Hydrostatics Density
Bodies of equal volume may have different weights. Among pieces of iron, lead and wood of equal volume, the piece of lead is heavier than the piece of iron, and the piece of wood is the lightest.
It leads to the concept of density. A body with greater density weighs more, and a body with lower density weighs less — if both the bodies are of the same volume.
Density Definition: Density is the mass per unit volume of a substance.
If a volume V of a substance has a mass M, then the density of the substance, D = \(\frac{M}{V}\). Often, the greek letter ρ(rho) is used as the symbol of density.
If a body is not homogeneous, the average density of the body can be found out by dividing the total mass of the body by its total volume.
Units of density:
- g • cm-3
- kg • m-3
Relation: 1 kg • m-3 = \(\frac{1 \mathrm{~kg}}{1 \mathrm{~m}^3}=\frac{10^3 \mathrm{~g}}{10^6 \mathrm{~cm}^3}=10^{-3} \mathrm{~g} \cdot \mathrm{cm}^{-3}\)
∴ 1g • cm-3 = 1000kg • m-3
Density and Specific Gravity in Everyday Life
Effect of pressure and temperature on density: The density of a substance depends on pressure and temperature. With change in pressure, the volume of a solid or a liquid does not change appreciably.
- So, to express the density of a solid or a liquid, the pressure need not be mentioned. But with change in pressure, the volume of a gaseous substance changes appreciably, and the density also changes accordingly.
- With change in temperature, the volume, and hence the density, of a substance changes. So, to express the density of a gaseous substance, both the temperature and the pressure of the substance have to be mentioned. Ib express the density of a solid or a liquid, the temperature needs to be mentioned to get sufficient accuracy.
- For example, at normal atmospheric pressure, air at 0°C has a density of 1.29 kg · m-3 but at 10°C, the density of air is 1.25 kg · m-3 which is slightly less.
Dimension of density: [D] = \(\frac{M}{L^3}=M L^{-3}\)

Density vs Specific Gravity Explained
Hydrostatics Specific Gravity
In many cases, the density of a substance is expressed with respect to the density of another substance. The density of a substance expressed in this way explains the concept of specific gravity. For the specific gravity of a liquid or a solid, the density of water at 4°C is taken as the standard. But, in the case of a gaseous substance, the standard is the density of hydrogen gas at STP.
Specific Gravity Definition: The ratio of the density of a solid or a liquid to that of water at 4°C is called the specific gravity of that substance.
Alternatively, the specific gravity of a solid or a liquid is defined as the ratio between the mass of a certain volume of the substance to the mass of an equal volume of water at 4°C.
If S denotes the specific gravity of a solid or a liquid, then according to the second definition,
S = \(\frac{\text { mass of volume } V \text { of the substance }}{\text { mass of volume } V \text { of water at } 4^{\circ} \mathrm{C}}\)
= \(\frac{\text { mass of unit volume of the substance }}{\text { mass of unit volume of water at } 4^{\circ} \mathrm{C}}\)
= \(\frac{\text { density of the substance }}{\text { density of water at } 4^{\circ} \mathrm{C}}\)
This is the earlier definition of specific gravity.
- Since the specific gravity of a substance is the ratio of two densities, it is also called relative density. Specific gravity (relative density) tells us how dense a substance is in comparison to water at 4°C.
- For example, when we say that the relative density of brass is 8.4, it means a piece of brass of any volume has mass 8.4 times that of an equal volume of water at 4°C.
- When specific gravity of a substance is greater than one, it is heavier than water. Hence it will sink in water. When specific gravity is less than one, it is lighter than water. Hence it will float in water.
- Specific gravity is only a number and has no unit. For this reason, the value of the specific gravity of a substance is equal in all systems of units. It is a dimensionless physical quantity.
Comparison of density and specific gravity in terms of magnitude: From the definition of specific gravity, we can write,
Density of a substance = specific gravity of the substance x density of water at 4°C
In the CGS system, the density of water at 4°C is 1 g • cm-3 and hence in this system, density of a substance = specific gravity of the substance x 1 g • cm-3. So, in the CGS system, the density of a substance and its specific gravity are numerically equal.
In SI, the density of water at 4°C is 1000 kg • m-3. So, in this system, density of a substance = specific gravity of the substance x 1000 kg • m-3. Therefore, in SI, the density of a substance is numerically 1000 times that of the specific gravity of the substance.
Applications of Density and Specific Gravity
Temperature correction of specific gravity: in determining the specific gravity of a solid or a liquid, we usually use water at room temperature. But, actually water at 4°C should be taken as standard because the density of water at 4°C only is 1 g • cm-3.
Since the density of water changes with temperature, a temperature correction of specific gravity should be considered for a better result.
Let the temperature of the water used be r°C. If the specific gravity of the substance is S, then,
S = \(\frac{\text { mass of volume } V \text { of the substance }}{\text { mass of volume } V \text { of water at } 4^{\circ} \mathrm{C}}\)
= \(\frac{\text { mass of volume } V \text { of the substance }}{\text { mass of volume } V \text { of water at } t^{\circ} \mathrm{C}}\)
x \(\frac{\text { mass of volume } V \text { of water at } t^{\circ} \mathrm{C}}{\text { mass of volume } V \text { of water at } 4^{\circ} \mathrm{C}}\)
This correction may be avoided where a fine measurement is not strictly necessary. This is because the change in the density of water with temperature is quite small.
Hydrostatics Differences Between Density And Specific Density
Differences Between Density And Specific Density
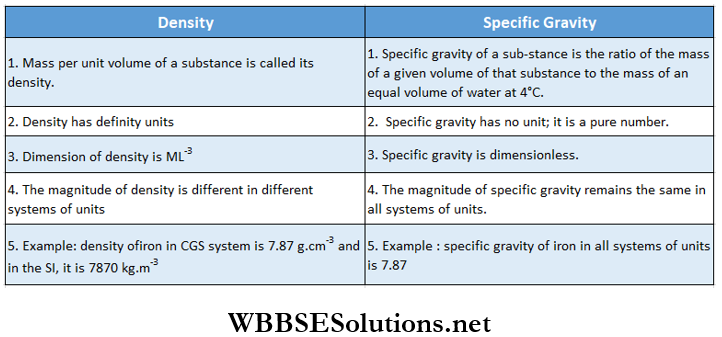
Differences Between Density And Specific Density Numerical Examples
Example 1. By mixing 210 g of salt in 1 L of water, 1.05 L of solution is produced. Determine the density of that solution.
Solution:
Given
By mixing 210 g of salt in 1 L of water, 1.05 L of solution is produced.
Mass of the solution = mass of water + mass of salt
= (1000 + 210) g [Mass of 1 L of water = 1000 g] = 1210 g
Volume of the solution = 1.05 L = 1050 cm3.
∴ Density of the solution = \(\frac{\text { mass of the solution }}{\text { volume of the solution }}\)
= \(\frac{1210}{1050}=1.152 \mathrm{~g} \cdot \mathrm{cm}^{-3}\)
Dimensionless Nature of Specific Gravity
Example 2. How much of a liquid of density 1.3 g • cm-3 can be stored in a container of 5 kg of kerosene oil? The density of kerosene = 0.8 g • cm-3. Give your answer in kg.
Solution:
Volume of kerosene oil = \(\frac{5000}{0.8}\) = 6250 cm3
∴ The container can hold 6250 cm3 of any liquid.
∴ Mass of the given liquid that can be stored in the container =6250 x 1.3 = 8125 g = 8.125 kg.
Example 3. A liquid of mass my and density ρ1 is mixed with another liquid of mass m2 and density ρ2. If the volume of the mixture does not change, then what will be the density of the mixture?
Solution:
Given
A liquid of mass my and density ρ1 is mixed with another liquid of mass m2 and density ρ2. If the volume of the mixture does not change
Mass of the mixture, m = m1 + m2
Volume of the first liquid, \(v_1=\frac{m_1}{\rho_1}\) and volume of the second liquid, \(v_2=\frac{m_2}{\rho_2}\)
∴ Volume of the mixture,
ν = \(v_1+v_2=\frac{m_1}{\rho_1}+\frac{m_2}{\rho_2}=\frac{m_1 \rho_2+m_2 \rho_1}{\rho_1 \rho_2}\)
∴ Density of the mixture, \(\frac{m}{v}=\frac{\left(m_1+m_2\right) \rho_1 \rho_2}{m_1 \rho_2+m_2 \rho_1}\).
Examples of Density and Specific Gravity Calculations
| Class 11 Physics | Class 12 Maths | Class 11 Chemistry |
| NEET Foundation | Class 12 Physics | NEET Physics |
Example 4. The specific gravity of a mixture of two substances of equal volumes is 4. The specific gravity of the mixture becomes 3 when these two substances are mixed in equal masses. Calculate the specific gravity of the substances.
Solution:
Given
The specific gravity of a mixture of two substances of equal volumes is 4. The specific gravity of the mixture becomes 3 when these two substances are mixed in equal masses.
Let the specific gravities of the two substances be S1 and S2 and the density of water at 4°C be ρ.
∴ Densities of the substances are S1ρ and S2ρ.
Let V = volume of each of the substances in the mixture.
∴ Total mass of the mixture = \(\left(V S_1 \rho+V S_2 \rho\right)\)
Total volume of the mixture = V+ V = 2 V
∴ Density of the mixture = \(\frac{V S_1 \rho+V S_2 \rho}{2 V}\)=\(\frac{\rho\left(S_1+S_2\right)}{2}\)
Specific gravity = \(\frac{S_1+S_2}{2}\) (according to the problem)
or, S1 + S2 = 8 ……….(1)
Again, let m = mass of each of the two substances in the mixture.
∴ Total mass of the mixture = m + m = 2 m
Total volume of the mixture = \(\left(\frac{m}{S_1 \rho}+\frac{m}{S_2 \rho}\right)\)
∴ Density of the mixture = \(\frac{2 m}{\frac{m}{S_1 \rho}+\frac{m}{S_2 \rho}}=\frac{2 S_1 S_2 \rho}{S_1+S_2}\)
∴ Specific gravity of the mixture = \(\frac{2 S_1 S_2}{S_1+S_2}=3\) (according to the problem)
or, \(S_1 S_2=\frac{3}{2}\left(S_1+S_2\right)=\frac{3}{2} \times 8=12\) ………(1)
∴ \(S_1-S_2=\sqrt{\left(S_1+S_2\right)^2-4 S_1 S_2}\)
= \(\sqrt{(8)^2-4 \times 12}=4\) ……..(2)
From equations (1) and (2), S1 = 6 and S2 = 2
∴ The specific gravities of the two given substances are 6 and 2.
Example 5. The ratio of the densities of three liquids is 1:2:3. If they are mixed in
- Equal volume,
- Equal mass, then what will be the densities of their mixtures?
Solution:
Given
The ratio of the densities of three liquids is 1:2:3.
1. Let the densities of the liquids be d, 2d and 3d.
Let volume V of each of the three liquids be mixed.
∴ Total volume of the mixture = V+ V+ V = 3 V and total mass of the mixture = V • d + V • 2d + V • 3d = 6 Vd.
∴ Density of the mixture = \(\frac{6 V d}{3 V}\) = 2d
So, the density of the mixture will be twice the density of the first liquid.
2. if the mixture is prepared by mixing m mass of each of the three liquids, then the total mass of the mixture m+ m + m = 3m.
Total volume of the mixture = \(\frac{m}{d}+\frac{m}{2 d}+\frac{m}{3 d}=\frac{11}{6} \cdot \frac{m}{d}\)
∴ Density of the mixture = \(\frac{3 m}{\frac{11}{6} \cdot \frac{m}{d}}=\frac{18}{11} d\)
So, the density of the mixture will be 18/11 times the density of the first liquid.
Example 6. What amount of concentrated sulphuric acid (specific gravity 1.8) should be mixed with 1 L of water so that the specific gravity of the mixture becomes 1.24?
Solution:
Let the required volume be ν cm3.
Mass of that volume of acid =1.8 ν g and mass of 1 L of water = 1000 g
∴ Total mass =(1.8 ν+ 1000) g and total volume = (ν+ 1000) cm3
∴ Specific gravity of the mixture = \(\frac{1.8 v+1000}{v+1000}\)
∴ \(\frac{1.8 v+1000}{v+1000}\) = 1.24
or, (1.8-1.24)ν = 1240-1000 or, 0.56 ν = 240
or, v= 428.6 cm3.
Example 7. The mass of 1 L of milk is 1032 g. The milk contains 4% butter by volume. If the specific gravity of butter is 0.865, then find the density of butter-free milk.
Solution:
Given
The mass of 1 L of milk is 1032 g. The milk contains 4% butter by volume. If the specific gravity of butter is 0.865
Volume of butter present in 1 L of milk = 40 cm3.
Its mass =40 x 0.865 = 34.6 g
∴ Mass of butter-free milk = 1032-34.6 = 997.4 g and its volume = 1000 – 40 = 960 cm3
∴ Density of butter free milk = \(\frac{997.4}{960}=1.039 \mathrm{~g} \cdot \mathrm{cm}^{-3}\)
Dimensionless Nature of Specific Gravity
Example 8. The mass of a coil of copper wire of diameter 1.2 mm is 150 g. If the density of copper is 8.9 g • cm-3, then find the length of the copper wire.
Solution:
Given
The mass of a coil of copper wire of diameter 1.2 mm is 150 g. If the density of copper is 8.9 g • cm-3
Let the length of the wire be l and its radius be r.
Its volume = πr²l= π(0.06)²l cm³, its mass = π(0.06)² l x 8.9 g = 150
∴ l = \(\frac{150}{\pi \times 0.0036 \times 8.9}=1490 \mathrm{~cm} \text { (approx.) }\)
Example 9. The volume and mass of a piece of iron-aluminium alloy are 100 cm3 and 588 g respectively. The specific gravities of iron and aluminium are 8 and 2.7 respectively. Calculate the ratio of the volumes of iron and aluminium in the alloy.
Solution:
Given
The volume and mass of a piece of iron-aluminium alloy are 100 cm3 and 588 g respectively. The specific gravities of iron and aluminium are 8 and 2.7 respectively.
Let the volume of iron = ν1 cm3, volume of aluminium = ν2 cm3; density of iron = 8 g • cm-3 and density of aluminium =2.7 g • cm-3.
According to the problem,
⇒ \(8 v_1+2.7 v_2=588\) ….(1)
and \(v_1+v_2=100\)
Solving (1) and (2) we get, ν1 = 60, ν2 = 40
∴ \(\frac{v_1}{v_2}=\frac{60}{40}=\frac{3}{2}\)
∴ The ratio of the volumes of iron and aluminium =3:2.
