Photoelectric Effect
Question 1. The number of photoelectrons emitted for a light of frequency v (higher than the threshold frequency v0) is proportional to
- The intensity of the light
- The threshold frequency (v0)
- v – v0 (d) the frequency of the light
- The frequency of the light
Answer: 1. The intensity of the light
The intensity (I) of light is proportional to the number of photons emitted per second. The greater the intensity, the greater will be the number of photoelectrons emitted (N). Hence, N ∝ I.

Question 2. When a photosensitive surface is illuminated with a radiation of wavelength λ, the stopping potential is V. If the same surface is illuminated with a radiation of wavelength 2λ, the stopping potential is V/4. The threshold wavelength for the given surface is
- 5λ
- 4λ
- 3λ
- \(\frac{5 \lambda}{2}\)
Answer: 3. 3λ
According to the equation of the photoelectric effect,
hv = Φ0 + eVs.
For the wavelength λ,
⇒ \(\frac{h c}{\lambda}=\phi_0+e V\) → (1)
For the wavelength 2λ,
⇒ \(\frac{h c}{2 \lambda}=\phi_0+\frac{e V}{4}\)
or \(\frac{2 h c}{\lambda}=4 \phi_0+e V\) → (2)
Subtracting (1) from (2),
⇒ \(\frac{h c}{\lambda}=3 \phi_0\)
But \(\phi_0=\frac{h c}{\lambda_0}\), where λ0 = threshold wavelength.
∴ \(\frac{h c}{\lambda}=3\left(\frac{h c}{\lambda_0}\right) \text { or } \lambda_0=3 \lambda\).
the charge of a photo electron is
Question 3. The number of ejected photoelectrons increases with an increase in
- The intensity of light
- The wavelength of light
- The frequency of light
- None of these
Answer: 1. The intensity of light
The emission of photoelectrons takes place due to the absorption of the incident photons. Hence, the number of photoelectrons ejected will be proportional to the intensity of the incident light.
Question 4. The threshold frequency for the photoelectric effect on sodium corresponds to a wavelength of 500 nm. Its work function is
- 4 x l0-19 J
- 3 x 10-19 J
- 1J
- 2 x 10-19 J
Answer: 1. 4 x l0-19 J
Photoelectric work function = \(\phi_0=h v_0=\frac{h c}{\lambda_0}\)
⇒ \(\frac{\left(6.67 \times 10^{-34} \mathrm{~J} \mathrm{~s}\right)\left(3 \times 10^8 \mathrm{~m} \mathrm{~s}^{-1}\right)}{500 \times 10^{-9} \mathrm{~m}}\)
∴ 4 x 10-19 J.
Question 5. A light of wavelength 500 nm falls on a photosensitive surface with a photoelectric work function of 1.9 eV. The kinetic energy of the fastest photoelectrons emitted will be
- 0.58 eV
- 2.48 eV
- 1.24 eV
- 1.16 eV
Answer: 1. 0.58 eV
According to Einstein’s photoelectric equation,
hv = Φ0 + KEmax
⇒ \(\frac{h c}{\lambda}=\phi_0+\frac{1}{2} m v_{\max }^2\)
Substituting the values,
⇒ \(\frac{h c}{500 \mathrm{~nm}}=1.9 \mathrm{eV}+\mathrm{KE}_{\max }\)
∴ \(\mathrm{KE}_{\max }=\frac{1242 \mathrm{eV} \mathrm{nm}}{500 \mathrm{~nm}}-1.9 \mathrm{eV}=2.48 \mathrm{eV}-1.9 \mathrm{eV}=0.58 \mathrm{eV}\).
Question 6. Einstein’s work on the photoelectric effect gives support to
- E = mc2
- \(E=\frac{h}{\lambda}\)
- E = hv
- \(h v=\frac{1}{2} m v^2\)
Answer: 3. E = hv
Einstein’s work on the photoelectric effect supports the particle nature of light, which consists of photons with the energy E = hv.
Question 7. If the threshold wavelength for a certain metal is 200 nm, the work function of the metal is
- 6.2 J
- 6.2 eV
- 6.2 MeV
- 6.2 keV
Answer: 2. 6.2 eV
Work function = \(\phi_0=h v_0=\frac{h c}{\lambda_0}\)
Substituting hc =1242 eV nm and λ0 = 200 nm in the above expression,
∴ \(\phi_0=\frac{1242 \mathrm{eV} \mathrm{nm}}{200 \mathrm{~nm}} \approx 6.2 \mathrm{eV}\).
the charge of a photoelectron is
Read And Learn Also NEET Physics Multiple Choice Question and Answers
Question 8. The photoelectric threshold wavelength of silver is 3250 x 10-10 m. The maximum velocity of the electron ejected from a silver surface by ultraviolet radiation of wavelength 2536 x 10-10 m is (given that h = 4.14 x 10-15 eV s and c = 3 x108 m s-1 )
- 0.3 x 106 m s-1
- 6 x l05 m s-1
- 0.6 x 106 m s-1
- 61 x 103 m s-1
Answer: 2. 6 x l05 m s-1
Given that threshold wavelength = λ0 = 3250 Å and wavelength of the incident radiation = λ = 2536 Å.
From the photoelectric equation,
⇒ \(\frac{h c}{\lambda}=\frac{h c}{\lambda_0}+\frac{1}{2} m v_{\max }^2\)
⇒ \(h c\left(\frac{1}{\lambda}-\frac{1}{\lambda_0}\right)=\frac{1}{2} m v_{\max }^2\)
∴ the maximum velocity of the ejected photoelectron is
⇒ \(v_{\max }=\sqrt{\frac{2 h c}{m}\left(\frac{1}{\lambda}-\frac{1}{\lambda_0}\right)}\)
⇒ \(\sqrt{\frac{2\left(6.67 \times 10^{-34} \mathrm{~J} \mathrm{~s}\right)\left(3 \times 10^8 \mathrm{~m} \mathrm{~s}^{-1}\right)}{9.1 \times 10^{-31} \mathrm{~kg}} \times \frac{(3250-2536) \times 10^{10}}{2536 \times 3250 \mathrm{~m}}}\).
≈ 6 x l05 m s-1.
Question 9. A metallic surface is illuminated with a monochromatic light of wavelength λ, The stopping potential for the photoelectric current for this light is 3V0. If the same surface is illuminated with a light of wavelength 2λ, the stopping potential is V0 The threshold wavelength for this photo-sensitive surface is
- 6λ
- 4λ
- \(\frac{\lambda}{4}\)
- \(\frac{\lambda}{6}\)
Answer: 2. 4λ
For the wavelength A,
⇒ \(\frac{h c}{\lambda}=\frac{h c}{\lambda_0}+e\left(3 V_0\right)\) → (1)
For the wavelength 2λ,
⇒ \(\frac{h c}{2 \lambda}=\frac{h c}{\lambda_0}+e V_0\) → (2)
Multiplying (2) by 3 and then subtracting it from (1), we obtain
∴ threshold wavelength = λ0= 4λ.
Question 10. Photons of ultraviolet radiation of 6.2 eV fall on an aluminum surface. The kinetic energy of the fastest electron emitted is (given that work function = 4.2 eV)
- 3.2 x 10-21 J
- 3.2 x 10-19 J
- 7 x 10-25J
- 9 x l0-32 J
Answer: 2. 3.2 x 10-19 J
Given that the energy of a photon = hv = 6: 2 eV and Φ0 = 4.2 eV.
From hv = Φ0 + KEmax , we have
KEmax = hv – Φ0 = 6.2 eV – 4.2 eV
= 2.0 eV = 2.0(1.6 x 10-19 J)
= 3.2 x 10-19 J.
Question 11. A monochromatic light of frequency 6.0 x 1014 Hz is produced by a laser beam. The power emitted is 2 x 10-3 W. The average number of photons emitted by the source per second is
- 5 x 1015
- 5 x l016
- 5 x l017
- 5 x l014
Answer: 1. 5 x 1015
Power of the light source = 2 x 10-3 W = 2 x 10-3 J s-1.
The energy of each photon = hv.
If N is the number of photons emitted per second,
N(hv) = 2 x 10-3 J
⇒ \(N=\frac{2 \times 10^{-3} \mathrm{~J}}{h \mathrm{v}}=\frac{2 \times 10^{-3} \mathrm{~J}}{\left(6.67 \times 10^{-34} \mathrm{~J} \mathrm{~s}\right)\left(6.0 \times 10^{14} \mathrm{~s}^{-1}\right)}=5 \times 10^{15}\).
the charge of a photo electron is
Question 12. During a photoelectric emission from a metal surface of work function 1.8 eV, the kinetic energy of the most energetic electron is 0.5 eV. The corresponding stopping potential is
- 1.3 V
- 2.3 V
- 0.5 V
- 1.8 V
Answer: 3. 0.5 V
Given that KEmax = 0.5 eV.
From the work-energy principle,
work done by the stopping voltage = change in KE
⇒ eVs = KEmax = 0.5 eV
⇒ \(V_{\mathrm{s}}=\frac{0.5 \mathrm{eV}}{e}=0.5 \mathrm{~V}\).
Question 13. A photoelectric emission occurs only when the incident light has more than a certain minimum
- Power
- Intensity
- Frequency
- Wavelength
Answer: 3. Frequency
For photoelectric emission, the frequency of the incident light must be greater than the threshold (minimum) frequency.
Question 14. Electrons of mass m with a de Broglie wavelength of λ fall on the target in an X-ray tube. The cutoff wavelength λ0 of the emitted X-rays is
- \(\lambda_0=\frac{2 m c \lambda^2}{h}\)
- \(\lambda_0=\frac{2 h}{m c}\)
- \(\lambda_0=\frac{2 m^2 c^2 \lambda^3}{h^2}\)
- \(\lambda_0=\lambda\)
Answer: 1. \(\lambda_0=\frac{2 m c \lambda^2}{h}\)
The cutoff wavelength (λ0) of the continuous X-rays corresponds to the maximum kinetic energy of an electron in the X-ray tube.
For an electron of de Broglie wavelength λ,
⇒ \(\mathrm{KE}=\frac{p^2}{2 m}=\frac{(h / \lambda)^2}{2 m}=\frac{1}{2 m} \cdot \frac{h^2}{\lambda^2}\)
Corresponding to this KE, the maximum energy of each photon is
⇒ \(h v_{\max }=\frac{h c}{\lambda_{\min }}, \text { where } \lambda_{\min }=\lambda_0=\text { cutoff wavelength }\)
∴ \(\frac{h c}{\lambda_0}=\frac{h^2}{2 m \lambda^2} \Rightarrow \lambda_0=\frac{2 m c \lambda^2}{h}\).
Question 15. A monochromatic radiation emitted during the electron transition in hydrogen from the first excited state to the ground state irradiates a photosensitive material. The stopping potential is measured to be 3.57 V. The threshold frequency of the material is
- 25 x 1015 Hz
- 4 x 1015Hz
- 1.6 x l015 Hz
- 5 x 1015 Hz
Answer: 3. 1.6 x l015 Hz
The energy of a photon emitted from the hydrogen atom is
⇒ \(\Delta E=h v=13.6\left(\frac{1}{1}-\frac{1}{2^2}\right) \mathrm{eV}=\frac{3}{4} \times 13.6 \mathrm{eV}=10.2 \mathrm{eV}\)
Now, hv = Φ0 + eVs
⇒ 10.2 eV = Φ0 + 3.57 eV
⇒ Φ0 = hv0 = (10.2- 3.57) eV = 6.63 eV.
Hence, the threshold frequency is
∴ \(\mathrm{v}_0=\frac{6.63 \times\left(1.6 \times 10^{-19} \mathrm{~J}\right)}{6.67 \times 10^{-34} \mathrm{Js}}=1.6 \times 10^{15} \mathrm{~Hz}\).
Question 16. When the energy of the incident radiation is increased by 20%, the maximum kinetic energy of the photoelectrons emitted from a metal surface increases from 0.5 eV to 0.8 eV. The work function of the metal is
- 0.65 eV
- 1.5 eV
- 1.3 eV
- 1.0 eV
Answer: 4. 1.0 eV
Initially, hv = Φ0 + = Emax + 0.5 eV. → (1)
With 20% increase in hv, we have
the charge of a photo electron is
⇒ \(h v+\frac{1}{5} h v=\phi_0+0.8 \mathrm{eV}\)
⇒ \(\frac{6}{5} h v=\phi_0+0.8 \mathrm{eV}\)
⇒ \(h v=\frac{5}{6} \phi_0+\frac{2}{3} e V\) → (2)
Equating (1) and (2),
⇒ \(\phi_0+0.5 \mathrm{eV}=\frac{5}{6} \phi_0+\frac{2}{3} \mathrm{eV}\)
∴ work function = Φ0 = 1.0 eV.
Question 17. A photocell employs the photoelectric effect to convert
- A change in the frequency of light into a change in the electric voltage
- A change in the intensity of illumination into a change in the photoelectric current
- A change in the intensity of illumination into a change in the work function of the photocathode
- A change in the frequency of light into a change in the electric current
Answer: 2. A change in the intensity of illumination into a change in the photoelectric current
In a photocell, the radiant energy of the incident light produces a stream of electrons constituting an electric current.
The change in the intensity of light changes the photoelectric current.
Question 18. Photons of energy 5 eV are incident on a cathode (C) in a photoelectric cell. The maximum kinetic energy of the emitted photoelectrons is 2 eV. When photons of energy 6 eV are incident on C, no photoelectrons will reach the anode (A) if the stopping potential of A relative to C is
- +4 V
- +3 V
- -3 V
- -1 V
Answer: 3. -3 V
From Einstein’s photoelectric equation,
hv = Φ0 + KEmax
In the first case,
hv = 5 eV and KEmax = 2 eV.
∴5 eV = Φ0 + 2eV ⇒ Φ0 = 3 eV.
In the second case,
hv = 6 eV.
∴ 6 eV = Φ0 + KEmax
⇒ (6-3) eV = KEmax = (-e)(Vs), where Vs is the stopping potential
⇒ 3eV = e(3V)= -eVs.
Hence, Vs =-3 V.
Question 19. A photosensitive surface is illuminated successively by monochromatic light waves of wavelengths λ and λ/2. If the maximum kinetic energy of the emitted photoelectrons in the second case is thrice that in the first case, the work function of the surface of the material is
- \(\frac{h c}{2 \lambda}\)
- \(\frac{h c}{\lambda}\)
- \(\frac{2 h c}{\lambda}\)
- \(\frac{h c}{3 \lambda}\)
Answer: 1. \(\frac{h c}{2 \lambda}\)
We know that hv = Φ0 + E1, where E1 = maximum KE of the emitted photoelectrons.
In the first case,
⇒ \(\frac{h c}{\lambda}=\phi_0+E_1\) → (1)
and in the second case,
⇒ \(\frac{h c}{\lambda / 2}=\phi_0+E_2\)
Given that E2 = 3E1. Hence,
⇒ \(\frac{2 h c}{\lambda}=\phi_0+3 E_1\)
⇒ \(\frac{2}{3} \cdot \frac{h c}{\lambda}=\frac{\phi_0}{3}+E_1\) → (2)
Subtracting (2) from (1),
⇒ \(\frac{h c}{3 \lambda}=\frac{2 \phi_0}{3}\)
Hence, work function = \(\phi_0=\frac{h c}{2 \lambda}\).
| Class 11 Physics | Class 12 Maths | Class 11 Chemistry |
| NEET Foundation | Class 12 Physics | NEET Physics |
Question 20. A monochromatic light of wavelength 667 nm is produced by a helium-neon laser. The power emitted is 9 mW. The average number of photons reaching per second at a target irradiated by the beam is
- 9 x 1017
- 3 x 1019
- 3 x l016
- 9 x l015
Answer: 3. 3 x l016
Given that λ = 667 run and power = P = 9 mW.
Let N = number of photons reaching per second
Power = \(P=\frac{\text { energy }}{\text { time }}=\frac{N(h v)}{1 \mathrm{~s}}\)
⇒ \(9 \times 10^{-3} \mathrm{~J} \mathrm{~s}^{-1}=N\left(\frac{h c}{\lambda}\right) \mathrm{s}^{-1}\)
⇒ \(N=\frac{9 \times 10^{-3} \mathrm{~J}}{h c / \lambda}=\frac{\left(9 \times 10^{-3}\right)\left(667 \times 10^{-9}\right)}{\left(6.67 \times 10^{-34}\right)\left(3 \times 10^8\right)}=3 \times 10^{16}\).
the charge of a photo electron is
Question 21. When a light beam of wavelength 300 nm falls on a photoelectric emitter, photoelectrons are just emitted. For another emitter, however, light of wavelength 600 nm is sufficient for liberating photoelectrons. The ratio of the work functions of the two emitters is
- 1:2
- 2:1
- 1:4
- 4:1
Answer: 2. 2:1
Just for the emission of photoelectrons, its KE is zero, so that
hv = Φ0 + 0
or \(\frac{h c}{300 \mathrm{~nm}}=\phi_0\) = work function.
Similarly, for the second surface,
⇒ \(\frac{h c}{600 \mathrm{~nm}}=\phi_0^{\prime}\)
∴ \(\frac{\phi_0}{\phi_0^{\prime}}=\frac{\frac{h c}{300 \mathrm{~nm}}}{\frac{h c}{600 \mathrm{~nm}}}=2 \Rightarrow \phi_0: \phi_0^{\prime}=2: 1\).
Question 22. When a light source is at a distance d from a photoelectric cell, the number of photoelectrons emitted from the cell is n. If the distance of the light source from the cell is reduced to d/2, the number of photoelectrons emitted will become
- \(\frac{n}{2}\)
- n
- 2n
- 4n
Answer: 4. 4n
We know that according to the inverse-square law, the intensity (I) of the incident radiation is inversely proportional to the square of the distance from the surface. Thus,
⇒ \(I \propto \frac{1}{d^2}\)
⇒ \(\frac{I_1}{I_2}=\frac{d_2^2}{d_1^2}\) → (1)
The number of photoelectrons emitted is directly proportional to the intensity of the incident radiation. Thus,
⇒ \(\frac{I_1}{I_2}=\frac{n_1}{n_2}\) → (2)
⇒ \(\frac{n_1}{n_2}=\frac{d_2^2}{d_1^2}\)
∴ \(\frac{n}{n_2}=\frac{(d / 2)^2}{d^2}=\frac{1}{4} \Rightarrow n_2=4 n\) [From (1)]
Question 23. A radio transmitter operates at a frequency of 880 kHz and a power of 10 kW. The number of photons emitted per second is
- 1.327 x l037
- 1.327 x l025
- 1.7 x l031
- 1.327 x l045
Answer: 3. 1.7 x l031
Power P = \(\frac{\text { energy }}{\text { time }}=\frac{N(h v)}{1 \mathrm{~s}}\)
⇒ \(N=\frac{P}{h v}=\frac{10 \times 10^3 \mathrm{Js}^{-1}}{\left(6.67 \times 10^{-34} \mathrm{Js}\right)\left(880 \times 10^3 \mathrm{~s}^{-1}\right)}=1.7 \times 10^{31} \mathrm{~s}^{-1}\).
Question 24. A 200-W lamp emits a monochromatic light of wavelength 0.6 nm. Assuming it to be 25% efficient in converting the electrical energy to light, the number of photons of light emitted per second is
- 1.55 x l020
- 3 x l019
- 62 x l020
- 6 x l018
Answer: 1. 1.55 x l020
Power = \(\frac{\text { energy }}{\text { time }}=\frac{N}{t}(h v)\),
the charge of a photo electron is
where hv = energy of each photon and N/t = number of photons emitted per second.
For 25% efficiency,
⇒ \(P^{\prime}=\frac{P}{4}=\frac{200 \mathrm{~W}}{4}=50 \mathrm{~W}\)
⇒ \(50 \mathrm{~W}=\left(\frac{N}{t}\right)\left(\frac{h c}{\lambda}\right)\)
∴ \(\frac{N}{t}=\frac{\left(50 \mathrm{~J} \mathrm{~s}^{-1}\right)\left(0.6 \times 10^{-6} \mathrm{~m}\right)}{\left(6.6 \times 10^{-34} \mathrm{~J} \mathrm{~s}\right)\left(3 \times 10^8 \mathrm{~m} \mathrm{~s}^{-1}\right)}=1.55 \times 10^{20} \mathrm{~s}^{-1}\)
Question 25. A photosensitive metallic surface has a work function of hv0. If photons of energy 2hv0 fall on this surface, the electrons come out with a maximum velocity of 4 x 106 m s-1. When the photon energy is increased to 5hv0, the maximum velocity of the photoelectrons will be
- 8 x 105 m s-1
- 2 x l07 m s-1
- 2 x l06 m s-1
- 8 x l06 m s-1
Answer: 4. 8 x l06 m s-1
We know that hv = \(\phi_0+\frac{1}{2} m v_{\max }^2\).
For the incident photon of energy 2hv0,
vmax = 4 x 106 m s-1
⇒ \(2 h v_0=h v_0+\frac{1}{2} m\left(4 \times 10^6 \mathrm{~m} \mathrm{~s}^{-1}\right)^2\) → (1)
With photons of energy 5hv0,
⇒ \(5 h v_0=h v_0+\frac{1}{2} m v_{\max }^2\) → (2)
From (1) and (2),
⇒ \(h v_0=\frac{1}{2} m\left(4 \times 10^6 \mathrm{~m} \mathrm{~s}^{-1}\right)^2\) → (3)
and \(4 h v_0=\frac{1}{2} m v_{\max }^2\) → (4)
Now, dividing (3) by (4),
∴ \(\left(\frac{v_{\max }}{4 \times 10^6 \mathrm{~m} \mathrm{~s}^{-1}}\right)^2=4 \Rightarrow v_{\max }=8 \times 10^6 \mathrm{~m} \mathrm{~s}^{-1}\).
Question 26. Light beams of two different frequencies, whose photons have energies of 1 eV and 2.5 eV respectively, illuminate successively a metallic surface whose work function is 0.5 eV. The ratio of the maximum speeds of the emitted electrons will be
- 1:2
- 1:5
- 1 :1
- 1:4
Answer: 1. 1:2
For photons of energy 1 eV, we have
⇒ \(1 \mathrm{eV}=\phi_0+\frac{1}{2} m v_{\max }^2=0.5 \mathrm{eV}+\frac{1}{2} m v_1^2\)
⇒ \(0.5 \mathrm{eV}=\frac{1}{2} m v_1^2\) → (1)
Similarly, for 2.5-eV photons,
⇒ \(2.5 \mathrm{eV}=0.5 \mathrm{eV}+\frac{1}{2} m v_2^2\)
⇒ \(2.0 \mathrm{eV}=\frac{1}{2} m v_2^2\) → (2)
Dividing (2) by (1)
∴ \(\frac{v_2^2}{v_1^2}=\frac{2}{0.5}=4 \Rightarrow \frac{v_1}{v_2}=\frac{1}{2} \Rightarrow v_1: v_2=1: 2\).
Question 27. When ultraviolet rays are incident on a metal surface, photoelectrons are not emitted. The emission of electrons will occur by the incidence of
- Infrared rays
- X-rays
- Radio waves
- Lightwaves
Answer: 2. X-rays
For the emission of photoelectrons, the frequency of the incident radiations must be greater than the threshold frequency. No photoemission by the UV rays means that the frequency of the UV rays is less than the threshold frequency. Hence, photoemission will occur when the frequency is greater than that of the ultraviolet rays, which is true only for X-rays.
Question 28. The energy of a photon of a light beam is 3 eV. Then, the wavelength of the photon must be
- 4125 nm
- 414 nm
- 41250 nm
- 4nm
Answer: 2. 414 nm
The energy of a photon is
the charge of a photo electron is
⇒ \(h v=\frac{h c}{\lambda}=3 \mathrm{eV}\)
∴ Wavelength = \(\lambda=\frac{h c}{3 \mathrm{eV}}=\frac{1242 \mathrm{eV} \mathrm{nm}}{3 \mathrm{eV}}=414 \mathrm{~nm}\)
Question 29. In a photoemissive cell with an exciting wavelength of λ, the fastest electron has a speed of v. If the exciting wavelength is reduced to 3λ/4, the speed of the fastest electron will be
- \(\left(\frac{3}{4}\right)^{-1 / 2}\).v
- \(\left(\frac{4}{3}\right)^{1 / 2}\).v
- Less than \(\left(\frac{4}{3}\right)^{1 / 2}\).v
- Greater than \(\left(\frac{4}{3}\right)^{1 / 2}\).v
Answer: 4. Greater than \(\left(\frac{4}{3}\right)^{1 / 2}\).v
For photoemission,
⇒ \(\frac{h c}{\lambda}=h v=\phi_0+\frac{1}{2} m v_{\max }^2=\phi_0+\frac{1}{2} m v^2\).
Since the exciting wavelength is reduced to 3λ/4, let the maximum speed of an electron be vmax
∴ \(\frac{h c}{3 \lambda / 4}=\phi_0+\frac{1}{2} m v_{\max }^{\prime 2}\)
⇒ \(\frac{4 h c}{3 \lambda}=\phi_0+\frac{1}{2} m v_{\max }^{\prime 2}\)
∴ \(\frac{\frac{1}{2} m v_{\max }^{\prime 2}}{\frac{1}{2} m v^2}=\frac{\frac{4}{3} \cdot \frac{h c}{\lambda}-\phi_0}{\frac{h c}{\lambda}-\phi_0}=\frac{\frac{4}{3}\left(\frac{h c}{\lambda}-\phi_0\right)+\frac{\phi_0}{3}}{\frac{h c}{\lambda}-\phi_0}\)
⇒ \(\frac{4}{3}+\frac{\phi_0}{3\left(\frac{h c}{\lambda}-\phi_0\right)}=\frac{4}{3}+(\text { a positive constant })\)
⇒ \(\frac{v_{\max }^{\prime}}{v}>\sqrt{\frac{4}{3}}\)
∴ \(v_{\max }^{\prime}>\left(\frac{4}{3}\right)^{1 / 2} \cdot v\).
Question 30. The work function of the surface of a photosensitive material is 6.2 eV. The wavelength of the incident radiation for which the stopping potential is 5 V lies in the
- Ultraviolet region
- Visible region
- Infrared region
- X-ray region
Answer: 1. Ultraviolet region
Given that work function = Φ0 = 6.2 eV.
If stopping potential = Vs = 5V, from the photoelectric equation we have
⇒ \(h v=\phi_0+\frac{1}{2} m v_{\max }^2\)
⇒\(\frac{h c}{\lambda}=\phi_0+e V_s\)
∴ Wave length = \(\lambda=\frac{h c}{\phi_0+e V_s}=\frac{1242 \mathrm{eV} \mathrm{nm}}{6.2 \mathrm{eV}+5 \mathrm{eV}}=\frac{1242}{11.2} \mathrm{~nm}=110.9 \mathrm{~nm}\).
The wavelength range for UV rays is 10-310 nm.
Hence, the required wavelength lies in the ultraviolet region
Question 31. For the photoelectric emission from a certain metal, the cutoff frequency is v. If radiations of frequency 2v be incident on the metal plate, the maximum possible velocity of the emitted electron will be (given that me = mass of an electron)
- \(\sqrt{\frac{h v}{2 m_{\mathrm{e}}}}\)
- \(\sqrt{\frac{h v}{m_{\mathrm{e}}}}\)
- \(\sqrt{\frac{2 h v}{m_{\mathrm{e}}}}\)
- \(2 \sqrt{\frac{h v}{m_e}}\)
Answer: 3. \(\sqrt{\frac{2 h v}{m_{\mathrm{e}}}}\)
Given that cutoff frequency = v0 = v and frequency of the incident radiation = 2v.
⇒ \(h v=\phi_0+\mathrm{KE}_{\max }=\phi_0+\frac{1}{2} m_e v_{\max }^2\)
⇒ \(h(2 v)=h v+\frac{1}{2} m_e v_{\max }^2\)
∴ \(v_{\max }=\sqrt{\frac{2 h v}{m_e}}\)
the charge of a photo electron is
Question 32. When the intensity of an incident light beam increases, the
- Photocurrent increases
- Photocurrent decreases
- The kinetic energy of the emitted photoelectrons increases
- The kinetic energy of the emitted photoelectrons decreases
Answer: 1. Photocurrent increases
The maximum KE of photoelectrons depends on the frequency of the incident radiation and not on the intensity of the incident light. However, the rate of emission of electrons (= photocurrent) depends on its intensity. An increase in the intensity increases the photocurrent.
Question 33. The figure shows the plots of the photocurrent versus the anode potential for a photosensitive surface for three different radiations. Which of the following statements is true?
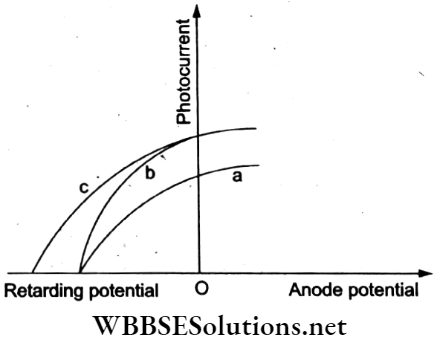
- The curves a and b represent the incident radiations of different frequencies and different intensities.
- The curves a and b represent the incident radiations of the same frequency but of different intensities.
- The curves b and c represent the incident radiations of different frequencies and different intensities.
- The curves b and c represent the incident radiations of the same frequency and the same intensity.
Answer: 2. The curves a and b represent the incident radiations of the same frequency but of different intensities.
The curves a and b have the same stopping potential V1. Hence, the incident radiations have the same frequency but different intensities. The curves b and c have the same intensity as the saturation current for both the stopping potentials. V1 and V2 are different Hence, the frequency of the incident radiations must be different.
Question 34. The work functions for the metals A, B, and C are respectively 1.92 eV, 2.0 eV, and 5eV. According to Einstein’s equation, the metal(s) which will emit photoelectrons for a radiation of wavelength 4100 A is/are
- A and B only
- All the three
- An only
- None of these
Answer: 1. A and B only
The work function of a photosensitive surface is given by
⇒ \(\phi_0=h v_0=\frac{h c}{\lambda_0}\)
∴ \(\lambda_0=\frac{h c}{\phi_0}\)
For photoeniission, λ ≤ λ0.
For A, \(\lambda_0=\frac{1242 \mathrm{eV} \mathrm{nm}}{1.92 \mathrm{e} V}=646.8 \mathrm{~nm}=6468\) Å
For B, \(\lambda_0=\frac{1242 \mathrm{eV} \mathrm{nm}}{2 \mathrm{eV}}=621 \mathrm{~nm}=6210\) Å
For C ,\(\lambda_0=\frac{1242 \mathrm{eV} \mathrm{nm}}{5 \mathrm{eV}}=248.4 \mathrm{~nm}=2484\) Å
Since the given value of λ (= 4100 Å) is less than that of A and B, the emission will be possible only for A and B.
Question 35. The photoelectric work function for a metal is 4.125 eV. The cut-off wavelength for this surface is
- 412.5 nm
- 301 nm
- 600 nm
- 206.2 nm
Answer: 2. 301 nm
Work function = \(\phi_0=\frac{h c}{\lambda_0}=4.125 \mathrm{eV}\)
the charge of a photo electron is
∴ cutoff wavelength = \(\lambda_0=\frac{1242 \mathrm{eV} \mathrm{nm}}{4.125 \mathrm{eV}} \approx 301 \mathrm{~nm}\)
Question 36. In the photoelectric effect, the work function of a metal is 3.5 eV. The emitted electrons can be stopped by applying a potential of -1.2 V. Then,
- The energy of the incident photons is 4.7 eV
- The energy of the incident photons is 2.3 eV
- If higher-frequency photons are used, the photoelectric current will rise
- In case the energy of photons is 3.5 eV, the photoelectric current will be the maximum
Answer: 1. The energy of the incident photons is 4.7 eV
Given that work function = Φ0 = 3.5 eV
and stopping potential = Vs = -1.2 V.
∴ KEmax=(-e)(-1.2V) =1.2eV.
From the photoelectric equation, the energy of the incident photon is
∴ hv = Φ0 + KEmax = 3.5 eV + 1.2 eV = 4.7 eV.
Question 37. A photoelectric cell is illuminated by a point source of light 1 m away. When the source is shifted to 2 m,
- Each emitted electron carries half the maximum initial energy
- The number of electrons emitted is a quarter of the initial number
- Each emitted electron carries one-quarter of the initial maximum kinetic energy
- The number of electrons emitted is half the initial number
Answer: 2. The number of electrons emitted is a quarter of the initial number
Varying the distance of the light source from a photoelectric cell will change the intensity but not the frequency. Hence, the KE of the photoelectrons will not change but their number will change according to the inverse-square law. Hence,
⇒ \(N \propto I \propto \frac{1}{d^2}\)
⇒ nd2 = n’d22 => n(1 m)2 = n'(2 m)2
⇒ \(n^{\prime}=\frac{n}{4}\)
Question 38. According to Einstein’s photoelectric equation, the graph between the maximum kinetic energy of the photoelectrons ejected and the frequency of the incident radiation is
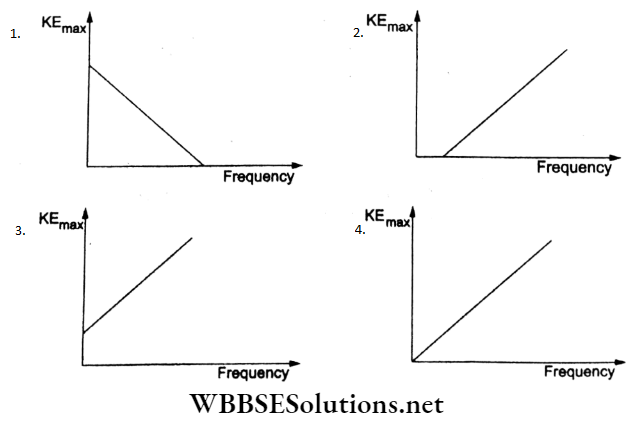
Answer: 2.
According to Einstein’s-photoelectric equation,
hv = Φ0 + KEmax
KEmax = hV – Φ0.
This represents a straight-line graph of the form y = mx-c, where the slope is h and the intercept on the x-axis is v0 (the threshold frequency) as in option (2).
Question 39. A beam of light is incident normally on a completely absorbing surface with an energy flux of 25 W cm-2. If the surface area by 25 cm2, the momentum transferred to the surface in a duration of 40 minutes will be
- 6.3 x 10-4 Ns
- 3.5 x 10-6 N s
- 5.0 x 10-3 N s
- 1.4 x l0-6 N s
Answer: 3. 5.0 x 10-3 N s
The intensity of the incident light = I = 25 W cm-2.
The rate at which energy is absorbed by the surface (of area = A = 25 cm-2) will be 625 W.
∴ momentum transferred = \(\Delta p=\frac{E}{c}=\frac{(625 \mathrm{~W})(40 \times 60 \mathrm{~s})}{3 \times 10^8 \mathrm{~m} \mathrm{~s}^{-1}}=5 \times 10^{-3} \mathrm{~N} \mathrm{~s}^2\).
Question 40. A 2-mW laser operates at a wavelength of 500 nm. The number of photons emitted per second is
- 5 x 1015
- 2 x 1016
- l x l016
- 1.5 x l016
Answer: 1. 5 x 1015
The power of the laser is
P = 2 mW = 2 x l0-3 J s-1.
This is the energy emitted per second associated with N photons each of energy hv.
∴ \(N\left(\frac{h c}{\lambda}\right)=2 \times 10^{-3} \mathrm{~J}\)
Hence, \(N=\frac{\left(2 \times 10^{-3} \mathrm{~J}\right)\left(500 \times 10^{-9} \mathrm{~m}\right)}{\left(6.6 \times 10^{-34} \mathrm{~J} \mathrm{~s}\right)\left(3 \times 10^8 \mathrm{~m} \mathrm{~s}^{-1}\right)}=5 \times 10^{15}\)
the charge of a photo electron is
Question 41. The magnetic field associated with an electromagnetic wave at the origin is given by B = \(B_0\left[\sin \left(3.14 \times 10^7\right) c t+\sin \left(6.28 \times 10^7\right) c t\right]\). If the wave is incident on a silver plate having a photoelectric work function of 4.7 eV, what will be the maximum kinetic energy of the emitted photoelectrons?
- 7.7 eV
- 6.82 eV
- 8.52 eV
- 12.5 eV
Answer: 1. 7.7 eV
Angular frequency = ω = (6.28 x107 )c
⇒ 2πv = 2π x 107 x (3 x108) Hz ⇒ v = 3 x l015Hz.
From the photoelectric equation,
hv = Φ0 + KEmax
⇒ KEmax = hv – Φ = (6.6 x10-34 J s)(3 x1015 s-1) – 4.7 eV
⇒ \(\frac{6.6 \times 3 \times 10^{-19}}{1.6 \times 10^{-19}} \mathrm{eV}-4.7 \mathrm{eV}=7.7 \mathrm{eV}\)
Question 42. In a photoelectric-effect experiment, the threshold wavelength of the photosensitive surface is 380 nm. If the wavelength of the incident light is 260 nm, the maximum kinetic energy of an emitted photoelectron will be [given that E (in eV) = 1237/λ, (in nm)]
- 1.5 eV
- 15.0 eV
- 3.0 eV
- 4.5 eV
Answer: 1. 1.5 eV
From the equation,
hv = hv0 + KEmax
⇒ \(\frac{h c}{\lambda}=\frac{h c}{\lambda_0}+\mathrm{KE}_{\max }\)
the charge of a photo electron is
⇒ \(\mathrm{KE}_{\max }=h c\left(\frac{1}{\lambda}-\frac{1}{\lambda_0}\right)=(1237 \mathrm{eV} \mathrm{nm})\left(\frac{1}{260 \mathrm{~nm}}-\frac{1}{380 \mathrm{~nm}}\right)\)
∴ \((1237 \mathrm{eV})\left(\frac{120}{260 \times 380}\right)=1.5 \mathrm{eV}\)
Question 43. The stopping potential corresponding to the incident radiation of wavelength λ, is V. If the wavelength of the incident light on the same photosensitive surface is increased to 3λ, the stopping potential becomes V/4. If the threshold wavelength be kλ then k is
- 6
- 3
- 9
- 2
Answer: 3. 9
According to Einstein’s photoelectric equation,
⇒ \(\frac{h c}{\lambda}=\phi+e V \text { and } \frac{h c}{3 \lambda}=\phi+\frac{e V}{4}\)
Eliminating V,
⇒ \(\frac{h c}{12 \lambda}=\frac{3}{4} \phi=\frac{3}{4}\left(\frac{h c}{\lambda_0}\right)\)
∴ threshold wavelength = X0 = 9X.
Hence, k = 9.
Question 44. The kinetic energy of the most energetic photoelectrons increases from K to 3K when the wavelength of the incident radiation changes from 500 nm to 200 nm. The photoelectric work function of the metallic surface is
- 0.50 eV
- 0.25 eV
- 0.62 eV
- 0.70 eV
Answer: 3. 0.62 eV
Einstein’s photoelectric equation is
⇒ \(\frac{h c}{\lambda}=\phi-\mathrm{KE}_{\max }\)
For λ = λ1 = 500 nm,
⇒ \(\frac{h c}{500 \mathrm{~nm}}=\phi+K\)
For λ = λ2 = 200 nm,
⇒ \(\frac{h c}{200 \mathrm{~nm}}=\phi+3 K\)
⇒ \(2 \phi=h c\left(\frac{3}{500 \mathrm{~nm}}-\frac{1}{200 \mathrm{~nm}}\right)=\frac{1240 \mathrm{eV} \mathrm{nm}}{1000 \mathrm{~nm}}=1.24 \mathrm{eV}\).
Hence, work function = Φ = 0.62 eV.
Question 45. The given graph shows the variation of the stopping potential (Vs) with the frequency (f) of the incident radiation. The photoelectric work function of the metallic surface is
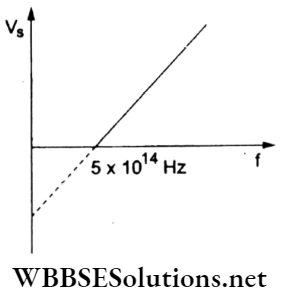
- 4.01 eV
- 2.07 eV
- 5.01 eV
- 3.01 eV
Answer: 2. 2.07 eV
hf = Φ + eVs,
∴ \(V_{\mathrm{s}}=\left(\frac{h}{e}\right) f-\frac{\phi}{e}\)
This represents the given straight-line graph. When Vs = 0,
⇒ \(\left(\frac{h}{e}\right) f-\frac{\phi}{e}=0\)
Hence, the work function is
∴ \(\phi=h f=\left(\frac{6.63 \times 10^{-34}}{1.6 \times 10^{-19}} \mathrm{eV} \mathrm{s}\right)\left(5 \times 10^{14} \mathrm{~s}^{-1}\right)=2.07 \mathrm{eV}\)
Question 46. In the case of the photoelectric effect, the graph showing the variation of the stopping potential (Vs) against the reciprocal of the wavelength (1/λ) of the incident radiation is given. What will happen to the graph on increasing the intensity of light?
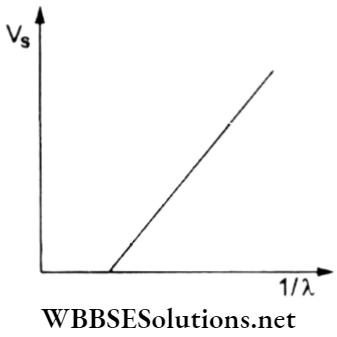
- The graph will not change.
- The intercept on the y-axis will change.
- The slope of the graph will increase.
- The graph will shift to the right remaining parallel to the given graph.
Answer: 1. The graph will not change.
The stopping potential depends on the frequency (and wavelength) of the incident radiation and is independent of the intensity.
Hence, the Vs – λ-1 graph does not change.
Question 47. In a photoelectric experiment, the potential difference between the plates increases while keeping the incident light on the photocathode unchanged. Which of the following is the correct statement about the saturation current?
- It increases.
- It decreases.
- It remains unchanged.
- It first increases and then decreases.
Answer: 3. It remains unchanged.
The saturation current depends on the intensity of the incident light only. An increase in the accelerating voltage will not affect the saturation current and it will remain unchanged.
Question 48. Two monochromatic sources emitting photons of λ1 = 500 nm and λ2 =1 nm have the same power of 200 W. What is the ratio of the densities of the photons emitted from the two sources?
- 200
- 500
- 300
- 0.8
Answer: 2. 500
Power is defined as the energy radiated per unit of time.
nhf = \(n\left(\frac{h c}{\lambda}\right)\) where n is the number of photons emitted per unit time.
∴ photon density = n = \(\frac{P \lambda}{h c} \propto \lambda\)
∴ \(\frac{n_1}{n_2}=\frac{\lambda_1}{\lambda_2}=\frac{500 \mathrm{~nm}}{1 \mathrm{~nm}}=500\).
Question 49. A beam of light with an average flux of 20 W cm-2 falls normally on a non-reflecting surface having a total surface area of 20 cm2. The energy received by the surface during a time span of 1 min is
- 12 kJ
- 24 kJ
- 48 kJ
- 10 kJ
Answer: 2. 24 kJ
Average flux = 20 J s-1 cm-2.
∴ the energy received in1 min on 20 cm2 will be
(20 J s-1 cm-2 X 60 s)(20 cm2) = 20 x 60 x 20 J = 24 kJ.
Question 50. A light beam of frequency 1.5 times the threshold frequency is incident on a photosensitive material. What will be the photoelectric current if the frequency is halved and the intensity is doubled?
- Four times
- One fourth
- Zero
- Doubled
Answer: 3. Zero
Let the threshold frequency be v0.
Initially, frequency = v = 1.5v0.
Finally, when the frequency is halved, it becomes
⇒ \(v^{\prime}=\frac{v}{2}=\frac{1.5 v_0}{2}=0.75 v_0<v_0\)
For frequencies v’ less than the threshold value, no photoemission is possible, so the photocurrent will be zero.
