Heat And Thermodynamics Synopsis
- Heat is a form of energy that exists in matter due to the constant random motion of molecules. Its SI unit is the joule (symbol: J) and cgs unit is the calorie (symbol: cal).1 cal = 4.2 J and 1 kcal = 4200 J.
- The temperature of a body indicates its thermal state and determines the direction of the flow of heat Heat always flows from a higher to a lower temperature.
- The absolute zero, or OK, corresponds to a temperature of -273.15 degrees on the Celsius temperature scale. The lower and upper fixed points in the Celsius scale correspond to 273.15 K and 373.15 K respectively.
- The triple point for pure water is 0.01°C (273.16 K) at 611 Pa, and is used to calibrate thermometers.
- Ideal gas equation of state: pV= nRT, where p = pressure (in pascals or newtons per meter squared), V = volume (in m³), n = amount of substance (in moles), and R = gas constant ≈ 8.3 J K-1 mole-1.
- van der Waals equation of state for real gases:
\(\left(p+\frac{a}{V^2}\right)\) (V – b) = nRT, where a and b are constants. - Thermal expansion:
- For length, L2θ = Lv(1 + αθ).
- For area, Aθ = A0(1 + βθ).
- For volume, Vθ = V0(1 + γθ).
- 6α = 3β = 2γ.
- Molar mass M = wNA, where m = mass of each molecule and NA = Avogadro constant ≈ 6.022 x 1023 mol-1.
- Boltzmann constant,
\(k=\frac{R}{N_{\mathrm{A}}} \approx \frac{8.3 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}}{6.022 \times 10^{23} \mathrm{~mol}^{-1}} \approx 1.38 \times 10^{-23} \mathrm{~J} \mathrm{~K}^{-1}\) - Gaseous pressure,
\(p=\frac{1}{3}\left(\frac{M}{V}\right) c_{\mathrm{rms}}^2=\frac{1}{3} \rho c_{\mathrm{rms}}^2\).

“heat thermodynamics “
In this equation:
-
- RMS speed, \(c_{\mathrm{rms}}=\sqrt{\frac{3 p}{\rho}}=\sqrt{\frac{3 p V}{M}}=\sqrt{\frac{3 n R T}{M}}=\sqrt{\frac{3 k T}{m}}\), where
m = mass of each molecule; - mean speed, \(\bar{c}=\sqrt{\frac{8 R T}{\pi M}}=\sqrt{\frac{8 k T}{\pi m}}\)
- RMS speed, \(c_{\mathrm{rms}}=\sqrt{\frac{3 p}{\rho}}=\sqrt{\frac{3 p V}{M}}=\sqrt{\frac{3 n R T}{M}}=\sqrt{\frac{3 k T}{m}}\), where
“thermodynamics class 12 physics notes “
- Most probable velocity, \(c_{\mathrm{mp}}=\sqrt{\frac{2 R T}{M}}=\sqrt{\frac{2 k T}{m}}\)
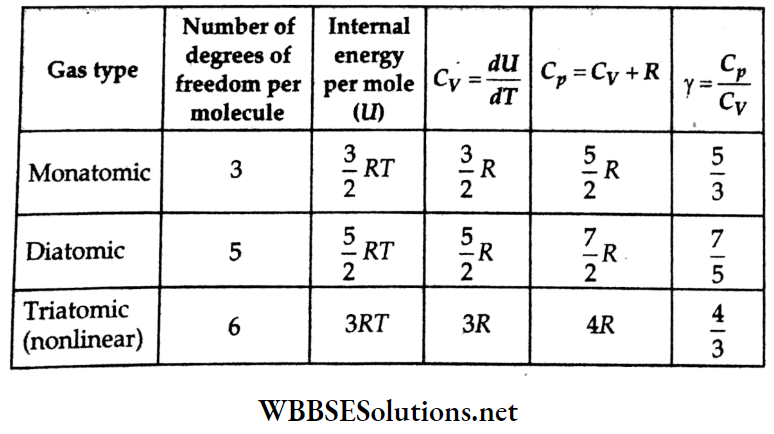
- Thus, cmp < vmean < crms.
- Heat absorbed (or expelled) Q = mcAT, where Q is in joules, specific heat capacity (unit: J kg-1K-1), and AT = change in temperature (in°C or K).
- Specific latent heat = L (SI unit: J kg-1).
- During a phase change, Q = mL.
- The molar heat capacity C (SI unit: J mol-1C-1) of gases is process dependent and is given by the equation Q = nCAT, where n = amount of substance (in moles).
- At a constant volume, \(C_V=\left.\frac{Q}{n \Delta T}\right|_{V=\text { constant }}\)
- At a constant pressure, \(C_p=\left.\frac{Q}{n \Delta T}\right|_{p=\text { constant }}\)
“heat and thermodynamics pdf “
- \(C_p-C_V=R, \frac{C_p}{C_V}=\gamma, C_p=\left(\frac{R \gamma}{\gamma-1}\right), p V^\gamma=\text { constant }\)
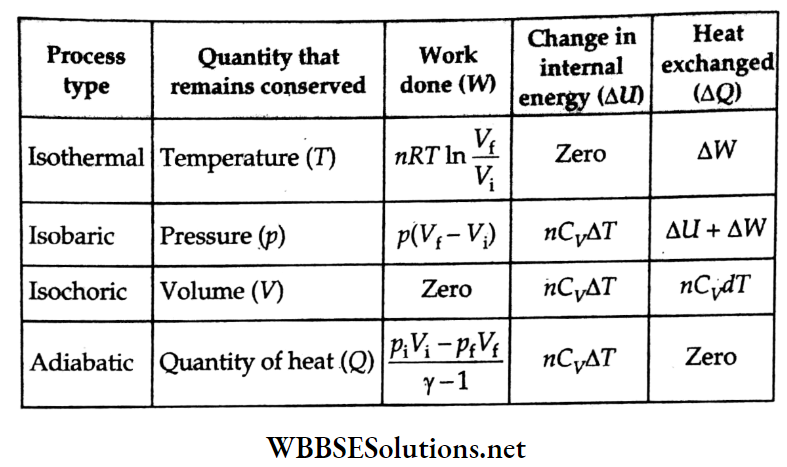
- Change in internal energy, AU = nCvΔT.
- The first law of thermodynamics: ΔQ = ΔU + ΔW.
- Thermodynamic processes:
- Molar heat capacity of a mixture of gases:
- \(\left(C_V\right)_{\text {mix }}=\frac{n_1 C_{V_1}+n_2 C_{V_2}+\ldots}{n_1+n_2+\ldots}\)
- \(\left(C_p\right)_{{mix}}=\frac{n_1 C_{p_1}+n_2 C_{p_2}+\ldots}{n_1+n_2+\ldots}\)
- \((\gamma)_{{mix}}=\frac{n_1 C_{p_1}+n_2 C_{p_2}+\ldots}{n_1 C_{V_1}+n_2 C_{V_2}+\ldots}\)
| Class 11 Physics | Class 12 Maths | Class 11 Chemistry |
| NEET Foundation | Class 12 Physics | NEET Physics |
“heat and thermodynamics definition
- Equipartition law: This law states that the average energy of a molecule in a gas associated with each degree of freedom is \(\frac{1}{2}\)kT, where k = Boltzmann constant and T = temperature (in kelvin).