U_{\mathrm{i}}\)
= \(\frac{3}{8} N_{\mathrm{A}} k_{\mathrm{B}}\left(T_2-T_1\right)\)
Question 17. At 10°C, the value of the density of a fixed mass of an ideal gas divided by its pressure is x. At 110°C this ratio is
- x
- \(\frac{283}{383} x\)
- \(\frac{10}{110} x\)
- \(\frac{383}{283} x\)
Answer: 2. \(\frac{283}{383} x\)
From the gas equation,
pV = nRT
or, pV = \(\frac{m}{M}\)RT, where m = mass ofgas and M = molar mass
or, \(p\left(\frac{V}{m}\right)=\left(\frac{R}{M}\right) T\)
or, \(\frac{p}{p}\) = constant x T.
∴ \(\frac{\rho}{p} \propto \frac{1}{T}\)
In the given situations,
⇒ \(\frac{\rho_1 / p_1}{\rho_2 / p_2}=\frac{T_2}{T_1}\)
or, \(\frac{x}{\rho_2 / p_2}=\frac{110+273}{10+273}=\frac{383}{283}\)
heat mcqs
∴ required ratio, \(\frac{\rho_2}{p_2}=\frac{283}{383} x\)
Question 18. The equation of state for 5g of oxygen at a pressure p and temperature T, when occupying a volume V, will be
- pV = \(\frac{5}{32}\) RT
- pV = 5 RT
- pV = \(\frac{5}{2}\) RT
- pV = \(\frac{5}{16}\) RT
Answer: 1. pV = \(\frac{5}{32}\) RT
For oxygen, molar mass = M = 32 g.
∴ number of moles in5 g is \(n=\frac{m}{M}=\frac{5}{32}\)
For gas equation,
pV=nRT
or PV = \(\frac{5}{32}\)
Question 19. A thermometer graduated according to a linear scale reads a value x0 when in contact with boiling water and x0/3 when in contact with ice. What is the temperature of an object in °C if this thermometer in contact with the object reads x0/2?
- 40
- 60
- 35
- 25
Answer: 4. 25
Since the graduation is linear, we have from the given scale,
⇒ \(\frac{100^{\circ} \mathrm{C}-0^{\circ} \mathrm{C}}{\theta-0^{\circ} \mathrm{C}}=\frac{x_0-\frac{x_0}{3}}{\frac{x_0}{2}-\frac{x_0}{3}}\)
or, \(\frac{100^{\circ} \mathrm{C}}{\theta}=\frac{2}{3} \times \frac{6}{1}=4\)
0 = 25°C
fig
Question 20. Two rods A and B of identical dimensions are at a temperature 30 °C. If A is heated up to 180°C and B up to 0°C then their new lengths are found to be the same. If the ratio of the coefficients of linear expansion of A and B is 4: 3 then the value of 0 is
- 220 °C
- 270 °C
- 230°C
- 250 °C
Answer: 3. 230°C
Since the change in length is the same for both A and B,
ΔLA = ΔLB
or αAL(180°C – 30°C) = αBL(θ – 30°C)
or, \(\frac{\alpha_{\mathrm{A}}}{\alpha_{\mathrm{B}}}\left(150^{\circ} \mathrm{C}\right)=\theta-30^{\circ} \mathrm{C}\)
Hence, θ = \(\frac{4}{3}\) x 150°C + 30°C
= 230°C.
Question 21. A thermally insulated vessel contains 150 g of water at 0°C. Then, die air from the vessel is pumped out adiabatically when a fraction of water turns into ice and the rest evaporates at 0°C itself. The mass of evaporated water will be closest to (given that Lice = 3.36 x 105 J kg-1, Lwater = 2.10 x 106J kg-1)
- 130 g
- 35g
- 20 g
- 150 g
Answer: 3. 20 g
Let x = mass of water frozen twice and y = mass of water that evaporates.
Thus, x + y = 150 g……(1)
The amount of heat Q1 released during freezing will be sufficient to evaporate water.
Thus,
xLice = yLwater => x(3.36 x 105) = y(2.10 x 106)
x = \(\frac{21.0}{3.36}\)y
= 6.25y.
Substituting in (1),
6.25y + y = 150g
y = 20.6 g
= 20g.
Question 22. When Mj g of ice at -10 °C (specific heat capacity = 0.5 cal g-1 °C-1) is added to M2 g of water at 50°C, finally no ice is left and the final temperature of the mixture is 0°C. The latent heat of ice (in cal g-1) is
- \(\frac{5 M_1}{M_2}-50\)
- \(\frac{50 M_2}{M_1}\)
- \(\frac{5 M_2}{M_1}-5\)
- \(\frac{50 M_2}{M_1}-5\)
Answer: 4. \(\frac{50 M_2}{M_1}-5\)
Heat gained by ice,
Q1 = M1Cice(Δθ) + M1Lice
⇒ \(M_1\left(\frac{1}{2} {cal~g}^{-1}{ }^{\circ} \mathrm{C}^{-1}\right)\left(10^{\circ} \mathrm{C}\right)+M_1 L \mathrm{cal} \mathrm{g}^{-1}\)
= 5M1cal g-1 + M1L cal g-1
= M1(L + 5) cal g-1.
Heat lost by water,
Q2 = M2Cwater Aθ
= M2(1 cal g-1 °C)50°C
= 50 M2cal g-1.
Since heat lost = heat gained,
heat mcqs
Ml(L + 5) = 50M2
⇒ \(L=\frac{50 M_2}{M_1}-5\)
Question 23. A rod is heated from 0°C to 10°C so that its length is changed by 0.02%. What is the percentage change in its mass density?
- 0.02
- 0.08
- 0.04
- 0.06
Answer: 4. 0.06
⇒ \(\alpha=\frac{1}{L} \frac{\Delta L}{\Delta T}\)
= \(\frac{1}{\Delta T}\left(\frac{\Delta L}{L}\right)\)
= \(\frac{1}{10^{\circ} \mathrm{C}}\left(\frac{0.02}{100}\right)\)
= \(2 \times 10^{-5}{ }^{\circ} \mathrm{C}^{-1}\)
∴ y = 3a = 6 x 10-5 °C-1.
Density \(=\rho=\frac{M}{V}\) Hence, the fractional change in density is,
⇒ \(\frac{\Delta \rho}{\rho}\)
= \(-\frac{\Delta V}{V}=-\gamma \Delta T\)
= \(-6 \times 10^{-5} \times 10\)
Hence, percentage change in density = 6 x 10-4 x 100
= 6 x 10-2
= 0.06.
Question 24. A cubical block at 273 K is compressed by an external pressure p uniformly from all directions. To bring the cube back to its original size by heating, the rise in temperature is (take α = coefficient of linear expansion, B = bulk modulus of elasticity)
- \(\frac{p}{2 \alpha B}\)
- \(\frac{p}{4 \alpha B}\)
- \(\frac{p}{3 \alpha B}\)
- \(\frac{p}{\alpha B}\)
Answer: 3. \(\frac{p}{3 \alpha B}\)
Bulk modulus is
⇒ \(B=\frac{\text { stress }}{\text { volume strain }}\)
= \(\frac{p}{\Delta V / V}\)
decrease in volume = AV = \(\frac{pV}{B}\)…..(1)
If ATbe the rise in temperature regains its original volume,
ΔV = yVΔT….(2)
Equating (1) and (2),
⇒ \(\gamma V \Delta T=\frac{p V}{B}\)
⇒ \(\Delta T=\frac{p}{\gamma B}=\frac{p}{3 \alpha B}\)
Question 25. Two rods of the same cross-section and of lengths l1 and l2 have coefficients of linear expansion α1 and α2 respectively. The equivalent coefficient of the linear expansion for their combination in series is
- \(\frac{\alpha_1+\alpha_2}{2}\)
- \(\sqrt{\alpha_1 \alpha_2}\)
- \(\frac{\alpha_1 L_1+\alpha_2 L_2}{L_1+L_2}\)
- \(\frac{\alpha_1 L_2+\alpha_2 L_1}{L_1+L_2}\)
Answer: 3. \(\frac{\alpha_1 L_1+\alpha_2 L_2}{L_1+L_2}\)
L = L2 + L1; ΔL = ΔL1 + ΔL2.
But ΔL1 = L1α1ΔT and ΔL2 = L2α2AT
∴ ΔL = ΔL1 + ΔL2
= (L1α1 + L2α2)ΔT…..(1)
For the series combination,
ΔL = (L1 + L2)αeqΔT……(2)
From (1) and (2),
⇒ \(\alpha_{e q}=\frac{L_1 \alpha_1+L_2 \alpha_2}{L_1+L_2}\)
