Kinetic Theory
Question 1. A gas behaves as an ideal gas at
- High pressure and low temperature
- Low pressure and high temperature
- High pressure and high temperature
- Low pressure and low temperature
Answer: 2. Low pressure and high temperature
For an ideal gas, no intermolecular force exists. Since at low pressure and high temperature, the molecules in a gas are far apart, the gas behaves like an ideal gas.

Question 2. According to the kinetic theory of gases, molecules of a gas behave like
- Inelastic spheres
- Perfectly elastic rigid spheres
- Inelastic nonrigid spheres
- Perfectly elastic nonrigid spheres
Answer: 2. Perfectly elastic rigid spheres
According to the fundamental postulates of kinetic theory, gas molecules undergo perfectly elastic collisions and thus act like perfectly elastic rigid shapers
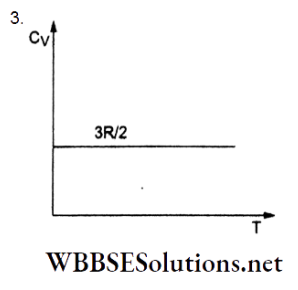
Question 3. The graph of molar heat capacity at constant volume (Cy) for a monatomic gas is given by
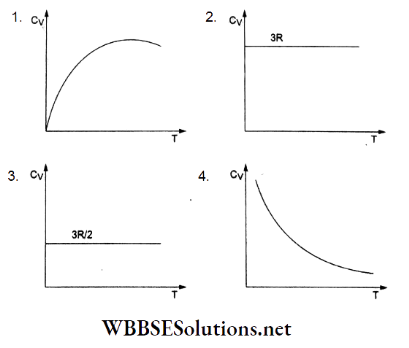
Answer: 3.
“ch 13 physics class 11 “
For an ideal monatomic gas, the internal energy for 1 molar temperature T is given by
⇒ \(U=\left(\frac{1}{2} k T\right)(3 N)=\frac{3}{2} \frac{R}{N} T N=\frac{3}{2} R T\)
But \(C_V=\frac{d U}{d T}=\frac{3}{2} R\)
Question 4. The degree(s) of freedom for a polyatomic gas molecule is
- < 4
- ≥ 5
- ≥ 6
- >7
Answer: 3. <= 6
The motion of a polyatomic gas molecule consists of 3 degrees of freedom of translational motion, 3 degrees of freedom of rotational motion, and a number of degrees of freedom for vibrational motion.
Thus, the total number is more than 6.
Question 5. The average kinetic energy of a gas molecule at 27 °C is 6.21 x 10-21 J. Its average KE at 227 °C will be
- 52.2 x 10-21 J
- 10.35 x 10-21 J
- 5.22 x 10-21 J
- 11.35 x 101 J
Answer: 2. 10.35 x 10-21 J
The kinetic energy of a gas molecule is given by
⇒ \(E=\frac{3}{2} k T \text { or } E \propto T\)
∴ \(\frac{E_1}{E_2}=\frac{T_1}{T_2}\)
⇒ \(\frac{6.21 \times 10^{-21} \mathrm{~J}}{E_2}\)
= \(\frac{27+273}{227+273}\)
= \(\frac{300}{500}\)
⇒ \(E_2=\frac{5}{3} \times 6.21 \times 10^{-21} \mathrm{~J}\)
= 10.35 x 10-21 J
Read And Learn Also NEET Physics Multiple Choice Question and Answers
Question 6. A perfect gas is contained in a closed cylindrical vessel kept in f vacuum. If the cylinder suddenly bursts then the temperature of the gas
- Is increased
- Becomes zero K
- Remains unchanged
- Is decreased
Answer: 4. Is decreased
Sudden expansion or compression is an adiabatic process. In an adiabatic expansion, cooling is produced; consequently, the temperature of the gas decreases.
Question 7. An ideal gas is heated from 27°C to 627°C at constant pressure. If its initial volume was 4 m³ then the final volume Of the gas will be
- 6 m³
- 4 m³
- 12 m³
- 2 m³
Answer: 3. 12 m³
According to Charles’s law, V ∝ T, when pressure is constant.
Thus,
⇒ \(\frac{V_1}{V_2}=\frac{T_1}{T_2}\)
or, \(\quad \frac{4 \mathrm{~m}^3}{V_2}\)
= \(\frac{27+273}{627+273}\)
= \(\frac{300}{900}\)
=\(\frac{1}{3}\)
V2 = 3(4m³)
=12m³
Question 8. Let a gas sample be heated from 27°C to 327°C when the initial KE of the molecules is E. What will be the average KE after heating?
- 2E
- 327E
- 300E
- V2E
Answer: 1. 2E
Given, T1 = (273 + 27) K = 300 K
and T2 = (327 + 273) K = 600 K.
Since average KE ∝ T, we have
⇒ \(\frac{E}{E_{\mathrm{f}}}=\frac{300}{600}\)
⇒ \(E_{\mathrm{f}}=2 E\)
“chapter 13 physics class 11 “
Question 9. The temperature corresponding to the energy of 1 eV is approximately
- 7.6 x 10³ K
- 7.2 x 10³ K
- 7.7 x 10³ K
- 7.1 x 10-2 K
Answer: 3. 7.7 x 10³ K
Given that average KE = 1 eV = 1.6 x 10-19 J.
The average KE of a molecule of a monatomic gas is \(\frac{3}{2}\), where k = Boltzmann constant = 1.38 x 10-23 J K-1 and T tires absolute temperature.
∴ \(\frac{3}{2} k T=1.6 \times 10^{-19} \mathrm{~J} \quad\)
or, \(\quad T=\frac{2\left(1.6 \times 10^{-19} \mathrm{~J}\right)}{3\left(1.38 \times 10^{-23} \mathrm{JK}^{-1}\right)}\)
= \(7.73 \times 10^3 \mathrm{~K}\)
| Class 11 Physics | Class 12 Maths | Class 11 Chemistry |
| NEET Foundation | Class 12 Physics | NEET Physics |
Question 10. A constant-pressure air thermometer gave a reading of 47.5 units of volume when immersed in ice-cold water, and 67 units in a boiling liquid. The boiling point of the liquid is
- 135°C
- 112°C
- 100°C
- 125°C
Answer: 2. 112°C
According to the pressure law, V ∝ T, when the pressure is constant.
Hence,
⇒ \(\frac{V_1}{V_2}=\frac{T_1}{T_2}\)
or, \(\frac{47.5}{67}=\frac{0+273}{\theta+273}\)
0 = 112°C
Question 11. A bulb contains one mole of hydrogen mixed with one mole of oxygen at temperature T. The ratio of rms values of the velocity of hydrogen molecules to that of oxygen molecules is
- 1:4
- 1:16
- 16:1
- 4:1
Answer: 4. 4:1
According to the kinetic theory of gaseous pressure,
⇒ \(p=\frac{1}{3} \frac{M}{V} c_{\mathrm{rms}}^2 \quad \text { or } \quad p V=\frac{1}{3} M c_{\mathrm{rms}}^2=R T\)
⇒ \(c_{\mathrm{rms}}^2=\frac{3 R T}{M} \quad\)
or, \(\quad c_{\mathrm{rms}} \propto \frac{1}{\sqrt{M}}\)
Hence,
⇒ \(\frac{\left(v_{\mathrm{rms}}\right)_{\mathrm{H}_2}}{\left(v_{\mathrm{rms}}\right)_{\mathrm{O}_2}}=\sqrt{\frac{(M)_{\mathrm{O}_2}}{(M)_{\mathrm{H}_2}}}\)
= \(\sqrt{\frac{32}{2}}=4\)
= \(\frac{4}{1}\)
Question 12. In a closed vessel, a gas is at pressure p. Ifthemass of all the molecules is halved and their speed is doubled then the resultant pressure will be
- p
- 2p
- 4p
- \(\frac{p}{2}\)
Answer: 2. 2p
Gaseous pressure is \(p=\frac{1}{3} \frac{M}{V} c_{\mathrm{rms}}^2\).
If the mass of each molecule is m and N is the number of molecules then M = mN.
∴ \(p=\frac{1}{3} \frac{m N}{V} c_{\mathrm{rms}}^2 \propto m c_{\mathrm{rms}}^2\)
Let \(m_1=m \text { and } m_2=\frac{m}{2}\)
∴ \(\left(c_{r m s}\right)_1=v \text { and }\left(c_{r m s}\right)_2=2 v \Rightarrow \frac{p_1}{p_2}=\frac{m v^2}{\frac{m}{2}(2 v)^2}=\frac{1}{2}\)
Hence, the resultant pressure = p2 = 2p1 = 2p.
Question 13. The temperature of a gas is held constant while its volume is decreased. The pressure exerted by the gas on the walls of the container increases because its molecules
- Strike the walls more frequently
- Strike the walls with higher velocities
- Are in contact with the wall for a shorter time
- Strike die walls with a larger force
Answer: 1. Strike the walls more frequently
The pressure exerted by the gas molecules on the walls of the container arises due to their impact on the walls. This impact causes the transfer of momentum from the molecules to the walls and hence, pressure is produced. With the decrease in volume, the impact is much more frequent which leads to an increase in gaseous pressure.
“chapter 13 physics class 11 “
Question 14. A column of mercury of length 10 cm is contained in the middle of a narrow horizontal tube of length 1 m closed at both ends. The air in both halves of the tube is under the pressure of 76 cm of mercury column. The tube is now slowly made vertical. The distance moved by the mercury will be
- 2.5 cm
- 4.5 cm
- 3.0 cm
- 1.2 cm
Answer: 3. 3.0 cm
In the horizontal position of the tube, the volume of air on both sides of the mercury pellet is 45 A and the pressure is 76 cm of the Hg column. In the vertical position, let the Hg pellet drop by x so the volume of air above mercury = (45 cm + x)A and the new pressure = p1
Similarly, for air below the Hg pellet,
volume = (45 cm- x)A and pressure = p2.
Applying Boyle’s law
76(45 cm)A = p1(45 cm + x)A
=> p1 = \(p_1=\frac{76 \times(45 \mathrm{~cm})}{(45 \mathrm{~cm}+x)}\)
Similarly, for the lower portion,
76(45 cm)A = p2(45 cm- x)A
p2 = \(\frac{76(45 \mathrm{~cm})}{(45 \mathrm{~cm}-x)}\)
Now, p2 > P1, so the difference (p2– p1) is the pressure produced by the Hg column.
⇒ \((10 \mathrm{~cm})=\frac{76 \times 45 \mathrm{~cm}}{(45 \mathrm{~cm}-x)}-\frac{76 \times 45 \mathrm{~cm}}{(45 \mathrm{~cm}+x)}\)
= \(\frac{(76 \times 45) 2 x}{45^2-x^2}\)
Solving, we get,
x = 2.9cm ≈ 3 cm.
Question 15. 3 mol of hydrogen is mixed with 1 mol of neon. The molar heat capacity at constant pressure is
- \(\frac{9}{4}\) R
- \(\frac{13}{2}\) R
- \(\frac{9}{2}\) R
- \(\frac{13}{4}\) R
Answer: 4. \(\frac{13}{4}\) R
For hydrogen: n = 3 mol, Cv = latex]\frac{5}{2}[/latex]R (diatomic).
For neon: n =1 mol, CV = \(\frac{3}{2}\)R (monatomic).
For the mixture,
⇒ \(C_V=\frac{n_1 C_{V_1}+n_2 C_{V_2}}{3+1}=\frac{3\left(\frac{5}{2} R\right)+1\left(\frac{3}{2} R\right)}{4}=\frac{9 R}{4}\)
Now, \(C_p-C_V=R\)
⇒ \(C_p=C_V+R\)
= \(\frac{9 R}{4}+R\)
= \(\frac{13}{4} R\)
Question 16. If vrms, vav, and Vmp be rms, average speed, and most probable speed of molecules of a gas obeying Maxwellian velocity distribution then which of the following statements is correct?
- vrms < vav < vmp
- vmp > vrms > vav
- vrms > vav > vmp
- vmp < vrms < vav
Answer: 3. vrms > vav > vmp
From Maxwell’s distribution, the expressions for velocities are as under:
RMS velocity = \(\sqrt{\frac{3 k T}{m}}=1.73 \sqrt{\frac{k T}{m}}\)
Average velocity = \(=\sqrt{\frac{8 k T}{\pi m}}\)
= \(\sqrt{\frac{8}{3.14}} \sqrt{\frac{k T}{m}}\)
= \(1.6 \sqrt{\frac{k T}{m}}\)
Most probable velocity = \(\sqrt{\frac{2 k T}{m}}=1.41 \sqrt{\frac{k T}{m}}\)
Thus, vrms>vav>vmp
Question 17. One mole of hydrogen gas is contained in a box of volume V = 1.00 m3 at T- 300 K. It is heated to 3000 K and gets converted to a gas of hydrogen atoms. The final pressure would be (assume the gas to be ideal)
- Same as the initial pressure
- Twice the initial pressure
- Ten times the initial pressure
- Twenty times the initial pressure
Answer: 4. Twenty times the initial pressure
From gas laws,pV = nRT
=> p1V1 = n1RT1; p2V2 = n2RT2
“chapter 13 physics class 11 “
Hence = \(\frac{p_1 V_1}{p_2 V_2}=\frac{n_1 T_1}{n_2 T_2}\)
V1 = V2 = V; n1 =1, n2 = 2 (as H2 molecule splitsinto atoms)
T1 = 300K, T2 = 3000 K.
These substitutions in (1) give
⇒ \(\frac{p_1}{p_2}=\frac{300}{2(3000)}\)
= \(\frac{1}{20}\)
⇒ \(p_2=20 p_1\)
Question 18. Two balloons are filled, one with pure He gas and the other with air. If the pressure and temperature of these balloons are the same then the number of molecules per unit volume is
- More in the He-filled balloon
- The same in both balloons
- More in an air-filled balloon
- In file ratio 1: 2
Answer: 2. The same in both balloons
From the gas laws, \(\frac{p_1 V_1}{T_1}=\frac{p_2 V_2}{T_2}\)
Since p1 = p2 and T1 = T2, the volumes V1 = V2.
Again, pV = nRT.
Hence, for the same values of p, V, and T, both hydrogen and air will have an equal number of molecules per unit volume.
Question 19. A gas mixture contains one mole of O2 and one mole of He gas. Find the ratio of the specific heat at constant pressure to that at constant volume of the gaseous mixture.
- 2.5
- 1.5
- 2
- 4
Answer: 2. 1.5
For oxygen: n = 1, Cv = \(\frac{5}{2}\)(diatomic).
For helium: n = 1, Cv = \(\frac{3}{2}\)R (monatomic).
⇒ \(\left(C_V\right)_{\text {mixture }}=\frac{\frac{5}{2} R+\frac{3}{2} R}{1+1}=2 R\)
(Cp)mixture = (CV)mixture + R = 2R + R = 3R.
⇒ \(\frac{C_p}{C_V}=\frac{3 R}{2 R}\)
= 1.5
Question 20. A closed cylindrical vessel contains 60 g of Ne and 64 g of 02. If the pressure of the gaseous mixture in the cylinder be 30 bar then the partial pressure of O2 (in bar) in the cylinder is
- 15
- 30
- 12
- 20
Answer: 3. 12
Number of moles2R in Ne = \(\frac{60 \mathrm{~g}}{20 \mathrm{~g} \mathrm{~mol}^{-1}}\) = 3 mol.
Number of moles in O2 = \(\frac{64 \mathrm{~g}}{32 \mathrm{~g} \mathrm{~mol}^{-1}}\) = 2 mol.
Total number of moles = (3 + 2) mol
= 5 mol.
∴ the partial pressure of oxygen
(P) = mole fraction x (32 bar)
= \(\frac{2}{5}\) (30 bar)
= 12 bar.
Question 21. A certain gas is taken to the five states represented by dots in the graph. The plotted curves are isothermals. The order of the most probable speed-up of the molecules at these five states is
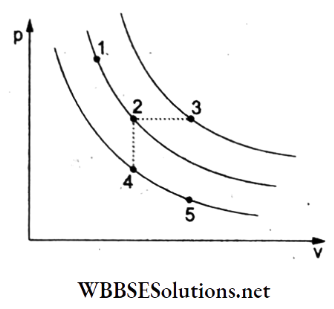
- vp at 3 > vp at 1 = vp at 2 > vp at 4 = vp at 5
- vp at 1 > vp at 2 = vp at 3 > vp at 4 = vp at 5
- vp at 3 > vp at 2 = vp at 4 > vp at 1 = vp at 5
- vp at 3 > vp at 2 = vp at 4 > vp at 1 = vp at 5
Answer: 1. vp at 3 > vp at 1 = vp at 2 > vp at 4 = vp at 5
Let T1 T2, …, T5 represent the temperatures and \(v_{p_1}, v_{p_2}, \ldots, v_{p_5}\) the corresponding values of the most probable velocities at these temperatures.
From the given set of isothermals, T1 = T2, T4 = T5, and T3 is the maximum temperature.
“chapter 13 physics class 11 “
Thus, T3 > T1 = T2 > T4
= T5
Since vp ∝ √T, hence vp3 > vp1 = vp2 > vp4 = vp5.
Question 22. The molecules of a given mass of a gas have an rms velocity of 200 ms’1 at 27°C and 1.0 x 105 N m_2 pressure. When the temperature and pressure of the gas are 127 °C and 0.05 x 105 N m“2 respectively, the rms velocity of its molecules in m s-1 is
- \(\frac{100 \sqrt{2}}{3}\)
- 100V2
- \(\frac{100}{3}\)
- \(\frac{400}{\sqrt{3}}\)
Answer: 4. \(\frac{400}{\sqrt{3}}\)
At T1 = 27 °C = (27 + 273) K = 300K, p1= 105 N m-1, vrms = 200m s-1.
At T2 = 127 ºC = (127 + 273) K = 400 K, p2 = 0.05N m-2.
vrms = ?
From kinetic theory, \(v_{\mathrm{rms}} \propto \sqrt{T}\)
⇒ \(\frac{v_2}{v_1}=\sqrt{\frac{T_2}{T_1}} \Rightarrow \frac{v_2}{200 \mathrm{~m} \mathrm{~s}^{-1}}=\sqrt{\frac{400}{300}}\)
∴ \(v_2=\left(200 \mathrm{~m} \mathrm{~s}^{-1}\right) \sqrt{\frac{4}{3}}=\frac{400}{\sqrt{3}} \mathrm{~m} \mathrm{~s}^{-1}\)
Question 23. A given sample of an ideal gas occupies a volume V at pressure p and absolute temperature T. The mass of each molecule of the gas is m. Which of the following gives the density of the gas?
- \(\frac{p}{kT}\)
- \(\frac{mp}{kT}\)
- \(\frac{p}{kTV}\)
- mkT
Answer: 2. \(\frac{mp}{kT}\)
From the gas equation, pV = nRT.
∴ \(\frac{p V}{R T}=n \text { (number of moles) }=\frac{\text { mass }}{\text { molar mass }}\)
Density = \(\rho=\frac{\text { mass }}{\text { volume }}\)
= \(\frac{p(\text { molar mass })}{R T}\)
= \(\frac{p}{R T} \cdot\left(m N_{\mathrm{A}}\right)\)
= \(\frac{m p}{T\left(R / N_{\mathrm{A}}\right)}\)
= \(\frac{m p}{k T}\)
Question 24. Keeping the volume constant, if the temperature of a .gas is increased, the
- Collision on the walls will be less
- The number of collisions per unit of time will increase
- Collisions will be in straight lines
- The rate of collision will not change
Answer: 2. Number of collisions per unit of time will increase
With the increase in temperature, the molecules move more rapidly at random and this leads to an increase in the collision rate.
Question 25. A polyatomic gas with n degrees of freedom has a mean energy per molecule given by
- \(\frac{n k T}{2}\)
- \(\frac{n k T}{2N}\)
- \(\frac{3 k T}{2}\)
- \(\frac{n k T}{N}\)
Answer: 1. \(\frac{n k T}{2}\)
According to the law of equipartition of energy, energy per degree of
freedom = \(\frac{1}{2}\)KT.
In a polyatomic gas, each molecule has n degrees of freedom (given),
So the average energy per molecule is,
⇒ \(\left(\frac{1}{2} k T\right) n=\frac{n k T}{2}\)
Question 26. According to the kinetic theory of gases, at absolute zero temperature,
- Water freezes
- Liquid helium freezes
- Molecular motion stops
- Liquid hydrogen freezes
Answer: 3. Molecular motion stops
According to the kinetic theory,
⇒ \(p=\frac{1}{3} \frac{m}{V} C_{\mathrm{rms}}^2 \Rightarrow p V=R T=\frac{1}{3} M C_{\mathrm{rms}}^2\)
At absolute zero,
⇒ \(C_{\mathrm{rms}}^2=0 \Rightarrow \frac{1}{n}\left(C_1^2+C_2^2+C_3^2+\ldots+C_n^2\right)=0\)
This is possible if C1 = C2 = C3 = ….. = 0.
Hence, molecular motion of all types stops.
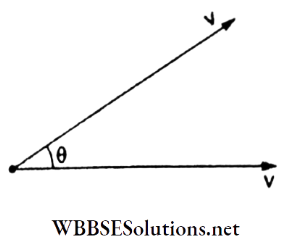
Question 27. In Maxwell’s speed distribution curve for nitrogen, the average relative velocity between two molecules at 300 K will be
- 300 ms-1
- 920 ms-1
- 606 ms-1
- zero
Answer: 3. 606 ms-1
The magnitude of relative velocity between two molecules is
⇒ \(\left|\vec{v}_{\text {rel }}\right|=\sqrt{v^2+v^2-2 v^2 \cos \theta}=2 v \sin \frac{\theta}{2}\)
Average value is
⇒ \(\mid \vec{v}_{\mathrm{rel}} \mathrm{lav}_{\mathrm{av}}=\frac{1}{\pi} \int_0^\pi 2 v \sin \frac{\theta}{2} d \theta=\frac{4 v_{\mathrm{av}}}{\pi}\)
According to.Maxwell’s velocity distribution,
⇒ \(v_{\mathrm{av}}=\sqrt{\frac{8 R T}{\pi m}}\)
average relative velocity
⇒ \(=\frac{4}{\pi} \sqrt{\frac{8 R T}{\pi m}}\)
⇒ \(\frac{4}{3.14} \sqrt{\frac{8\left(8.3 \mathrm{~J} \mathrm{~mol}^{-1} \mathrm{~K}^{-1}\right)(300 \mathrm{~K})}{3.14\left(28 \times 10^{-3} \mathrm{~kg} \mathrm{~mol}^{-1}\right)}}\)
= \(606 \mathrm{~m} \mathrm{~s}^{-1}\)
Question 28. The radius of an oxygen molecule is 40 A. Its time of relaxation at atmospheric pressure and a temperature of 27°C will be
- 10-12 s
- 10-10 s
- 10-16 s
- 10-14 s
Answer: 1. 10-12s
The rms velocity of gaseous molecules is
⇒ \(v_{\mathrm{rms}}=\frac{\text { mean free path }(\lambda)}{\text { relaxation time }(\tau)}\)
⇒ \(\tau=\frac{\lambda}{v_{\mathrm{rms}}}\)
= \(\frac{1}{\sqrt{2} \pi n d^2} \sqrt{\frac{m_{\mathrm{O}}}{3 R T}}\)
Now, number density = \(\), where p = number of moles.
But \(n=\frac{N}{V}=\frac{\mu N_A}{V}\)
∴ \(p V=\mu R T \text { or } \frac{\mu}{V}=\frac{p}{R T} \text {. Hence, } n=\frac{N_{\mathrm{A}} p}{R T}\)
Substituting the given values, x = 0.01 x 10-10s
= 10-12 s.
Question 29. If 1022molecules of a gas, each of mass 10-26 kg, collide elastically and perpendicularly with a surface per second over an area of 1 m2 with a speed of 104 m s-1, the pressure exerted by the gas molecules will be of the order of
- 4 Pa
- 3 Pa
- 2 Pa
- 5 Pa
Answer: 3. 2 Pa
For an elastic collision, the change in momentum per collision = 2mu,
and in 1 s, n molecules collide. Hence, net force F = 2mun.
This force acts on an area of 1 m2. Hence, pressure is
⇒ \(p=\frac{F}{A}\)
= \(\frac{2 m u n}{1 \mathrm{~m}^2}\)
= \(\frac{2\left(10^{-26} \mathrm{~kg}\right)\left(10^4 \mathrm{~m} \mathrm{~s}^{-1}\right)\left(10^{22}\right)}{1 \mathrm{~m}^2}\)
= \(2 \mathrm{Nm}^{-2}\)
= 2Pa.
Question 30. 15 g of nitrogen is enclosed in a vessel at a temperature of 27°C. For the rms speed of its molecules to get doubled, the amount of heat absorbed by the gas is about
- 10 kJ
- 14 kJ
- 6 kJ
- 0.5 kJ
Answer: 1. 10 kJ
The rms velocity \(v_{\mathrm{rms}}=\sqrt{\frac{3 R T}{M}}\)
When vrms is doubled, temperature increases from T to 4T.
Hence, increase in temperature = AT = 4T-T
= 3T
= 3(273 + 27) K
= 900 K.
Heat absorbed (at constant volume) = ΔQ = nCvΔT.
Here,
n = \(\frac{15g}{28g}\)
= \(\frac{15}{28}, C_V=\frac{5}{2} R\)
⇒ \(\Delta Q=\frac{15}{28} \cdot \frac{5}{2}\) (8-3).900
= 104J
= 10kJ.
Question 31. An increase in the temperature of a gas-filled in container would lead to
- An increase in its mass
- An increase in its kinetic energy
- A decrease in its pressure
- A decrease in intermolecular separation
Answer: 2. An increase in its kinetic energy
The energy of an ideal gas is purely kinetic, and is \(\frac{1}{2}\)kT per degree of freedom.
Hence, an increase in temperature leads to an increase in its kinetic energy.
Question 32. A gas mixture consists of 3 mol of oxygen and 5 mol of argon at temperature T. Considering only translational and rotational modes, the total internal energy of the gaseous mixture will be
- 12 RT
- 20 RT
- 15 RT
- 4 RT
Answer: 3. 15 RT
Oxygen is diatomic, so it has 5 degrees of freedom for each molecule.
Hence, 3 mol of oxygen has total degrees of freedom = (3NA)5 = 15NA.
Similarly, argon (monatomic) has 3 degrees of freedom per molecule, and thus for 5 mol, the number of degrees of freedom = (5NA)3 = 15NA.
∴ total numberofdegrees offreedom = 30NA.
According to the equipartition law, the total energy is
⇒ \(E=\left(30 N_A\right)\left(\frac{1}{2} k T\right)\)
Question 33. An ideal gas occupies a volume of 2 m3 at a pressure of 3 x 106 Pa. The energy of the gas is
- 6 x 104 J
- 9 x 106 J
- 3 x 103 J
- 107J
Answer: 2. 9 x 106 J
According to the kinetic theory, the pressure is
⇒ \(p=\frac{1}{3} \frac{M}{V} C_{\mathrm{rms}}^2\)
= \(\frac{2}{3 V}\left(\frac{1}{2} M C_{\mathrm{rms}}^2\right)\)
= \(\frac{2}{3 V}(\mathrm{KE})\)
∴ energy of the gas = E = \(\frac{3}{2}\) pV.
Substituting the given values,
E = \(\frac{3}{2}\) (3 x 106 Pa)(2 m3)
= 9 x 106 J.
Question 34. The temperature at which the rms speed of hydrogen molecules equals their escape velocity from the earth is closest to (given that Boltzmann constant, kB = 1.38 x 10-23 J K-1; Avogadro’s number, NA = 6.02 x 1023 mol-1; earth’s radius = 6400 km; acceleration due to gravity = 10 m s-2)
- 104K
- 650 K
- 800 K
- 3 x 105 K
Answer: 1. 104K
The rms speed \(C_{\mathrm{rms}}=\sqrt{\frac{3 R T}{M}}\)
where M = molar mass ofhydrogen = 2 g mol-1 = 2 x 103 kgmol-1.
Given that = escape velocity = \(\sqrt{2 g r}\), where r is earth’s radius
Substituting the given values,
⇒ \(\sqrt{\frac{3 R T}{M}}=\sqrt{2 g r}\)
⇒ \(T=\frac{2 M g r}{3 R}\)
1.02 x 104K ≈ 104K
Question 35. If the temperature of an ideal gas enclosed in a box is increased then the
- Mean free path decreases
- The mean free path remains unchanged
- Relaxation time decreases
- Relaxation time remains unchanged
Answer: 2. Mean free path remains unchanged
Mean free path = \(\lambda=\frac{1}{\sqrt{2} \pi n d^2}, \text { where } n=\frac{N}{V}\) = number density,
d = diameter of molecule.
With the increase in temperature, the rate of collision increases, so relaxation time decreases, but the mean free path remains unchanged.
