Matter Waves
Question 1. An electron beam has a kinetic energy equal to 100 eV. The wavelength associated with the beam is
- 24.6 Å
- 0.12 Å
- 1.2 Å
- 6.3 Å
Answer: 3. 1.2 Å
The kinetic energy of an electron is E = \(E=\frac{p^2}{2 m}\)
∴ its momentum = p = \(\sqrt{2 m E}\)
The associated wavelength is
⇒ \(\lambda=\frac{h}{p}=\frac{h}{\sqrt{2 m E}}\)
Substituting the values,
∴ \(\lambda=\frac{6.6 \times 10^{-34} \mathrm{~J} \mathrm{~s}}{\sqrt{2\left(9.1 \times 10^{-31} \mathrm{~kg}\right)\left(100 \times 1.6 \times 10^{-19} \mathrm{~J}\right)}}=1.2 \times 10^{-10} \mathrm{~m}=1.2\) A.
Alternative method
KE =100 eV means accelerating voltage = V =100 V, i.e.,
∴ \(\lambda=\frac{1.2 \mathrm{~nm}}{\sqrt{V}}=1.2\) A.
wave nature of matter

Question 2. Radiant energy E falls normally on a perfectly reflecting surface. The momentum transferred to the surface is (given that c = velocity of light)
- \(\frac{E}{c}\)
- \(\frac{2 E}{c^2}\)
- \(\frac{E}{c^2}\)
- \(\frac{2 E}{c}\)
Answer: 4. \(\frac{2 E}{c}\)
Energy of a photon = E = hv = \(\frac{h c}{\lambda}\) and de Broglie wavelength = \(\lambda=\frac{h}{p}\).
∴ momentum = \(p=\frac{h}{\lambda}=\frac{h c}{\lambda c}=\frac{E}{c}\)
The momentum transferred to the surface is the change in momentum, i.e.,
⇒ \(\overrightarrow{\Delta p}=\vec{p}-(-\vec{p})=2 \vec{p}\)
∴ \(|\overrightarrow{\Delta p}|=2 p=\frac{2 E}{c}\)
Read And Learn Also NEET Physics Multiple Choice Question and Answers
Question 3. The wavelength associated with an electron accelerated through a potential difference of 100 V is of the order
- 1000 Å
- 100 AÅ
- 10.5 Å
- 1.2 Å
Answer: 4. 1.2 Å
The KE gained by the electron is
⇒ \(E=\frac{p^2}{2 m}=e(100 \mathrm{~V})=100 \cdot \mathrm{eV}=100\left(1.6 \times 10^{-19} \mathrm{~J}\right)\)
∴ associated wavelength = \(\lambda=\frac{h}{p}=\frac{h}{\sqrt{2 m E}}\)
Substituting the values,
∴ \(\lambda=\frac{6.6 \times 10^{-34} \mathrm{~J} \mathrm{~s}}{\sqrt{2\left(9.1 \times 10^{-31} \mathrm{~kg}\right)\left(1.6 \times 10^{-17} \mathrm{~J}\right)}}=1.2 \times 10^{-10} \mathrm{~m}=1.2\) A
Alternative method
∴ \(\lambda=\frac{1.227 \mathrm{~nm}}{\sqrt{V}}=\frac{1.227 \mathrm{~nm}}{\sqrt{100}}=1.2\) A.
Question 4. A particle of mass 1 mg has the same wavelength as that of an electron moving with a velocity of 3 x 106 m s-1. The velocity of the particle is (given that the mass of an electron = 9.1 x 10-31 kg)
- 2.7 x10-18 m s-1
- 2.7 x10-21 m s-1
- 3 xl0-31 m s-1
- 9 x10-2 m s-1
Answer: 1. 2.7 x10-18 m s-1
The wavelength associated with the moving particle of mass m is
⇒ \(\lambda=\frac{h}{p}=\frac{h}{m v}=\frac{h}{(1 \mathrm{mg}) v}\)
The wavelength of an electron is
⇒ \(\lambda_{\mathrm{e}}=\frac{h}{m_{\mathrm{e}} v_{\mathrm{e}}}=\frac{h}{\left(9.1 \times 10^{-31} \mathrm{~kg}\right)\left(3 \times 10^6 \mathrm{~m} \mathrm{~s}^{-1}\right)}\)
Given that λ = λe. Thus
(1 x 10-6 kg)v = (9.1 x 10-31 kg)(3 x 106 m s-1)
⇒ v = 27.3 x 10-19 m s-1 = 2.7 x 1018 m s-1
wave nature of matter
Question 5. Electrons, each of mass m and having the de Broglie wavelength λ, fall on the target in an X-ray tube. The cutoff wavelength of the emitted X-rays is
- \(\lambda_0=\frac{2 m c \lambda^2}{h}\)
- \(\lambda_0=\frac{2 h}{m c}\)
- \(\lambda_0=\frac{2 m^2 c^2 \lambda^3}{h^2}\)
- \(\lambda_0=\lambda\)
Answer: 1. \(\lambda_0=\frac{2 m c \lambda^2}{h}\)
The kinetic energy of the electron incident on the target is
⇒ \(E=\frac{p^2}{2 m}=\frac{1}{2 m}\left(\frac{h}{\lambda}\right)^2=\frac{h^2}{2 m \lambda^2}\)
Its total absorption produces X-ray photons of the maximum energy. Hence,
⇒ \(E=h v_{\max }=\frac{h c}{\lambda_{\min }}=\frac{h c}{\lambda_0}\)
∴ \(\frac{h^2}{2 m \lambda^2}=\frac{h c}{\lambda_0} \Rightarrow \lambda_0=\frac{2 m c \lambda^2}{h}\)
Question 6. Which of the following graphs correctly represents the variation of a particle’s momentum (p) with the associated de Broglie wavelength?
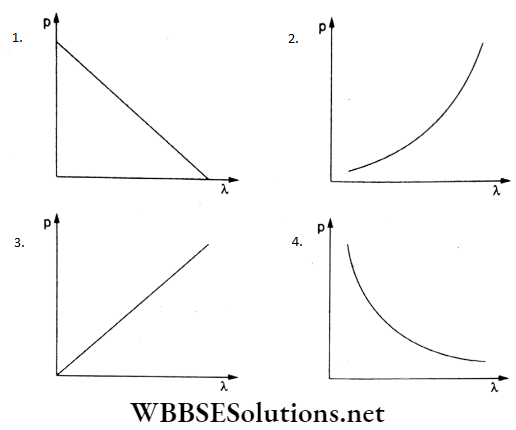
Answer: 4.
The de Broglie wavelength is λ = λ/p, where p is the momentum.
Thus, pλ = constant.
Like Boyle’s law(pV = constant), the p-λ graph represents a rectangular hyperbola, as depicted in (d).
| Class 11 Physics | Class 12 Maths | Class 11 Chemistry |
| NEET Foundation | Class 12 Physics | NEET Physics |
Question 7. If the kinetic energy of a particle is increased to 16 times its previous value, the percentage change in the de Broglie wavelength of the particle is
- 25%
- 75%
- 60%
- 50%
Answer: 2. 75%
Initial wavelength = \(\lambda=\frac{h}{p}=\frac{h}{\sqrt{2 m E}}\)
and final wavelength = A.’ = \(\frac{h}{p^{\prime}}=\frac{h}{\sqrt{(2 m)(16 E)}}=\frac{\lambda}{4}\)
∴ % change in wavelength = \(\frac{\lambda-\lambda^{\prime}}{\lambda} \times 100 \%\)
∴ \(\left(1-\frac{1}{4}\right) \times 100 \%=75 \%\)
wave nature of matter
Question 8. The wavelengths λe of an electron and λph of a photon of the same energy E are related by
- \(\lambda_{\mathrm{ph}} \propto \sqrt{\lambda_{\mathrm{e}}}\)
- \(\lambda_{\mathrm{ph}} \propto \frac{1}{\sqrt{\lambda_{\mathrm{e}}}}\)
- \(\lambda_{\mathrm{ph}} \propto \lambda_{\mathrm{e}}^2\)
- \(\lambda_{\mathrm{ph}} \propto \lambda_{\mathrm{e}}\)
Answer: 3. \(\lambda_{\mathrm{ph}} \propto \lambda_{\mathrm{e}}^2\)
The wavelength associated with an electron is \(\lambda_{\mathrm{e}}=\frac{h}{\sqrt{2 m_{\mathrm{e}} E_{\mathrm{e}}}}\)
∴ KE of an electron = \(E_{\mathrm{e}}=\frac{h^2}{2 m_{\mathrm{e}} \lambda_{\mathrm{e}}^2}\) → (1)
Energy of a photon = \(E_{\mathrm{ph}}=h v=\frac{h c}{\lambda_{\mathrm{ph}}}\) → (2)
Since Ee = Eph (given), then, equating (1) and (2), we have
⇒ \(\frac{h c}{\lambda_{\mathrm{ph}}}=\frac{h^2}{2 m_{\mathrm{e}} \lambda_{\mathrm{e}}^2}\)
⇒ \(\lambda_{\mathrm{ph}}=\left(\frac{2 m_{\mathrm{e}} c}{h}\right) \lambda_{\mathrm{e}}^2\)
∴ \(\lambda_{\mathrm{ph}} \propto \lambda_{\mathrm{e}}^2\)
Question 9. The velocity of a particle P is thrice that of an electron, whereas the ratio of the de Broglie wavelength of the particle to that of an electron is 1.824 x 10-4. Then, the particle will be
- A neutron
- Adeuteron
- An alpha particle
- A tritium nucleus
Answer: 1. A neutron
For the given, particle, Up = 3ve and wavelength = \(\lambda_P=\left(1.824 \times 10^{-4}\right) \lambda_e\)
∴ \(\frac{\lambda_{\mathrm{P}}}{\lambda_{\mathrm{e}}}=\frac{h / m_{\mathrm{P}} v_{\mathrm{P}}}{h / m_{\mathrm{e}} v_e}=\left(\frac{m_{\mathrm{e}}}{m_{\mathrm{P}}}\right)\left(\frac{v_{\mathrm{e}}}{v_{\mathrm{P}}}\right)\)
⇒ \(1.824 \times 10^{-4}=\left(\frac{m_{\mathrm{e}}}{m_{\mathrm{P}}}\right)\left(\frac{1}{3}\right)\)
∴ the mass of the particle is
⇒ \(m_{\mathrm{P}}=\frac{m_{\mathrm{e}}}{3\left(1.824 \times 10^{-4}\right)}=\frac{\left(9.1 \times 10^{-31} \mathrm{~kg}\right) \times 10^4}{3 \times 1.824}\)
⇒ \(1.663 \times 10^{-27} \mathrm{~kg} \approx 1.67 \times 10^{-27} \mathrm{~kg}\)
This mass corresponds to that of a neutron.
Question 10. An electron of mass m and a photon have the same energy E. The ratio of the de Broglie wavelengths associated with them is (c being the velocity of light)
- \(c(2 m E)^{1 / 2}\)
- \(\frac{1}{c}\left(\frac{2 m}{E}\right)^{1 / 2}\)
- \(\frac{1}{c}\left(\frac{E}{2 m}\right)^{1 / 2}\)
- \(\left(\frac{E}{2 m}\right)^{1 / 2}\)
Answer: 3. \(\frac{1}{c}\left(\frac{E}{2 m}\right)^{1 / 2}\)
For an electron, wavelength = \(\lambda_{\mathrm{e}}=\frac{h}{p}=\frac{h}{\sqrt{2 m E}}\)
For a photon,
⇒ \(E=h v=\frac{h c}{\lambda} \Rightarrow \lambda_{\mathrm{ph}}=\frac{h c}{E}\)
∴ \(\frac{\lambda_{\mathrm{e}}}{\lambda_{\mathrm{ph}}}=\frac{h}{\sqrt{2 m E}} \times \frac{E}{h c}=\frac{1}{c}\left(\frac{E}{2 m}\right)^{1 / 2}\)
wave nature of matter
Question 11. A light beam of wavelength 500 run is incident on a metal with a work function of 2.28 eV. The de Broglie wavelength of the emitted electron is
- λ ≥ 2.8 x 10-9 m
- λ ≤ 2.8 x l0-12 m
- λ < 2.8 x l0-10 m
- λ < 2.8 x 10-9 m
Answer: 1. λ ≥ 2.8 x 10-9 m
According to Einstein’s photoelectric equation,
⇒ \(h v=\phi_0+\mathrm{KE}_{\max } \Rightarrow \frac{h c}{\lambda}=\phi_0+\frac{p_{\max }^2}{2 m_{\mathrm{e}}}\)
Substituting the values,
⇒ \(\frac{1242 \mathrm{eV} \mathrm{nm}}{500 \times 10^{-9} \mathrm{~m}}=2.28 \mathrm{eV}+\frac{p_{\max }^2}{2 m_{\mathrm{e}}}\)
⇒ \(p_{\max }^2=(2.484 \mathrm{eV}-2.28 \mathrm{eV})\left(2 m_{\mathrm{e}}\right)\)
⇒ \(p_{\max }=\sqrt{\left(0.204 \times 1.6 \times 10^{-19} \mathrm{~J}\right)\left(2 \times 9.1 \times 10^{-31} \mathrm{~kg}\right)}\)
= 2.4 x 10-25 kg m s-1.
∴ the associated wavelength is
⇒ \(\lambda_{\min }=\frac{h}{p_{\max }}=\frac{6.6 \times 10^{-34} \mathrm{~J} \mathrm{~s}}{2.4 \times 10^{-25} \mathrm{~kg} \mathrm{~m} \mathrm{~s}^{-1}}=2.75 \times 10^{-9} \mathrm{~m}\)
∴ λ > 2.8 x 10-9 m
Question 12. The momentum of a photon of energy 1 MeV will be
- 5 x 10-22 kg m s-1
- 0.33 x l06 kg ms-1
- 7 x 10-24 kg m s-1
- 10-22 kg m s-1
Answer: 1. 5 x 10-22 kg m s-1
The momentum of a photon is
⇒ \(p=\frac{E}{c}=\frac{1 \mathrm{MeV}}{3 \times 10^8 \mathrm{~m} \mathrm{~s}^{-1}}=\frac{\left(1 \times 10^6\right)\left(1.6 \times 10^{-19} \mathrm{~J}\right)}{3 \times 10^8 \mathrm{~m} \mathrm{~s}^{-1}}\)
∴ 5 x 10-22 kg m s-1
Question 13. An electron of mass me, when accelerated through a potential difference V, has a de Broglie wavelength of λe. The de Broglie wavelength associated with a proton of mass mp accelerated through the same potential difference will be
- \(\lambda_{\mathrm{e}}\left(\frac{m_{\mathrm{p}}}{m_{\mathrm{e}}}\right)\)
- \(\lambda_{\mathrm{e}}\left(\frac{m_{\mathrm{e}}}{m_{\mathrm{p}}}\right)\)
- \(\lambda_{\mathrm{e}} \sqrt{\frac{m_{\mathrm{p}}}{m_{\mathrm{e}}}}\)
- \(\lambda_{\mathrm{e}} \sqrt{\frac{m_{\mathrm{e}}}{m_{\mathrm{p}}}}\)
Answer: 4. \(\lambda_{\mathrm{e}} \sqrt{\frac{m_{\mathrm{e}}}{m_{\mathrm{p}}}}\)
KE gained = \(e V=\frac{p^2}{2 m}\) and momentum = p = \(\sqrt{2 m e V}\)
For an electron, \(\lambda_{\mathrm{e}}=\frac{h}{p_{\mathrm{e}}}=\frac{h}{\sqrt{2 m_{\mathrm{e}} e V}}\)
For a proton, \(\lambda_{\mathrm{p}}=\frac{h}{p_{\mathrm{p}}}=\frac{h}{\sqrt{2 m_{\mathrm{p}} e V}}\)
∴ \(\frac{\lambda_{\mathrm{p}}}{\lambda_{\mathrm{e}}}=\sqrt{\frac{m_{\mathrm{e}}}{m_{\mathrm{p}}}} \Rightarrow \lambda_{\mathrm{p}}=\lambda_{\mathrm{e}} \sqrt{\frac{m_{\mathrm{e}}}{m_{\mathrm{p}}}}\)
wave nature of matter
Question 14. What is the kinetic energy of an electron associated with a de Broglie wavelength of 1 nm?
- 1.5 eV
- 4.2 eV
- 2.1 eV
- 3.1 eV
Answer: 1. 1.5 eV
The wavelength of an electron accelerated through a potential difference of V volts is given by \(\lambda=\frac{1.227}{\sqrt{V}}\).
For λ =1 nm, √V =1.227 ⇒ V =1.5.
∴ kinetic energy = e(1.5 V) =1.5 eV
Question 15. If the following particles move with the same speed, which has the maximum de Broglie wavelength?
- A proton
- An α-particle
- A β-particle
- A neutron
Answer: 3. A β-particle
The de Broglie wavelength is given by
⇒ \(\lambda=\frac{h}{m v}\)
∴ \(\lambda \propto \frac{1}{m}\)
We know that m∝ > mn > mp > me.
The particle with the least mass has the maximum value of the wavelength λ. Thus, β-particles have the maximum de Broglie wavelength.
Question 16. The de Broglie wavelength of a neutron of mass m in thermal equilibrium with heavy water at a temperature T is
- \(\frac{h}{\sqrt{3 m k T}}\)
- \(\frac{2}{\sqrt{3 m k T}}\)
- \(\frac{2 h}{\sqrt{m k T}}\)
- \(\frac{h}{\sqrt{m k T}}\)
Answer: 1. \(\frac{h}{\sqrt{3 m k T}}\)
A neutron has 3 degrees of freedom.
At die temperature T, its energy is
⇒ \(E=3\left(\frac{1}{2} k T\right)=\frac{3}{2} k T\)
But \(\mathrm{KE}=E=\frac{p^2}{2 m}\)
∴ \(p=\sqrt{2 m E}=\sqrt{2 m\left(\frac{3}{2} k T\right)}\)
⇒ \(\lambda=\frac{h}{p}=\frac{h}{\sqrt{3 m k T}}\)
Question 17. The momentum of a photon of a visible light beam of wavelength 500 nm is about
- 1.33 x 10-27 kg m s-1
- 2.46 x l0-27 kg m s-1
- 1.84 x l0-27 kg m s-1
- 3.67 x l0-27 kg m s-1
Answer: 1. 1.33 x 10-27 kg m s-1
The momentum of a photon is
⇒ \(p=\frac{E}{c}=\frac{h c / \lambda}{c}=\frac{h}{\lambda}\)
∴ \(p=\frac{6.67 \times 10^{-34} \mathrm{~J} \mathrm{~s}}{500 \mathrm{~nm}}=1.33 \times 10^{-27} \mathrm{~kg} \mathrm{~m} \mathrm{~s}^{-1}\).
wave nature of matter
Question 18. A monochromatic source of light having a power of 200 W emits 4 x 1020 photons per second. The wavelength of the light is
- 200 nm
- 800 run
- 400 nm
- 600 nm
Answer: 3. 400 nm
Energy of each photon = \(\frac{200 \mathrm{~J} \mathrm{~s}^{-1}}{4 \times 10^{20} \mathrm{~s}^{-1}}=5 \times 10^{-19} \mathrm{~J}\)
The corresponding wavelength is
∴ \(\lambda=\frac{h}{p}=\frac{h}{E / c}=\frac{h c}{E}=\frac{\left(6.6 \times 10^{-34} \mathrm{~J} \mathrm{~s}\right)\left(3 \times 10^8 \mathrm{~m} \mathrm{~s}^{-1}\right)}{5 \times 10^{-19} \mathrm{~J}}\)
⇒ 4.0 x 10-7 m = 400 nm.
Question 19. If a hydrogen atom (of mass m) at rest emits a photon of wavelength λ, the recoil speed of the atom will be
- \(\frac{m h}{\lambda}\)
- \(\frac{\lambda h}{m}\)
- \(m h \lambda\)
- \(\frac{h}{m \lambda}\)
Answer: 4. \(\frac{h}{m \lambda}\)
Conserving the linear momentum,
| momentum of the photon | = | momentum of the atom |
⇒ \(\frac{h}{\lambda}=m v\)
∴ recoil speed of the atom = v = \(\frac{h}{m \lambda}\).
Question 20. Two particles of masses m and 2m have equal kinetic energies. Their de Broglie wavelengths are in the ratio
- 1:2
- 1:1
- √2:1
- 1: √2
Answer: 3. √2:1
Kinetic energy = E = \(\frac{p^2}{2 m} \Rightarrow p=\sqrt{2 m E}\)
∴ \(\frac{\lambda_1}{\lambda_2}=\frac{h / p_1}{h / p_2}=\frac{p_2}{p_1}=\sqrt{\frac{2(2 m) E}{2 m E}}=\sqrt{2}\)
λ1 : λ2 ⇒ √2:1
Question 21. An electron is accelerated through a potential difference of 10000 V. Its de Broglie wavelength is nearly
- 12.27 x l0-12 m
- 12.2 x 10-14 m
- 12.2 x l0-13 m
- 12.2 nm
Answer: 1. 12.27 x l0-12 m
The de Broglie wavelength linked with an electron accelerated through a potential difference of V volts is given by
⇒ \(\lambda=\frac{12.27}{\sqrt{V}} Å=\frac{12.27 \times 10^{-10}}{\sqrt{10000}} \mathrm{~m}=12.27 \times 10^{-12} \mathrm{~m}\)
Question 22. A particle P is formed due to a completely inelastic collision of two particles x and y having de Broglie wavelengths λx and λy respectively. If x and y are moving in opposite directions, the de Broglie wavelength of P is
- \(\lambda_x-\lambda_y\)
- \(\frac{\lambda_{\mathrm{x}} \lambda_{\mathrm{y}}}{\lambda_{\mathrm{x}}-\lambda_{\mathrm{y}}}\)
- \(\lambda_x+\lambda_y\)
- \(\frac{\lambda_{\mathrm{x}} \lambda_{\mathrm{y}}}{\lambda_{\mathrm{x}}+\lambda_{\mathrm{y}}}\)
Answer: 2.
The de Broglie wavelengths associated with x and y are λx = h/px and λy = h/py respectively.
Conserving the momentum,
Px – Py = P [v moving in opposite directions]
⇒ \(\frac{h}{\lambda_x}-\frac{h}{\lambda_y}=\frac{h}{\lambda}\)
⇒ \(\lambda=\frac{\lambda_x \lambda_y}{\lambda_x-\lambda_y}\).
wave nature of matter
Question 23. Two particles move at a right angle to each other. Their de Broglie wavelengths are λ1 and λ2 respectively. The particles suffer a perfectly inelastic collision. The de Broglie wavelength (λ) of the final particle is given by
- \(\lambda=\frac{\lambda_1+\lambda_2}{2}\)
- \(\frac{2}{\lambda}=\frac{1}{\lambda_1}+\frac{1}{\lambda_2}\)
- \(\lambda=\sqrt{\lambda_1 \lambda_2}\)
- \(\frac{1}{\lambda^2}=\frac{1}{\lambda_1^2}+\frac{1}{\lambda_2^2}\)
Answer: 4. \(\frac{1}{\lambda^2}=\frac{1}{\lambda_1^2}+\frac{1}{\lambda_2^2}\)
The de Broglie wavelengths associated with the colliding particles are λ1 = h/p1 and λ2 = h/p2 respectively. Hence,
⇒ \(p_1=\frac{h}{\lambda_1} \text { and } p_2=\frac{h}{\lambda_2}\)
Conserving the momentum,
⇒ \(\vec{p}=p_1 \hat{i}+p_2 \hat{j}\)
⇒ \(p^2=p_1^2+p_2^2\)
∴ \(\frac{h^2}{\lambda^2}=\frac{h^2}{\lambda_1^2}+\frac{h^2}{\lambda_2^2} \Rightarrow \frac{1}{\lambda^2}=\frac{1}{\lambda_1^2}+\frac{1}{\lambda_2^2}\)
Question 24. If the de Broglie wavelength of an electron is equal to 10 3 times the wavelength of a photon of frequency 6 x1014 Hz then the speed of the electron is equal to (given that c = 3 x l08 m s-1, h = 6.64 x 10-34 Js and me = 9.1 x 10-31 kg)
- 1.45 x l06 m s-1
- 1.7 x 106 m s-1
- 1.1 x l06 m s-1
- 1.8 x l06 m s-1
Answer: 1. 1.45 x l06 m s-1
Given that \(\lambda_{\mathrm{e}}=10^{-3} \lambda_{\mathrm{ph}}=10^{-3}\left(\frac{c}{6 \times 10^{14} \mathrm{~s}^{-1}}\right)\)
But \(\lambda_{\mathrm{e}}=\frac{h}{m_{\mathrm{e}} v_{\mathrm{e}}} \Rightarrow v_{\mathrm{e}}=\frac{h}{m_{\mathrm{e}} \lambda_{\mathrm{e}}}\)
Substituting the given values, the velocity of an electron becomes
∴ \(v_{\mathrm{e}}=\frac{\left(6.63 \times 10^{-34} \mathrm{~J} \mathrm{~s}\right)\left(6 \times 10^{14} \mathrm{~s}^{-1}\right)}{\left(9.1 \times 10^{-31} \mathrm{~kg}\right)\left(10^{-3} \times 3 \times 10^8 \mathrm{~m} \mathrm{~s}^{-1}\right)}=1.45 \times 10^6 \mathrm{~m} \mathrm{~s}^{-1}\).
Question 25. A particle A of mass m and charge q is accelerated by a potential difference of 50 V. Another particle B of mass 4m and charge q is accelerated by a potential difference of 2500 V. The ratio of their de Broglie wavelengths λA and λB is close to
- 14.14
- 10.00
- 0.07
- 4.47
Answer: 1. 14.14
Kinetic energy = \(\frac{p^2}{2 m}=q V\)
For A, \(p_{\mathrm{A}}=\sqrt{2 m q V}=\sqrt{2 m q(50 \mathrm{~V})}\)
For B, \(p_{\mathrm{B}}=\sqrt{2(4 m) q(2500 \mathrm{~V})}\)
∴ \(\frac{\lambda_{\mathrm{A}}}{\lambda_{\mathrm{B}}}=\frac{h / p_{\mathrm{A}}}{h / p_{\mathrm{B}}}=\frac{p_{\mathrm{B}}}{p_{\mathrm{A}}}=\sqrt{\frac{2 \times 4 \times 2500}{2 \times 50}}=10 \sqrt{2}=14.14\).
Question 26. A beam of electrons moving with an energy of E gets scattered from a target having an atomic spacing of 1 Å. The first maximum intensity occurs at θ = 60°. The value of E is
- 20 eV
- 40 eV
- 50 eV
- 60 eV
Answer: 3. 50 eV
For the first maximum intensity in the diffraction of electrons,
2dsinθ = λ ⇒ 2(1 Å)\(\left(\frac{\sqrt{3}}{2}\right)\) = λ = √3 Å.
The de Broglie wavelength for an electron accelerated through a potential of V volts is
⇒ \(\lambda=\sqrt{\frac{150}{V}} Å=\sqrt{3}\) Å.
∴ V volts = 50 V
Hence, the energy of an electron is E = 50 eV
Question 27. A particle A of mass mA = m/2 moving along the x-axis with a velocity v0 collides elastically with another particle B at rest having a mass of mB = m/3. If the particles continue to move along the x-axis after the collision, the change in the de Broglie wavelength of A in terms of λ0 is
- \(\Delta \lambda=2 \lambda_0\)
- \(\Delta \lambda=\frac{3}{2} \lambda_0\)
- \(\Delta \lambda=4 \lambda_0\)
- \(\Delta \lambda=\frac{5}{2} \lambda_0\)
Answer: 3. \(\Delta \lambda=4 \lambda_0\)
Conserving the momentum,
⇒ \(\left(\frac{m}{2}\right) v_0=\left(\frac{m}{2}\right) v_{\mathrm{A}}+\left(\frac{m}{3}\right) v_{\mathrm{B}} \Rightarrow v_0=v_{\mathrm{A}}+\frac{2}{3} v_{\mathrm{B}}\) → (1)
For an elastic collision, e = 1. Hence,
VB – VA = V0 ⇒ VB = V0 + VA → (2)
Substituting uB from (2) in (1)
⇒ \(v_0=v_{\mathrm{A}}+\frac{2}{3}\left(v_0+v_{\mathrm{A}}\right) \Rightarrow v_{\mathrm{A}}=\frac{v_0}{5}\)
Hence, after the collision,
⇒ \(\lambda_{\mathrm{A}}=\frac{h}{m_{\mathrm{A}} v_{\mathrm{A}}}=\frac{5 h}{m_{\mathrm{A}} v_0}=5 \lambda\)
∴ Δλ = 5λ0 – λ0 = 4λ0.
wave nature of matter
Question 28. An electron is accelerated from rest through a potential difference of V volts. If the de Broglie wavelength of the electron is 1.227 x10-2 nm, the potential difference is
- 102 V volts
- 104 V volts
- 103 V volts
- 10V volts
Answer: 3. 103 V volts
The de Broglie wavelength of the electron is
⇒ \(\lambda=\frac{1.227}{\sqrt{V}} \mathrm{~nm}\)
Given that \(\lambda=1.227 \times 10^{-2} \mathrm{~nm}=\frac{1.227}{\sqrt{V}} \mathrm{~nm}\)
√V = 102 ⇒ V = 104 V.
