Bohr Model And Hydrogen Spectrum
Question 1. The energy of a hydrogen atom in the ground state is -13.6 eV. The energy of an He+ ion in the first excited state will be
- -13.6 eV
- -54.4 eV
- -27.2 eV
- -6.8 eV
Answer: 1. -13.6 eV
In the ground state (n = 1) of hydrogen, the total energy is
⇒ \(E_1=-\frac{13.6 \mathrm{eV}}{1^2}=-13.6 \mathrm{eV}\)
In the first excited state (n = 2), the total energy of an He+ ion (Z = 2) will be
∴ \(E_1^{\prime}=-\frac{Z^2(13.6 \mathrm{eV})}{2^2}=-\frac{4(13.6 \mathrm{eV})}{4}=-13.6 \mathrm{eV}\).
Read And Learn Also NEET Physics Multiple Choice Question and Answers
Question 2. The ground-state energy of a hydrogen atom is -13.6 eV. When the electron is in the first excited state, its excitation energy is
- 3.4 eV
- 6.8 eV
- 10.2 eV
- Zero
Answer: 3. 10.2 eV
In the ground state (n =1),
energy = E1 = \(-\frac{13.6 \mathrm{eV}}{1^2}=-13.6 \mathrm{eV}\)
In the first excited state(n = 2),
energy = E2 = \(-\frac{13.6 \mathrm{eV}}{2^2}=-3.4 \mathrm{eV}\)
The excitation energy is the energy required to raise the electron from the initial state (ni = 1) to the final state (nf = 2).
Thus, excitation energy = E2 – E1 = -3.4 eV -(-13.6 eV)
= (13.6- 3.4) eV = 10.2 eV.

Question 3. The total energy of an electron in the first excited state of a hydrogen atom is about -3.4 eV. Its kinetic energy in this state is
- -3.4 eV
- 3.4 eV
- 6.8 eV
- -6.8 eV
Answer: 2. 3.4 eV
In a hydrogen atom, both the total energy and the potential energy are negative such that
Etot = -KE and PE = -2KE.
∴ total energy = \(E_{\text {tot }}=\mathrm{KE}+\mathrm{PE}=\mathrm{KE}+(-2 \mathrm{KE})=-\mathrm{KE}=\frac{1}{2} \mathrm{PE}\)
Given that Etot = -3.4 eV.
∴ KE = -Etot = 3.4 eV.
Question 4. The ground-state energy of an H atom is -13.6 eV. The energy required to ionize an H atom from its second excited state is
- 13.6 eV
- 3.4 eV
- 12.1 eV
- 1.51 eV
Answer: 4. 1.51 eV
Given that E1 = -13.6 eV.
The energy in the second excited state (n = 3) is
⇒ \(E_3=-\frac{13.6 \mathrm{eV}}{3^2}=-\frac{13.6}{9} \mathrm{eV}=-1.51 \mathrm{eV}\)
Ionization means the extraction of the electron from the given state to n = ∞.
∴ the energy required to ionize is
E = E∞ – E3 = 0- (-1.51 eV) =1.51 eV
Question 5. The ionization energy of a hydrogen atom in its ground state is 13.6 eV. Following Bohr’s theory, the energy corresponding to a transition between the third and fourth orbits is
- 3.40 eV
- 0.66 eV
- 0.85 eV
- 1.51 eV
Answer: 2. 0.66 eV
Given that ionization energy = 13.6 eV.
In the third orbit,
⇒ \(E_3=-\frac{13.6 \mathrm{eV}}{n^2}=-\frac{13.6 \mathrm{eV}}{9}\)
In the fourth orbit,
⇒ \(E_4=\frac{-13.6 \mathrm{eV}}{4^2}=\frac{-13.6 \mathrm{eV}}{16}\)
The energy required for the transition from the state n = 3 to the state n = 4 is
⇒ \(\Delta E=E_4-E_3=\frac{-13.6 \mathrm{eV}}{16}-\left(-\frac{13.6 \mathrm{eV}}{9}\right)\)
∴ \(13.6\left(\frac{1}{9}-\frac{1}{16}\right) \mathrm{eV}=\frac{13.6 \times 7}{9 \times 16} \mathrm{eV}=0.66 \mathrm{eV}\)
Question 6. The ratio of the longest wavelengths corresponding to the Lyman series and the Balmer series in the hydrogen spectrum is
- \(\frac{5}{27}\)
- \(\frac{7}{29}\)
- \(\frac{9}{31}\)
- \(\frac{2}{23}\)
Answer: 1. \(\frac{5}{27}\)
The energy difference during the transition is
⇒ \(\Delta E=h \mathrm{v}=\frac{h c}{\lambda}\)
For the Lyman series,
⇒ \(\frac{h c}{\lambda_1}=E_2-E_1=13.6\left(1-\frac{1}{4}\right)\)
For the Balmer series,
⇒ \(\frac{h c}{\lambda_2}=E_3-E_2=13.6\left(\frac{1}{4}-\frac{1}{9}\right)\)
∴ \(\frac{\lambda_1}{\lambda_2}=\frac{E_3-E_2}{E_2-E_1}=\frac{5 / 36}{3 / 4}=\frac{5}{27}\).
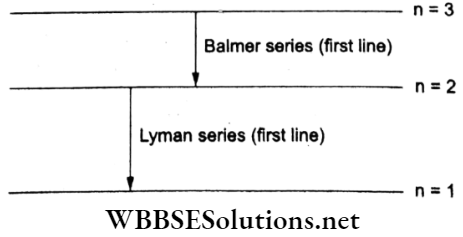
in the bohr model of the hydrogen atom
Question 7. The electron in a hydrogen atom first jumps from the third excited state to the second excited state and then from the second excited state to the first excited state. The ratio of the wavelengths λ1 and λ2 emitted in the two cases is
- \(\frac{27}{20}\)
- \(\frac{20}{7}\)
- \(\frac{7}{5}\)
- \(\frac{27}{5}\)
Answer: 2. \(\frac{20}{7}\)
For the electronic transition from the third excited state (n = 4) to the second excited state (n = 3), the wavelength λ1 is given by
⇒ \(\frac{h c}{\lambda_1}=E_4-E_3=13.6\left(\frac{1}{9}-\frac{1}{16}\right) \mathrm{eV}\)
For the transition from the second excited state(n = 3) to the first excited state (n = 2), the wavelength λ2 is given by
⇒ \(\frac{h c}{\lambda_2}=E_3-E_2=13.6\left(\frac{1}{4}-\frac{1}{9}\right) \mathrm{eV}\)
⇒ \(\frac{\lambda_1}{\lambda_2}=\frac{13.6\left(\frac{5}{36}\right) \mathrm{eV}}{13.6\left(\frac{7}{144}\right) \mathrm{eV}}=\frac{5}{36} \times \frac{144}{7}=\frac{20}{7}\).
Question 8. The wavelength of the first line of the Lyman series for a hydrogen atom is equal to that of the second line of the Balmer series for a hydrogen-like ion. The atomic number Z of this hydrogenlike ion is
- 1
- 3
- 2
- 4
Answer: 3. 2
For the first line of the Lyman series for an H atom, the transition takes place from n = 2 to n = 1. Thus,
⇒ \(\Delta E=\frac{h c}{\lambda_1}=E_2-E_1=13.6\left(1-\frac{1}{4}\right) \mathrm{eV}=13.6 \times \frac{3}{4} \mathrm{eV}\)
For the second line of the Balmer series, the energy needed for the transition for the atomic number Z is
⇒ \(\Delta E=\frac{h c}{\lambda_2}=13.6 \mathrm{Z}^2\left(\frac{1}{2^2}-\frac{1}{4^2}\right) \mathrm{eV}=13.6 \mathrm{Z}^2\left(\frac{3}{16}\right) \mathrm{eV}\)
Since λ1 = λ2 (given),
⇒ \(13.6 Z^2\left(\frac{3}{16}\right)=13.6 \times \frac{3}{4}\)
∴ Z2 = 4 ⇒ Z = 2.
Question 9. Which of the following transitions gives photons of the maximum energy?
- From n = 1 to n = 2
- From n = 2 to n = 1
- From n = 2 to n = 6
- From n = 6 to n = 2
Answer: 2. From n = 2 to n = 1
The energy of a photon depends on the change in the principal quantum number (n).
For the transition from n = 2 to n = 1,
⇒ \(\Delta E_1=(13.6 \mathrm{eV})\left(\frac{1}{1^2}-\frac{1}{2^2}\right)=\frac{3}{4}(13.6 \mathrm{eV})\)
For the transition from n = 6 to n = 2,
⇒ \(\Delta E_2=(13.6 \mathrm{eV})\left(\frac{1}{4}-\frac{1}{36}\right)=\frac{2}{9}(13.6 \mathrm{eV})\)
∵ ΔE1 > ΔE2,
∴ the transition from n = 2 to n = 1 will give photons of the maximum energy.
Question 10. The energy of the ground state of a hydrogen atom is -13.6 eV. The energy of the first excited state will be
- -6.8 eV
- -3.4 eV
- -27.2 eV
- -54.4eV
Answer: 2. -3.4 eV
Given that in the ground state,
⇒ \(E_1=-\frac{13.6}{1^2} \mathrm{eV}=13.6 \mathrm{eV}\)
In the first excited state (n = 2),
⇒ \(E_2=-\frac{13.6 \mathrm{eV}}{2^2}=-3.4 \mathrm{eV}\)
Question 11. Consider the third orbit of an He+ ion. The speed of the electron in this orbit will be (given that 1/4πε0 = 9 x 109 SI units, Z = 2, and h = 6.6 X 10-34J S)
- 2.92 x l06 m s-1
- 0.73 x 106 m s-1
- 1.45 x l06 m s-1
- 3.0 x l08 m s-1
Answer: 2. 0.73 x 106 m s-1
The electrostatic attraction provides the centripetal force. Hence,
⇒ \(\frac{m v^2}{r}=\frac{1}{4 \pi \varepsilon_0} \cdot \frac{(\mathrm{Z} e) e}{r^2}\)
⇒ \(m v^2 r=\frac{1}{4 \pi \varepsilon_0}\left(Z e^2\right)\) → (1)
From the quantization rule,
⇒ \(m v r=\frac{n h}{2 \pi}\) → (2)
Dividing (1) by (2)
⇒ \(v=\frac{1}{4 \pi \varepsilon_0} \cdot \frac{\left(Z e^2\right)(2 \pi)}{n h}\)
Substituting the velocity,
∴ \(v=\left(9 \times 10^9\right) \times \frac{2\left(1.6 \times 10^{-19}\right)^2 \times 2(3.14)}{3\left(6.67 \times 10^{-34}\right)} \mathrm{m} \mathrm{s}^{-1} \approx 1.45 \times 10^6 \mathrm{~m} \mathrm{~s}^{-1}\)
Question 12. A hydrogen atom in its ground state is excited by a monochromatic radiation of wavelength 975 Å. The number of spectral lines in the resulting spectrum emitted will be
- 2
- 6
- 10
- 3
Answer: 2. 6
The energy of the photon absorbed is
⇒ \(E=\frac{h c}{\lambda}=\frac{12420 \mathrm{eV} Å}{975 Å}=12.74 \mathrm{eV}\)
This energy gap corresponds to the transition from n = 4 to n = 1.
∴ the number of spectral lines in the spectrum is
∴ \(E=\frac{h c}{\lambda}=\frac{12420 \mathrm{eV} Å}{975 Å}=12.74 \mathrm{eV}\)
“atomic model of hydrogen “
Question 13. The ionization energy of the electron in a hydrogen atom in its ground state is 13.6 eV. The atoms are excited to high energy levels to emit radiations of six wavelengths. The maximum wavelength of the emitted radiations corresponds to the transition between the states
- n = 3 and n = 2
- n = 3 and n = l
- n = 2 and n = 1
- n = 4 and n = 3
Answer: 4. n = 4 and n = 3
As seen in the preceding question, six spectral lines exist in the spectrum for the transition from n = 4 to n = 3. The maximum wavelength corresponds to the minimum energy gap, which is from n = 4 to n = 3.
Question 14. When an electron jumps from the n = 4 orbit to the n = 2 orbit, we get
- The second line of the Lyman series
- The second line of the Balmer series
- The second line of the Paschen series
- An absorption line of the Balmer series
Answer: 2. The second line of the Balmer series
From the energy-level diagram, transition I corresponds to the first line of the Balmer series; transition 2 corresponds to the second line of the Balmer series; and transition 3 corresponds to the first line of the Paschen series.
Hence, the option (2) is true.
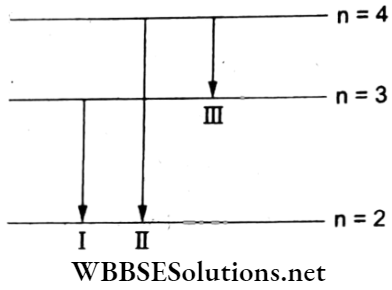
Question 15. The radius of a hydrogen atom in its ground state is 5.3 x 10-11 m. After a collision with an electron, it is found to have a radius of 21.2 x l0-11 m. What is the value of the principal quantum number (n) of the final state of the atom?
- 3
- 2
- 4
- 16
Answer: 2. 2
The radius of Bohr’s stationary orbit in the nth state (rn) varies as n2.
∴ \(\frac{r_n}{r_1}=\frac{n^2}{1} \Rightarrow \frac{21.2 \times 10^{-11} \mathrm{~m}}{5.3 \times 10^{-11} \mathrm{~m}}=n^2 \Rightarrow n^2=4\)
The principal quantum number in the final state is n = 2
Question 16. The energy levels A, B, and C of a certain atom correspond to increasing values of energy, i.e., EA < EB < EC. If λ1, λ2, and λ3 are the wavelengths of the radiations corresponding to the transitions C→ B, B → A, and C→ A respectively, which of the following relations is correct?
- λ1 + λ2 + λ3 = 0
- \(\lambda_3=\frac{\lambda_1 \lambda_2}{\lambda_1+\lambda_2}\)
- \(\lambda_3=\sqrt{\lambda_1^2+\lambda_2^2}\)
- λ3 = λ1+ λ2
Answer: 2. \(\lambda_3=\frac{\lambda_1 \lambda_2}{\lambda_1+\lambda_2}\)
From the energy-level diagram,
⇒ \(E_{\mathrm{C}}-E_{\mathrm{B}}=\frac{h c}{\lambda_1}\) → (1)
⇒ \(E_{\mathrm{B}}-E_{\mathrm{A}}=\frac{h c}{\lambda_2}\) → (2)
and \(E_{\mathrm{C}}-E_{\mathrm{A}}=\frac{h c}{\lambda_3}\) → (3)
Adding (1) and (2)
⇒ \(E_{\mathrm{C}}-E_{\mathrm{A}}=h c\left(\frac{1}{\lambda_1}+\frac{1}{\lambda_2}\right)\) (4)
Equating (3) and (4),
∴ \(\frac{h c}{\lambda_3}=h c\left(\frac{\lambda_1+\lambda_2}{\lambda_1 \lambda_2}\right) \Rightarrow \lambda_3=\frac{\lambda_1 \lambda_2}{\lambda_1+\lambda_2}\)
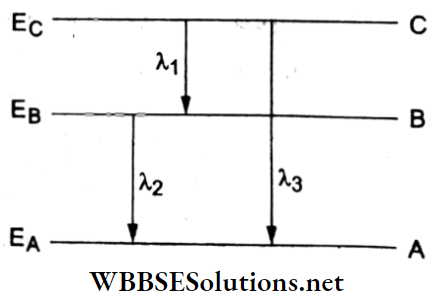
Question 17. If an electron in a hydrogen atom jumps from the third orbit to the second orbit, it emits a photon of wavelength λ. When it jumps from the fourth orbit to the third orbit, the corresponding wavelength of the emitted photon will be
- \(\frac{9 \lambda}{16}\)
- \(\frac{20 \lambda}{7}\)
- \(\frac{20 \lambda}{13}\)
- \(\frac{9 \lambda}{16}\)
Answer: 2. \(\frac{20 \lambda}{7}\)
For the transition from n = 3 to n = 2,
⇒ \(\Delta E=\frac{h c}{\lambda}=(13.6 \mathrm{eV})\left(\frac{1}{2^2}-\frac{1}{3^2}\right)=13.6\left(\frac{5}{36}\right) \mathrm{eV}\) → (1)
For the transition from n = 4 to n = 3,
⇒ \(\Delta E=\frac{h c}{\lambda^{\prime}}=(13.6 \mathrm{eV})\left(\frac{1}{3^2}-\frac{1}{4^2}\right)=13.6\left(\frac{7}{144}\right) \mathrm{eV}\) → (2)
Dividing (1) by (2),
∴ \(\frac{\lambda^{\prime}}{\lambda}=\frac{5}{36} \times \frac{144}{7}=\frac{20}{7} \Rightarrow \lambda^{\prime}=\frac{20 \lambda}{7}\).
Question 18. The ratio of the wavelengths of the last line of the Balmer series and the last line of the Lyman series is
- 1
- 4
- 2
- 0.5
Answer: 2. 4
The last line of any series corresponds to the transition from n = ∞ to any value of n.
Hence, for the last line of the Balmer series,
⇒ \(\Delta E=\frac{h c}{\lambda_1}=(13.6 \mathrm{eV})\left(\frac{1}{2^2}-\frac{1}{\infty}\right)=\frac{13.6}{4} \mathrm{eV}\)
For the last line of the Lyman series,
⇒ \(\Delta E=\frac{h c}{\lambda_2}=(13.6 \mathrm{eV})\left(\frac{1}{1^2}-\frac{1}{\infty}\right)=13.6 \mathrm{eV}\)
∴ \(\frac{\lambda_1}{\lambda_2}=\frac{13.6}{13.6 / 4}=4\)
Question 19. The ionization energy of a hydrogen atom is 13.6 eV. The ionization energy of an He+ ion would be
- 13.6 eV
- 27.2 eV
- 6.8 eV
- 54.4 eV
Answer: 4. 54.4 eV
The ionization energy of an H atom in its ground state is the energy required to knock out the electron from n = 1 to n = ∞.
Thus, for the H atom,
⇒ \(E_{\mathrm{H}}=(13.6 \mathrm{eV})\left(\frac{1}{1^2}-\frac{1}{\infty}\right)=13.6 \mathrm{eV}\).
For the He+ ion,
⇒ \(E_{\mathrm{He}^{+}}=(13.6 \mathrm{eV}) \mathrm{Z}^2\left(\frac{1}{1^2}-\frac{1}{\infty}\right)=(13.6 \times 4) \mathrm{eV}=54.4 \mathrm{eV}\)
Question 20. Consider an electron in the nth orbit of a hydrogen atom in the Bohr model. The circumference of the orbit can be expressed in terms of the de Broglie wavelength (λ) of that electron as
- 0.529nλ
- \(\sqrt{n \lambda}\)
- nλ
- 13.6λ
Answer: 3. nλ
According to the de Broglie relation,
wavelength = \(\lambda=\frac{h}{m v}\) → (1)
From Bohr’s quantization condition,
angular momentum = \(\frac{n h}{2 \pi}\)
\(m v r=\frac{n h}{2 \pi} \Rightarrow \frac{h}{m v}=\frac{2 \pi r}{n}\) → (2)
Equating (1) and (2),
⇒ \(\lambda=\frac{2 \pi r}{n}\)
∴ the circumference of the orbit is 2πr = nλ.
Question 21. When a hydrogen atom is raised from the ground state to an excited state,
- The PE decreases and the KE increases
- The PE increases and the KE decreases
- Both PE and KE decrease
- An absorption spectrum is obtained
Answer: 2. The PE increases and the KE decreases
The total energy of an H atom is negative, as a result of a positive value of the \(\mathrm{KE}\left(=\frac{1}{4 \pi \varepsilon_0} \cdot \frac{Z e^2}{2 r}\right)\) and a negative value of the \(\mathrm{PE}\left(=-\frac{1}{4 \pi \varepsilon_0} \cdot \frac{Z e^2}{r}\right)\).
When the atom is raised to the higher excited state, its orbital radius (r) increases, hence the KE decreases; and the PE becomes less negative, i.e., it increases.
Question 22. Some hydrogen atoms are excited from their ground states to the states having the principal quantum number 4. Then, the number of spectral lines observed will be
- 3
- 2
- 5
- 6
Answer: 4. 6
The total number of spectral lines observed in an H atom for all possible transitions from n = 4 is \(N=\frac{n(n-1)}{2}=\frac{4 \times 3}{2}=6\)
This is also shown in the adjoining energy-level diagram.
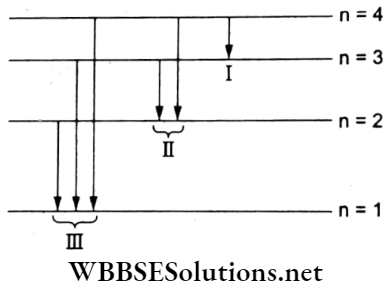
Question 23. In terms of the Bohr radius a0, the value of the second Bohr orbit of a hydrogen atom is
- 2a0
- 4a0
- √2a0
- 8a0
Answer: 2. 4a0
The radius of the nth orbit in an H atom is rn = n2a0, where a0 = Bohr radius (radius of the first orbit).
∴ for the second orbit, r2 = 22a0 = 4a0
“hydrogen bohr model “
| Class 11 Physics | Class 12 Maths | Class 11 Chemistry |
| NEET Foundation | Class 12 Physics | NEET Physics |
Question 24. The ionization potential of a hydrogen atom is 13.6 V. Some hydrogen atoms in their ground states are excited by a monochromatic radiation of photons of energy 12.1 eV. According to Bohr’s theory, the number of spectral lines emitted by the hydrogen atoms will be
- 2
- 4
- 3
- 1
Answer: 3. 3
The ionization potential in the ground state is 13.6 V. So, the ionization energy is 13.6 eV, and the energy in the ground state is E1 = -13.6 eV. Let the absorption of the 12.1 eV energy by the atom raise the electron to the nth state. Thus,
E1 + ΔE = En
⇒ \(-13.6 \mathrm{eV}+12.1 \mathrm{eV}=-\frac{13.6 \mathrm{eV}}{n^2} \Rightarrow-1.5=-\frac{13.6}{n^2} \Rightarrow n=3\)
Hence, the number of spectral lines is
∴ \(N=\frac{n(n-1)}{2}=\frac{3 \times 2}{2}=3\)
Question 25. An electron of a stationary hydrogen atom passes from the fifth energy level to the ground level. The velocity that the atom acquires as a result of the emission of photons will be (given that m = mass of an electron, R∞ = Rydberg constant arid h = Planck constant)
- \(\frac{24 R_{\infty} h}{25 m}\)
- \(\frac{24 m}{25 R_{\infty} h}\)
- \(\frac{25 m}{24 R_{\infty} h}\)
- \(\frac{25 R_{\infty} h}{24 m}\)
Answer: 1. \(\frac{24 R_{\infty} h}{25 m}\)
The energy of an H atom in its nth state in terms of the Rydberg constant (R∞) is given by
⇒ \(E_n=-\frac{R_{\infty} h c}{n^2}\)
The energy of the photon emitted during the transition 5 → 1 will be
⇒ \(\Delta E=E_5-E_1=R_{\infty} h c\left(\frac{1}{1}-\frac{1}{5^2}\right)=\frac{24}{25} R_{\infty} h c\)
⇒ \(\frac{h c}{\lambda}=\frac{24}{25} R_{\infty} h c \Rightarrow \frac{1}{\lambda}=\frac{24}{25} R_{\infty}\)
∴ \(\frac{p}{h}=\frac{m v}{h}=\frac{24 R_{\infty}}{25} \Rightarrow v=\frac{24 R_{\infty} h}{25 m}\)
Question 26. The maximum wavelength λ which can ionize a hydrogen atom in its ground state is
- 150.2 nm
- 100.5 nm
- 91.3 nm
- 110 nm
Answer: 3. 91.3 nm
The ionization of an H atom from its ground state results in a transition from n =1 to n = ∞. The required energy is
⇒ \(\Delta E=\frac{h c}{\lambda_0}=(13.6 \mathrm{eV})\left(\frac{1}{1^2}-\frac{1}{\infty}\right)=13.6 \mathrm{eV}\)
∴ \(\lambda_0=\frac{h c}{13.6 \mathrm{eV}}=\frac{1242 \mathrm{eV} \mathrm{nm}}{13.6 \mathrm{eV}}=91.3 \mathrm{~nm}\)
Question 27. Which of the following parameters is the same for an H+ ion, an He+ ion, and a Li2+ ion in their ground states?
- Energy
- Speed of an electron
- The orbital angular momentum of an electron
- The radius of the orbit
Answer: 3. Orbital angular momentum of an electron
According to Bohr’s quantum condition, the angular momentum of an orbital electron in the nth orbit is quantized as nh/2π. Hence, the orbital angular momentum is the same for all ions in the same state.
Question 28. What can be the maximum photoelectric work function of a metal from which photoelectrons can be ejected by the light of the Balmer series of hydrogen?
- 13.6 eV
- 3.4 eV
- 1.5 eV
- None of these
Answer: 2. 3.4 eV
In the Balmer series, the maximum energy of a photon corresponds to the transition from n = ∞ to n = 2.
∴ \(\Delta E=\frac{h c}{\lambda}=(13.6 \mathrm{eV})\left(\frac{1}{2^2}-\frac{1}{\infty}\right)=\frac{13.6 \mathrm{eV}}{4}=3.4 \mathrm{eV}\)
This maximum value is equal to the maximum work function.
“hydrogen bohr model “
Question 29. Consider the radiations from an H-atom, an He+ ion, and a Li2+ ion. The ratio of the smallest wavelengths radiated from them will be
- 1 : 4: 9
- 9: 4: 1
- 36: 9: 4
- 4: 9: 36
Answer: 3. 36: 9: 4
The smallest wavelength corresponds to the maximum energy of radiations, which corresponds to the transition from n = ∞ to n = 1. Thus,
⇒ \(\Delta E=h v=\frac{h c}{\lambda}=13,6\left(\frac{1}{1^2}-\frac{1}{\infty}\right) Z^2 \mathrm{eV}\)
For H – atom, \(\lambda_{\mathrm{H}}=\frac{h c}{13.6 \mathrm{eV}}\)
For He+, \(\lambda_{\mathrm{He}^{+}}=\frac{h c}{4(13.6 \mathrm{eV})}\)
For Li2+, \(\lambda_{\mathrm{L}^{2+}}=\frac{h c}{9(13.6 \mathrm{eV})}\)
∴ H+ : He+: Li2+ = 36: 9: 4.
Question 30. A hydrogen atom emits ultraviolet radiation of wavelength 102.5 ran. The values of the principal quantum number for the stationary states involved in this transition are
- n = 2 and n = 1
- n = 3 and n = 1
- n = 3 and n = 2
- n = 4 and n = 2
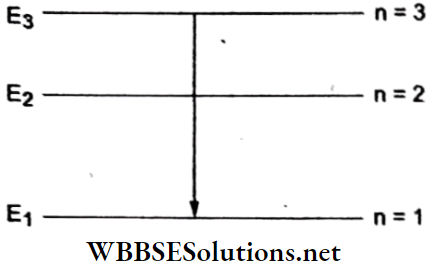
Answer: 2. n = 3 and n = 1
The possible transitions are shown in the adjoining energy-level diagram in which E1 = -13.6 eV, E2 = -3.4 eV, and E3 = -1.5 eV.
The energy of the photon emitted is
⇒ \(\Delta E=\frac{h c}{\lambda}=\frac{1242 \mathrm{eV} \mathrm{nm}}{102.5 \mathrm{~nm}}=121 \mathrm{eV}\)
This energy corresponds to the transition 3 → 1.
Question 31. A photon of wavelength 50 nm is absorbed by an H atom in its ground state and is ionized. The kinetic energy associated with the ejected electron is
- 13.6 eV
- 10.6 eV
- 11.24 eV
- 9.12 eV
Answer: 3. 11.24 eV
Given that λ = 50 nm. Hence, the energy of the photon is
⇒ \(E=\frac{h c}{\lambda}=\frac{1242 \mathrm{eV} \mathrm{nm}}{50 \mathrm{~nm}}=24.84 \mathrm{eV}\)
The energy in the ground state of hydrogen is E1 = -13.6 eV.
∴ ionization energy = (energy spent in the extraction of an electron)
= 13.6 eV.
The energy remaining in the electron is
KE = 24.84 eV – 13.6 eV = 11.24 eV.
Question 32. Find the magnetic field at the center of a hydrogen atom in its ground state. (Given that Bohr radius = a0 = 5 x 10-11 m).
- 20 T
- 14 T
- 15.7 T
- 10.75 T
Answer: 2. 14 T
An electron revolving around the nucleus constitutes an electric current given by
⇒ \(I=\frac{e}{T}=\frac{e v}{2 \pi r}\)
the magnetic field at the center is
⇒ \(B=\frac{\mu_0 I}{2 r}=\frac{\mu_0 e v}{4 \pi r^2}\) →(1)
From Bohr’s quantum condition,
⇒ \(m v r_n=\frac{n h}{2 \pi}\)
For the ground state (n = 1),
⇒ \(m v r=\frac{h}{2 \pi} \Rightarrow v=\frac{h}{2 \pi m r}\)
Substituting v in (1),
⇒ \(B=\frac{\mu_0 e}{4 \pi r^2} \cdot \frac{h}{2 \pi m r}=\frac{\mu_0 e h}{4 \pi\left(2 \pi m r^3\right)}\)
Substituting the standard values,
∴ \(B \approx \frac{10^{-7}\left(1.6 \times 10^{-19}\right)\left(6.62 \times 10^{-34}\right)}{2(3.14)\left(9.1 \times 10^{-31}\right)\left(5 \times 10^{-11}\right)^3} \mathrm{~T} \approx 14 \mathrm{~T}\).
Question 33. How many spectral lines exist in the visible region Of the Balmer series of hydrogen atoms?
- 5
- 4
- 2
- 3
Answer: 2. 4
Transitions in the Balmer series are given by
⇒ \(\Delta E=\frac{h c}{\lambda}=13.6\left(\frac{1}{2^2}-\frac{1}{n^2}\right) \mathrm{eV}, \text { where } n=3,4,5, \ldots\)
For n = 3, \(\lambda=\frac{h c(4 \times 9)}{13.6 \times 5}=\frac{1242 \mathrm{eV} \mathrm{nm} \times 36}{13.6 \mathrm{eV} \times 5}=657.5 \mathrm{~nm}\)
For n = 4, \(\lambda=\frac{1242 \mathrm{eV} \mathrm{nm} \times 64}{13.6 \mathrm{eV} \times 12}=487 \mathrm{~nm}\)
For n = 5, \(\lambda=\frac{1242 \times 100}{13.6 \times 21} \mathrm{~nm}=434.8 \mathrm{~nm}\)
For n = 6, \(\lambda=\frac{1242 \times 36 \times 4}{13.6 \times 32} \mathrm{~nm}=411 \mathrm{~nm}\)
The line for n = 7 lies in the ultraviolet region. So, only four lines exist in the visible region
Question 34. The energy of the orbital electron in the first excited state of an He+ ion is
- -10.6 eV
- -25.6 eV
- 15.6 eV
- -13.6 eV
Answer: 4. -13.6 eV
For the first excited state, n = 2. So, the energy of an electron is
∴ \(E_2=-\frac{13.6 \mathrm{Z}^2}{n^2} \mathrm{eV}=-\frac{13.6\left(2^2\right)}{2^2} \mathrm{eV}=-13.6 \mathrm{eV}\)
Question 35. If the energy of the electron in the ground state of an H atom is -13.6 eV, find the orbital speed of the electron in the third excited state.
- 5.45 x l03 m s-1
- 5.45 x l04 m s-1
- 5.45 x l06 m s-1
- 5.46 x 105 m s-1
Answer: 4. 5.46 x 105 m s-1
In the hydrogen atom,|PE| = 2KE and total energy = KE + (-2KE) = -KE.
In the ground state,
⇒ \(-13.6 \mathrm{eV}=\frac{1}{2} m v^2\)
For the third excited state, n = 4. So,
⇒ \(\left|E_4\right|=+\frac{13.6}{4^2} \mathrm{eV}=\frac{1}{2} m v^2\)
∴ \(v=\sqrt{\frac{2\left|E_4\right|}{m}}=\sqrt{\frac{2\left(13.6 \times 1.6 \times 10^{-19} \mathrm{~J}\right)}{16\left(9.1 \times 10^{-31} \mathrm{~kg}\right)}}=5.46 \times 10^5 \mathrm{~m} \mathrm{~s}^{-1}\)
“hydrogen bohr model “
Question 36. If an energy of 15 eV is given to a hydrogen atom with the electron in the fourth orbit, the final KE of the electron when it leaves the atom will be
- 13.6 eV
- 12.08 eV
- 14.15 eV
- 15.85 eV
Answer: 3. 14.15 eV
The energy of the electron in the fourth orbit is
⇒ \(E_4=-\frac{13.6}{4^2} \mathrm{eV}=-0.85 \mathrm{eV}\)
For ionization (extraction of the electron from the atom), an energy of 0.85 eV is now required. Adding 15 eV to the atom will eject the electron with
KE = 15 eV- 0.85 eV = 14.15 eV
Question 37. The total energy of an electron in an atom in an orbit is -3.4 eV. Its kinetic and potential energies are respectively
- -3.4 eV and -3.4 eV
- -3.4 eV and -6.8 eV
- 3.4 eV and -6.8eV
- 3.4 eV and 3.4 eV
Answer: 3. 3.4 eV and -6.8eV
According to Bohr’s theory, the total energy and the potential energy of an atom are negative.
But KE of an electron = |total energy| = \(\mid \text { total energy }\left|=\frac{1}{2}\right| \mathrm{PE} \mid\)
Given that Etot = -3.4 eV.
∴ KE =|Etot| = 3.4eV
and PE = 2(-KE) = -2(3.4 eV) = -6.8 eV.
Question 38. Taking the wavelength of the first Balmer line in the hydrogen spectrum (from n = 3 to n = 2) as 660 runs, the wavelength of the second Balmer line (from n = 4 to n = 2) will be
- 642.7 nm
- 889.2 nm
- 488.9 nm
- 388.9 run
Answer: 3. 488.9 nm
For the first Balmer line,
⇒ \(\Delta E=\frac{h c}{\lambda_1}=13.6\left(\frac{1}{2^2}-\frac{1}{3^2}\right) \mathrm{eV}=13.6 \times \frac{5}{4 \times 9} \mathrm{eV}\) → (1)
For the second Balmer line,
⇒ \(\frac{h c}{\lambda_2}=13.6\left(\frac{1}{2^2}-\frac{1}{4^2}\right) \mathrm{eV}=13.6 \times \frac{12}{4 \times 16} \mathrm{eV}\) → (2)
Dividing (1) by (2), we have
⇒ \(\frac{\lambda_2}{\lambda_1}=\frac{5}{36} \times \frac{64}{12}=\frac{20}{27}\)
∴ \(\lambda_2=\frac{20}{27} \lambda_1=\frac{20}{27}(660 \mathrm{~nm})=488.88 \mathrm{~nm} \approx 488.9 \mathrm{~nm}\)
Question 39. A hydrogen atom initially in the ground state is excited by absorbing a photon of wavelength 980 Å. The radius of the atom in the excited state in terms of its Bohr radius a0 will be (assuming that he = 12500 eV Å)
- 4a0
- 16a0
- 9a0
- 25a0
Answer: 2. 16a0
The energy of the photon of wavelength λ = 980 A is
⇒ \(h c=\frac{12500 \mathrm{eV} Å}{980 Å}=12.76 \mathrm{eV}\)
This energy gap corresponds to the transition from n =1 (E1 = -13.6 eV) to n = 4 (E4 = -0.85 eV). Hence, the radius of the orbit of the electron in the state n = 4 is R4 = a0(42) =16a0.
Question 40. A singly ionized helium ion (He+) is in its first excited state. Its ionization energy is
- 54.40 eV
- 13.60 eV
- 48.36 eV
- 6.04 eV
Answer: 2. 13.60 eV
The ionization energy is the energy required to extract an electron from the atom. It is the positive value of the energy of the electron in the given quantum state.
For a singly ionized helium atom in its first excited state (n = 2), its energy is
⇒ \(E_2=-\frac{13.6 Z^2}{2^2} \mathrm{eV}=-\frac{13.6 \times 4}{4} \mathrm{eV}=-13.6 \mathrm{eV}\)
∴ ionization energy = \(-E_2=-(-13.6 \mathrm{eV})=13.6 \mathrm{eV}\).
Question 41. Consider an electron in a hydrogen atom revolving in its second excited state (having a radius of 4.65 Å). The de Broglie wavelength of this electron is
- 6.6 Å
- 9.7 Å
- 3.5 Å
- 12.9 Å
Answer: 2. 9.7 Å
According to Planck’s quantum condition,
⇒ \(m v r=\frac{n h}{2 \pi} \Rightarrow p=\frac{n h}{2 \pi r}\)
∴ the de Broglie wavelength is
⇒ \(\lambda=\frac{h}{p}=\frac{2 \pi r}{n}=\frac{2(3.14)(4.65 Å)}{3}=9.7 Å\)
Question 42. For n > > 1, the frequency of a photon emitted from a hydrogen atom 1 during the transition from the (n + 1)th state to the wth state is directly proportional to
- n
- \(\frac{1}{n}\)
- \(\frac{1}{n^2}\)
- \(\frac{1}{n^3}\)
Answer: 4. \(\frac{1}{n^3}\)
The energy in the nth state of an H atom is
⇒ \(E_n=-\frac{13.6}{n^2} \mathrm{eV}\)
Hence, during the transition (n +1)-» n, the energy difference is
⇒ \(\Delta E=h v=13.6\left[\frac{1}{n^2}-\frac{1}{(n+1)^2}\right] \mathrm{eV}\)
∴ \(\mathrm{v} \propto \frac{1}{n^2}-\frac{1}{n^2}\left(1+\frac{1}{n}\right)^{-2}\)
⇒ \(\mathrm{v} \propto \frac{1}{n^2}-\frac{1}{n^2}\left(1-\frac{2}{n}\right)\)
⇒ \(v \propto \frac{1}{n^3}\).
“hydrogen bohr model “
Question 43. If the difference λmax – λmin for the Lyman series is 340 Å, the corresponding difference for the Paschen series is
- 12502 Å
- 13802 Å
- 11802 Å
- 10000 Å
Answer: 3. 11802 Å
For the Lyman series,
⇒ \(\frac{1}{\lambda_{\min }}=R_{\infty}\left(\frac{1}{1^2}-\frac{1}{\infty}\right)=R_{\infty}\)
and \(\frac{1}{\lambda_{\max }}=R_{\infty}\left(\frac{1}{1^2}-\frac{1}{2^2}\right)=\frac{3}{4} R_{\infty}\)
∴ \(\lambda_{\max }-\lambda_{\min }=\frac{4}{3 R_{\infty}}-\frac{1}{R_{\infty}}=\frac{1}{3 R_{\infty}}\)
⇒ \(\frac{1}{3 R_{\infty}}=340 Å \Rightarrow \frac{1}{R_{\infty}}=3 \times 340 Å\)
For the Paschen series,
⇒ \(\frac{1}{\lambda_{\min }}=R_{\infty}\left(\frac{1}{3^2}-\frac{1}{\infty}\right)=\frac{R_{\infty}}{9}\)
and \(\frac{1}{\lambda_{\max }}=R_{\infty}\left(\frac{1}{3^2}-\frac{1}{4^2}\right)=\frac{7 R_{\infty}}{9 \times 16}\)
∴ \(\lambda_{\max }-\lambda_{\min }=\frac{9}{R_{\infty}}\left(\frac{16}{7}-1\right)=\frac{81}{7 R_{\infty}}=\frac{81}{7}(340 \times 3 Å)=11802 Å\).
Question 44. If λ is the maximum wavelength in the Lyman series in the spectrum of a hydrogen atom, the minimum wavelength in the Balmer series of the spectrum of an He+ ion is
- \(\frac{\lambda}{4}\)
- \(\frac{3 \lambda}{4}\)
- \(\frac{2 \lambda}{3}\)
- \(\frac{\lambda}{3}\)
Answer: 2. \(\frac{3 \lambda}{4}\)
For the He+ ion, the minimum wavelength corresponds to the maximum energy difference. Hence, for the Balmer series,
⇒ \(\frac{1}{\lambda_{\mathrm{He}^{+}}}=R_{\infty} \mathrm{Z}^2\left(\frac{1}{2^2}-\frac{1}{\infty}\right)=\frac{R_{\infty}}{4} \cdot 4=R_{\infty}\)
For the Hatom, the maximum wavelength corresponds to the minimum energy difference. Hence, for the Lyman series,
⇒ \(\frac{1}{\lambda_{\mathrm{H}}}=R_{\infty}\left(\frac{1}{1^2}-\frac{1}{2^2}\right)=\frac{3}{4} R_{\infty}=\frac{1}{\lambda} \text { (given). }\)
∴ \(\lambda_{\mathrm{He}^{+}}=\frac{1}{R_{\infty}}=\frac{3 \lambda}{4}\).
Question 45. A particle of mass 200 MeV/c2 collides with a hydrogen atom at rest. Soon after the collision, the colliding particle comes to rest, and the atom recoils and goes to its first excited state. The initial kinetic energy of the particle is (N/4) eV, where N is (given that mass of a hydrogen atom =1 GeV/c2)
- 24
- 48
- 51
- 73
Answer: 3. 51
The mass of the colliding particle is
⇒ \(m=\frac{200 \mathrm{MeV}}{c^2}=200 \times 10^6 \mathrm{u}\)
The mass of the hydrogen atom is
⇒ \(m_{\mathrm{H}}=\frac{1 \mathrm{GeV}}{c^2}=10^9 \mathrm{u}\)
∴ \(\frac{m_{\mathrm{H}}}{m}=\frac{1 \times 10^9 \mathrm{u}}{200 \times 10^6 \mathrm{u}}=\frac{1000}{200}=5\)
⇒ mH = 5m.
Conserving the momentum,
⇒\(m v=m_{\mathrm{H}} v_{\mathrm{H}} \Rightarrow v_{\mathrm{H}}=\left(\frac{m}{m_{\mathrm{H}}}\right) v=\frac{v}{5}\)
∴ loss of energy = \(\frac{1}{2} m v^2-\frac{1}{2} m_{\mathrm{H}} v_{\mathrm{H}}^2\)
⇒ \(\frac{1}{2} m v^2-\frac{1}{2}(5 m)\left(\frac{v}{5}\right)^2=\frac{1}{2} m v^2\left(\frac{4}{5}\right)\)
This energy loss is spent in exciting the H atom in its first excited state, which is
\(\Delta E=E_2-E_1=13.6\left(1-\frac{1}{4}\right) \mathrm{eV}=13.6 \times \frac{3}{4} \mathrm{eV}=10.2 \mathrm{eV}\)∴ \(\left(\frac{1}{2} m v^2\right)\left(\frac{4}{5}\right)=10.2 \mathrm{eV}\)
∴ initial KE = \(=\frac{1}{2} m v^2=(10.2 \mathrm{eV}) \frac{5}{4}=\frac{51}{4} \mathrm{eV}=\frac{N}{4} \mathrm{eV}\).
Hence, N = 51.
