Thermodynamics
- Elasticity Questions and Answers
- Hydrostatics Question And Answers
- Change Of State Of Matter Long Answer Questions
- Circular Motion Long Answer Questions
- Doppler Effect In Sound and Light Question and Answers
- Expansion Of Solid And Liquids Long Answer Questions
- For Calorimetry Questions and Answers
- Friction Long Answer Type Questions
- Kinetic Theory Of Gases Question and Answers
- Measurement And Dimension Of Physical Quantity Long Answer Questions
- Newton Law Of Motion Long Answer Questions
- Newtonian Gravitation And Planetary Motion Long Answer Questions
- One-Dimensional Motion Long Answer Questions
- Simple Harmonic Motion Long Answer Type Questions
- Statics Long Answer Questions
- Superposition Of Waves Question and Answers
- Thermometry Question and Answers
- Transmission Of Heat Long Answer Questions
- Vector Question and Answers
- Viscosity And Surface Tension Long Answer Questions
- Wave Motion Question and Answers
First And Second Law Of Thermodynamics Introduction
Position, displacement, velocity, acceleration, etc., are external properties of a body. From them, we get no information about the nature of the body. They are discussed in the subject of mechanics.
- On the other hand, properties like mass (M), volume (V), pressure (p), temperature (T), density (ρ), etc., are internal properties of a body. These are bulk (macroscopic) properties and can be measured by simple experiments.
- Any change in these properties generally involves the transfer of energy in the form of heat or work. All of these are studied in a branch of Physics, known as thermodynamics. The above-mentioned properties and similar others are called thermodynamic properties.
- The subject is based only on experi¬mental data and every thermodynamic formula comes from the analysis of this data. For example, suppose a fixed mass of an ideal gas is studied at constant temperature.
- Any change of pressure produces a change in volume and different sets of p and V are obtained. An analysis of these measured values shows that the product pV = constant. This is the well-known Boyle’s law. As it is experiment-based, it is a thermodynamic law.

Thermodynamic Systems: A body or, a part of a body, or a combination of a number of bodies that is being studied is usually called a thermodynamic system. In general, such a system interacts with its surroundings or, environment and exchanges energy and mass.
The environment or surroundings include all external objects that have some influence on the system. For example, if a room in a house is considered as a system and its thermal properties are to be studied, the sun at a distance of 1.5 x 108 km is an important component of the environment, but another house only 100 m away need not be included as a component. Thermodynamic systems are of three types.
Isolated system: This system does not exchange any energy or mass with the surroundings.
Closed system: Energy is exchanged with the surroundings, but the mass remains fixed.
Open system: Both energy and mass are exchanged. We shall not discuss open systems in this chapter.
Properties like a number of molecules, molecular velocity, etc., are internal microscopic properties of a body. They are not directly measurable and experimental data is absent. Hence, the study of this subject is based on theoretical assumptions and is known as kinetic theory.
Thermodynamics – First And Second Law Of Thermodynamics Nature Of Heat
Heat is a form of energy which can be identified only when it is in transit. It can be converted into other forms of energy and vice versa. To measure the amount of heat energy, different units are used:
CGS System: calorie: (cal)
SI: Joule (J)
- In SI, the unit of energy is directly used to measure some quantity of heat. So, Joule (J) is the unit of heat in SI, as it is the SI unit of energy. Similarly, other energy units like erg may also be used.
- Immediately it becomes clear that the energy units must have definite relations with the conventional units of heat. This topic is discussed in the next section.
- In thermodynamics, the definition of temperature comes earlier from the zeroth law of thermodynamics. The definition of heat is based on the concept of temperature.
Nature of heat Definition: Heat is a form of energy which is transmitted from one place to another due to their temperature differences only.
It is interesting to note the difference between this and the calorimetric definition of heat.
Thermodynamics – First And Second Law Of Thermodynamics Mechanical Equivalent Of Heat Joules Law
The widely used unit of heat is calorie (or cal, in CGS system). We know that heat is a form of energy; so energy units like erg (CGS system) and joule (J in SI) should somehow be related to the units of heat.
- James Prescott Joule was the first experimentalist who accurately measured the mechanical energy equivalent to some amount of heat energy i.e., the mechanical equivalent of heat.
- This measurement determines the amount of mechanical energy expressed in joule (or erg) which is equivalent to heat energy of 1 cal.
- Mechanical energy may be used to do work and work produces heat. On the other hand, heat may be converted to work or mechanical energy. This indicates that there is a natural relationship between work and heat. This relation is known as Joule’s law.
Joule’s law: If some amount of work is entirely converted to heat, then the work done and the heat produced are proportional to each other.
If W=workdoneand H=heat produced,then from Joule’slaw,
W ∝ H or, W = JH…..(1)
Here, J is a constant. This constant is called the mechanical equivalent of heat or Joule’s equivalent.
Definition of J and Hs magnitudes in different systems: In equation (1), if H = 1, then W = J. So mechanical equivalent of heat is defined as the amount of work done to produce a unit amount of heat.
Clearly, the unit and the magnitude of J depends on the unit of heat and work in different systems of units.
CGS system: The unit of H is calorie and the unit of W is erg.
As J = \({W}{H}\) the unit of J is erg · cal-1 and the measured value of J = 4.2 x 107 erg · cal-1.
As 1 J = 107 erg, the value of J = 4.2 J · cal-1.
The units like erg · cal-1 and J · cal-1 indicate that the mechanical equivalent of heat J actually denotes the conversion factor between two units. For example, J = 4.2 J · cal-1.
This means that 1 cal = 4.2 J, i.e., each calorie should be multiplied by 4.2 to express the energy in joule.
In SI: Here W and H are expressed in the same unit, which is joule (J). So, no conversion factor is necessary. The mechanical equivalent of heat is J = 1. This means that the concept of J is unnecessary in SI.
Relation of calories with erg and joule: 1 cal = 4.2 x 107 erg = 4.2 J.
Thermodynamics – First And Second Law Of Thermodynamics Mechanical Equivalent Of Heat Numerical Examples
Second Law of Thermodynamics Explained
Example 1. 800 g lead balls are kept in a 1 m long, vertical tube which is a bad conductor and the tube is then closed at both ends. The tube is suddenly inverted so that the balls fall from one end to the other. The I temperature of the lead balls increases by 3.89°C after 50 such inversions. Find out the mechanical equivalent of heat assuming that the lead balls have retained the entire amount of heat produced.
Solution:
Given
800 g lead balls are kept in a 1 m long, vertical tube which is a bad conductor and the tube is then closed at both ends. The tube is suddenly inverted so that the balls fall from one end to the other. The I temperature of the lead balls increases by 3.89°C after 50 such inversions.
Work done, W= loss of potential energy of the balls = n x mgh
= 50 x 800 x 980 x 100 erg (1 m = 100 cm)
Heat produced, H = mst = 800 x 0.03 x 3.89 cal
∴ J = \(\frac{W}{H}=\frac{50 \times 800 \times 980 \times 100}{800 \times 0.03 \times 3.89}=4.2 \times 10^7 \mathrm{erg} \cdot \mathrm{cal}^{-1}\).
Example 2. Water drops from a height of 50 m in a waterfall. Find out the rise in temperature of water, if 75% of its energy is converted into heat and absorbed by water. (\(J=4.2 \times 10^7 \mathrm{erg} \cdot \mathrm{cal}^{-1} ; \mathrm{g}=9.8 \mathrm{~m} \cdot \mathrm{s}^{-2}\))
Solution:
Given
Water drops from a height of 50 m in a waterfall.
Here, W = mgh x 75/100 and H = mst
Now, W = JH
or, \(m g h \times \frac{75}{100}=J m s t\)
or, \(t=\frac{m g h \times \frac{75}{100}}{J m s}\)
[s = \(J \mathrm{cal} \cdot \mathrm{g}^{-1} \cdot{ }^{\circ} \mathrm{C}^{-1}\) for water, g = \(9.8 \mathrm{~m} \cdot \mathrm{s}^{-2}\)=980 \(\mathrm{~cm} \cdot \mathrm{s}^{-2}, h=50 \mathrm{~m}=50 \times 100 \mathrm{~cm}\))
= \(\frac{g h}{J s} \times \frac{75}{100}=\frac{980 \times 50 \times 100}{4.2 \times 10^7 \times 1} \times \frac{75}{100}=0.0875^{\circ} \mathrm{C}\)
Real-Life Applications of the Second Law of Thermodynamics
Example 3. The velocity of a 42 kg celestial body reduces from 20 km · min-1 to 5 km · min-1 due to its passage through the earth’s atmosphere. Find out the heat produced in calories. (J = 4.2 x 107 erg · cal-1)
Solution:
Given
The velocity of a 42 kg celestial body reduces from 20 km · min-1 to 5 km · min-1 due to its passage through the earth’s atmosphere.
The initial velocity of the celestial body,
u = \(20 \mathrm{~km} \cdot \mathrm{min}^{-1}=\frac{20 \times 1000}{60} \mathrm{~m} \cdot \mathrm{s}^{-1}\)
Final velocity of the celestial body,
v = \(5 \mathrm{~km} \cdot \min ^{-1}=\frac{5 \times 1000}{60} \mathrm{~m} \cdot \mathrm{s}^{-1}\)
Work done, W = change in kinetic energy
= \(\frac{1}{2} m u^2-\frac{1}{2} m v^2=\frac{1}{2} m\left(u^2-v^2\right)\)
= \(\frac{1}{2} \times 42 \times\left(\frac{1000}{60}\right)^2\left(20^2-5^2\right)\)
= \(\frac{42 \times 10^4 \times 375}{2 \times 36} \text { joule }\)
∴ Heat produced,
H = \(\frac{W}{J}=\frac{42 \times 10^4 \times 375}{2 \times 36 \times 4.2} \)
(J = \(4.2 \times 10^7 \mathrm{erg} \cdot \mathrm{cal}^{-1}=4.2 \mathrm{~J} \cdot \mathrm{cal}^{-1}\))
= \(5.2 \times 10^5 \mathrm{cal}\).
Example 4. Find out the amount of work done to convert 100 g ice at 0°C to water at 100°C. (Latent heat of fusion of ice = 80 cal · g-1; the mechanical equivalent of heat = 4.2 J · cal-1).
Solution:
Total heat supplied,
H = 100 x 80 + 100 x 1 x (100-0)
= 8000 + 10000 = 18000 cal
∴ Work done, W = JH = 4.2 x 18000 = 75600 J.
Example 5. What will be the temperature difference between the top and the bottom of a 400 m high waterfall, assuming that 80% of the heat produced is retained by the water?
Solution:
Second Law of Thermodynamics and Entropy
Work done, W = loss in potential energy
= mg(h- 0) = mgh
∴ Heat produced, H = \(\frac{W}{J}=\frac{m g h}{J}\)
∴ Amount of heat retained by water = \(\frac{m g h}{J} \times \frac{80}{100}\)
If the increase in temperature of water is t, required heat = mst
According to the question,
mst = \(\frac{m g h}{J} \times \frac{80}{100}\)
or, t = \(\frac{g h}{J s} \times \frac{80}{100}=\frac{9.8 \times 400}{4.2 \times 1000} \times \frac{80}{100}\)
(specific heat of water in SI s = \(1000 \mathrm{cal} \cdot \mathrm{kg}^{-1} \cdot{ }^{\circ} \mathrm{C}^{-1}\))
or, t = 0.747 °C
Example 6. Find out the minimum height from which a piece of ice at 0°C should be dropped so that it melts completely due to its impact with the ground. Assume that half of the energy loss due to the fall is responsible for the fusion of ice. (Latent heat of fusion of ice = 80 cal · g-1, g = 980 cm · s-2, J = 4.2 J · cal-1)
Solution:
Let the piece of ice of mass m be allowed to fall from height h.
Now, energy loss due to the fall = loss in potential energy = mgh = work done, W.
Half of it, i.e., W/2 amount of energy is converted into heat and it is responsible for the fusion of ice.
∴ Heat produced, \(H=\frac{W / 2}{J}=\frac{m g h}{2 J}\)
As mg of ice melts into water, required latent heat = 80 m cal
So, \(\frac{m g h}{2 J}=80 \mathrm{~m}\)
or, \(h=\frac{80 \times 2 J}{g}=\frac{80 \times 2 \times 4.2 \times 10^7}{980}\)
(J = \(4.2 \mathrm{~J} \cdot \mathrm{cal}^{-1}=4.2 \times 10^7 \mathrm{erg} \cdot \mathrm{cal}^{-1}\))
= \(6.857 \times 10^6 \mathrm{~cm}=68.57 \mathrm{~km}\)
| Class 11 Physics | Class 12 Maths | Class 11 Chemistry |
| NEET Foundation | Class 12 Physics | NEET Physics |
Example 7. A piece of ice at 0°C is dropped to the ground from some height. The piece of ice melts completely due to its impact on the ground. Find the height from which the piece was dropped considering that 60% of its energy is converted into heat. (J = 4.2 J · cal-1)
Solution:
Given
A piece of ice at 0°C is dropped to the ground from some height. The piece of ice melts completely due to its impact on the ground.
The potential energy of the piece of ice of mass m at height h = mgh, kinetic energy = 0
So, total mechanical energy = mgh
This energy is conserved till it touches the ground. Due to the impact with the ground, 60% of this energy,
i.e., mgh x 60/100 or 0.6 mgh is converted into heat energy.
∴ Heat produced = \(\frac{0.6 \mathrm{mgh}}{J}\)
Again, heat required to melt m g of ice = mL
[L = latent heat of fusion of ice]
∴ \(\frac{0.6 m g h}{J}=m L\)
or, h = \(\frac{J L}{0.6 g}\left[L=80 \mathrm{cal} \cdot \mathrm{g}^{-1}=80 \times 1000 \mathrm{cal} \cdot \mathrm{kg}^{-1}\right]\)
= \(\frac{4.2 \times(80 \times 1000)}{0.6 \times 9.8}=5.71 \times 10^4 \mathrm{~m}=57.1 \mathrm{~km}\)
Example 8. A stirrer rotates in 1 l of water against a damping force of 0.1 N at 360 rpm in a circle of radius 5 cm. Calculate the rise in water temperature in 1 h, neglecting heat loss due to radiation. (J = 4.2 J · cal-1)
Solution:
Given
A stirrer rotates in 1 l of water against a damping force of 0.1 N at 360 rpm in a circle of radius 5 cm.
Mass of 1 l of water, m = 1 kg.
If t is the rise in temperature of water in 1 h, then heat produced, H = mst.
Now, circumference of the circle =2πr; number of rotations in 1 h = 360 x 60
∴ Displacement of the stirrer, d = 360 x 60 x 2πr
Work done, W = force x displacement = Fd
∴ W = JH or, Fd = Jmst
or, t = \(\frac{F d}{J m s}=\frac{0.1 \times(360 \times 60 \times 2 \times \pi \times 0.05)}{4.2 \times 1 \times 1000}\)
(F = \(0.1 \mathrm{~N} ; r=5 \mathrm{~cm}=0.05 \mathrm{~m}\))
s = \(1000 \mathrm{cal} \cdot \mathrm{kg}^{-1} \cdot{ }^{\circ} \mathrm{C}^{-1}\)
= 0.162°C.
Understanding Heat Transfer in Thermodynamics
Example 9. 10 l of water is dropped from a height of 250 m. How much heat (in calories) will be generated when the water reaches the bottom? Assuming that the entire heat will be retained by the mass of water, what will be the rise in temperature of the water? (Given J = 4.18 J · cal-1)
Solution:
Given
10 l of water is dropped from a height of 250 m.
Mass of 10 l of water, m = 10 kg.
Kinetic energy on impact with the ground
= initial potential energy = mgh = work done (W)
So, heat generated,
H = \(\frac{W}{J}=\frac{m g h}{J} \mathrm{cal}\)
The specific heat of water,
s = \(1000 \mathrm{cal} \cdot \mathrm{kg}^{-1} \cdot{ }^{\circ} \mathrm{C}^{-1}\)
If t is the rise in temperature, then
mst = H = \(\frac{m g h}{J}\)
or, t = \(\frac{g h}{J s}=\frac{9.8 \times 250}{4.18 \times 1000}=0.586^{\circ} \mathrm{C}\)
Example 10. What will be the time required to heat a 151 bucket full of water from 20°C to 40°C using a 1500W immersion heater?
Solution:
Mass of 15 1 of water, m = 15 kg specific heat of water, s = 1000 cal · kg-1 · °C-1
Now, W = JH or, Pt = Jmsθ
where P = power of the heater = 1500W; t = time required; θ = rise in temperature = 40 – 20 = 20°C.
So, t = \(\frac{J m s \theta}{P}=\frac{4.2 \times 15 \times 1000 \times 20}{1500}=840 \mathrm{~s}=14 \mathrm{~min} \text {. }\)
Example 11. The temperature of a piece of lead is 27°C. Find out the minimum velocity of its impact with a wall so that it melts completely. Suppose 58% of the heat generated is dissipated. Given, J = 4.2 J · cal-1; melting point, specific heat, and latent heat of fusion of lead are 327°C, 0.03 cal · g-1 · °C-1 and 5 cal · g-1, respectively.
Solution:
Given
The temperature of a piece of lead is 27°C.
⇒ \(H \times \frac{100-58}{100}=m s t+m l\)
or, \(H=(m s t+m l) \times \frac{100}{42}\)
Again, W = \(\frac{1}{2} m v^2\)
Now, W=J H
or, \(\frac{1}{2} m v^2=J(m s t+m l) \times \frac{100}{42}\)
or, \(v^2=2 J(s t+l) \times \frac{100}{42}\)
= \(2 \times\left(4.2 \times 10^7\right) \times[0.03 \times(327-27)+5] \times \frac{100}{42}\)
= \(28 \times 10^8\)
So, \(v=5.29 \times 10^4 \mathrm{~cm} \cdot \mathrm{s}^{-1}=529 \mathrm{~m} \cdot \mathrm{s}^{-1}\).
Example 12. A body of mass 2 kg is pulled with a velocity of 2 m · s-1 on a horizontal surface. What will be the heat produced in 5s, if the coefficient of friction between the body and the surface is 0.2? Given, J = 4.21 • cal-1; g = 9.8 m• s-2.
Solution:
Given
A body of mass 2 kg is pulled with a velocity of 2 m · s-1 on a horizontal surface.
Force of friction on the body, F = μmg
So the work done, W = Fs = μ mg · vt
∴ Heat produced, H = \(\frac{W}{J}=\frac{\mu \mathrm{mg \nu t}}{J}\)
= \(\frac{0.2 \times 2 \times 9.8 \times 2 \times 5}{4.2}\)
= 9.33 cal
Example 13. Two pieces of ice, of equal mass moving towards each other with the same velocity, collide head-on and are vaporized due to this collision. Find out the minimum initial velocity of the pieces of ice. Given, that the initial temperature, specific heat, and latent heat of fusion of ice are -12°C, 0.5 cal · g-1 · °C-1, and 80 cal · g-1 respectively latent heat of steam is 540 cal · g-1.
Solution:
Given
Two pieces of ice, of equal mass moving towards each other with the same velocity, collide head-on and are vaporized due to this collision.
The initial temperature, specific heat, and latent heat of fusion of ice are -12°C, 0.5 cal · g-1 · °C-1, and 80 cal · g-1 respectively latent heat of steam is 540 cal · g-1
Let m = mass of each piece of ice; v = minimum initial velocity.
Here, work done due to collision, W
= loss in kinetic energy = 2 x 1/2 mv² = mv²
Heat produced, H = 2[m x 0.5 x 12 + m x 80 + m x 1 x 100 + m x 540]
= 1452 m cal
∴ W = JH or, mv² = J x 1452m
or, v² = (4.2 x 107)x 1452 = 6.1 x 1010
So, v = 2.47 x 105 cm · s-1 = 2.47 km · s-1.
Example 14. The top of a waterfall is at a temperature 0.49°C below that of the bottom. The work done by water due to its fall is converted entirely into heat. Find the height of the waterfall. Given, g = 980 cm · s-2, J = 4.2 J · cal-1.
Solution:
Given
The top of a waterfall is at a temperature 0.49°C below that of the bottom. The work done by water due to its fall is converted entirely into heat.
Let m = mass of water falling from height h.
Work done, W = kinetic energy of impact
= initial potential energy = mgh.
Now, W = JH or, mgh = Jmst
or, h = \(\frac{J s t}{g}\)
= \(\frac{\left(4.2 \times 10^7\right) \times 1 \times 0.49}{980}\)
= 21000 cm =210 m.
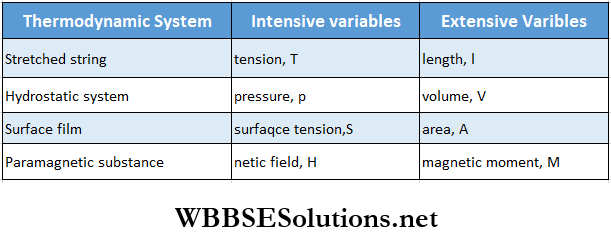
Thermodynamics – First And Second Law Of Thermodynamics Intensive And Extensive Variables
Examples of the Second Law in Nature
Let us consider a wooden table and a wooden chair. The mass (m) and volume (V) of them are different because such properties depend on the whole body. These are the extrinsic thermodynamic properties.
On the other hand, the density (ρ) is the same for both the bodies. This property does not depend on the whole body, but only on the material (in this case, wood) of the body. This is an intrinsic thermodynamic property.
Generally, thermodynamic variables are of two types
- Intensive variable and
- Extensive variable.
The variables that do not depend on the amount of matter or mass in a thermodynamic system are called intensive variables. Pressure, temperature, density, surface tension, etc., are intensive variables.
- The variables that are proportional to the amount of matter or mass in a thermodynamic system are called extensive variables. Volume, magnetic moment, internal energy, entropy, etc., are extensive variables.
- As an illustration we may consider some amount of gas of mass m, volume V, pressure p, temperature T, and density ρ, enclosed in a container.
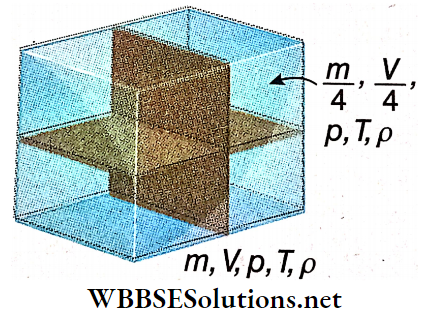
Now we divide the gas into 4 equal parts. Clearly, mass, volume, pressure, temperature, and density of each part arc \(\frac{m}{4}, \frac{V}{4}, p, T \text { and } \rho\), respectively.
- It means that m and V depend on the whole amount of the gas; they are extensive variables. But ρ, p, T do not depend on the amount; they are intensive variables.
- We consider a relation like mass = density x volume or, m = ρV. On the left-hand side, mass m changes proportionally with the amount of matter in a system. On the right-hand side, volume V changes similarly.
- Thus, the two sides remain equal only if ρ remain the same. So, whereas m and V are extensive, ρ is intensive. In general, in a product of two or more thermodynamic properties, only one is an extensive variable and the others are intensive variables.
So we can have a product like ρV which is extensive (V is extensive, but ρ is intensive). But a product like mV is not allowed, as both m and V are extensive.
Intensive and extensive variable in thermodynamics systems
State Function and Path Function: If a system is in equilibrium, the variables like its volume (V), pressure (p), temperature (T), etc. have definite values. So these values refer to the state of the system and do not depend on the path followed to reach that state. So variables like volume, pressure, temperature, etc. are called state functions or properties of the system.
- On the other hand, the two functions—work (W) and heat (Q) are not related to any state of the system, rather they are relevant to any process of the system. A statement—WA is the amount of work done in the state A—has no meaning.
- Rather the statement ‘WAB is the amount of work done in die process A → B’ is meaningful. There can be various paths from the state A to the state B. The values of WAB or QAB in each path are different, i.e., these values depend on the paths between the states A and B. So W and Q are called path functions.
- They are not state functions. It is to be noted that if VA and VB are the volumes of the system in the states A and B respectively, then the change in volume in the process A → B = VB– VA.
- Whatever may be the path taken from A to B the change in volume, VB-VA, remains the same. It does not depend on the path. So any change of a state function is independent of the path.
Thermodynamics – First And Second Law Of Thermodynamics Thermodynamic Equilibrium And Process
Thermodynamic equilibrium: The macroscopic properties that are used to describe a system may change spontaneously or due to an external influence. During such a change, the system and its surroundings interact with each other.
- The absence of unbalanced force or torque in the interior of a system or between a system and its surroundings implies mechanical equilibrium has been established.
- For a system in mechanical equilibrium, when there is no spontaneous change of internal structure (by means of a chemical reaction) or transfer of matter from one part of the system to another (by means of diffusion) chemical equilibrium is said to be established.
- For a system in mechanical and chemical equilibrium, thermal equilibrium is set to be attained if no exchange of heat occurs between the system and its surroundings. Hence it is obvious that in thermal equilibrium, the temperature remains the same throughout the system and is identical with that of the surroundings.
When all the three types of equilibrium stated above an attained by a system, it is said to be in a thermodynami equilibrium in an equilibrium state, or simply, in state.
- A closed thermodynamic system, i.e., a system having a fixed mass, can be described completely by three of its properties—volume (V), pressure (p), and temperature (T). All other properties of the system depend on these properties and therefore are functions of, V, p, and T.
- In thermodynamic equilibrium none of the tires properties of a system— volume, pressure, or temperature—changes with time. In our study, we shall deal with equilibrium states, and V, p, and T will not be treated as functions of time. So, the quantity time will never appear in our formulation of thermodynamic relations.
Moreover, volume, pressure, and temperature are related among themselves by an equation of state. Thus, if two of them are known, the third can be calculated using that equation of state. Hence, there are only two independent properties for a closed thermodynamic system. For example, let a fixed mass of 1 mol of a gas be taken at STP. Then,
p = 1 atm = 76 cm of mercury
= 76 x 13.6 x 980 dyn · cm-2
T = 0 °C = 273K
If die gas is assumed to be ideal, then from the equation of state pV = RT, we have
V = \(\frac{R T}{p}=\frac{8.31 \times 10^7 \mathrm{erg} \cdot \mathrm{mol}^{-1} \cdot \mathrm{K}^{-1} \times 273 \mathrm{~K}}{76 \times 13.6 \times 980 \mathrm{dyn} \cdot \mathrm{cm}^{-2}}\)
= \(22400 \mathrm{~cm}^3 \cdot \mathrm{mol}^{-1}=22.4 \mathrm{l} \cdot \mathrm{mol}^{-1}\)
Here, p and T played the role of independent properties and V could be calculated from them.
Thermodynamic process: Let a system be initially at an equilibrium state A. Now, if the system exchanges energy, in the form of work or heat, with its surroundings, then the values of V, p, and T would change, in general. So, the system would deviate from the state A.
- But eventually, when the energy exchange stops, the system would attain a new equilibrium state, say B. The transition of a system from an initial state A to a final state B is known as a thermodynamic process and is usually denoted as A → B.
- It should be noted that the transition between two states may occur along different paths 1, 2, …. Each of these paths corresponds to a separate process.
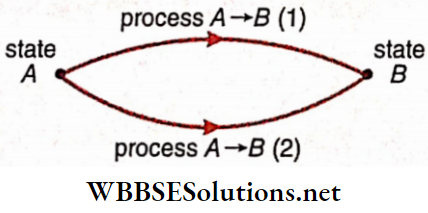
A process, in general, involves simultaneous changes of all the three properties—volume (V), pressure (p), and temperature (T). However, for simplicity of analysis, some special processes are often considered:
- Isochoric process: The volume of a system remains constant in this process. But, both pressure and temperature undergo some changes.
- Isobaric process: Here, p remains constant and Vand T change their values.
- Isothermal process: T is a constant, whereas there are changes in V and p.
- Adiabatic process: The heat exchange between the system and its surroundings remains zero there are changes in all of V, p, and T.
Thermodynamics – First And Second Law Of Thermodynamics External Work
Hydrostatic system: A system that obeys Pascal’s law is called a hydrostatic system. Important characteristics of such a system are:
- The pressure is uniform throughout the system and acts normally outwards at every point on the walls.
- Any additional pressure applied at any point gets transmitted throughout the system in such a way that the pressure everywhere continues to remain uniform.
These conditions are obeyed by
- Small amounts of liquids for which the effect of gravity may be neglected and
- Gaseous systems.
However, all liquids are nearly incompressible, i.e., any change in pressure does not produce an appreciable change in volume. Due to this reason, for the thermodynamic analysis of a hydrostatic system, it is convenient to take a closed gaseous system.
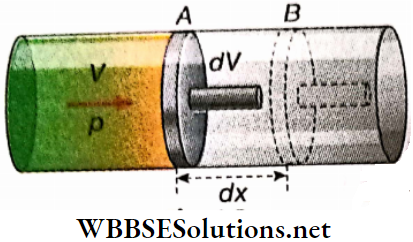
Hydrostatic work: An amount of gas is enclosed by an airtight cylinder-piston arrangement. where the piston can move without friction along the inner walls of the cylinder.
These conditions are obeyed by
- Small amounts of liquids for which the effect of gravity may be neglected and
- Gaseous systems.
However, all liquids are nearly incompressible, i.e., any change in pressure does not produce an appreciable change in volume. Due to this reason, for the thermodynamic analysis of a hydrostatic system, it is convenient to take a closed gaseous system.
Hydrostatic work: An amount of gas is enclosed by an airtight cylinder-piston arrangement. where the piston can move without friction along the inner walls of the cylinder.
Let p = pressure of the enclosed gas,
a = area of cross-section of the piston.
So, the force acting on the piston = pa.
- Now, suppose the piston goes through a very small displacement outwards from position A to position B; displacement AB = dx.
- This displacement occurs when a slight difference develops between the pressures of this system and its surroundings. A small outwards pull on the piston, a gain of a small amount of heat from the surroundings, etc., may initiate such a displacement.
- During this displacement, however small, the pressure p of the system may change. But at even instant, the pressure must be uniform throughout the system. A sufficiently slow motion of the piston is required to satisfy this hydrostatic condition.
Due to the small displacement dx, the gas expands from volume V to V+dV. Clearly, dV = adx. So, the small work done in this infinitesimal process is,
dW = force x displacement
= (pα)(dx) = p(αdx) = pdV
or concisely, dW = pdV …(1)
Work done in a finite process, for which the volume changes from V1 to V2, is
W = \(\int d W=\int_{V_1}^{V_2} p d V\)…(2)
The relation (1) can be interpreted as:
1. When there is no change in volume, V = constant and dV = 0. So, dW = 0 no work is done in such a process.
2. When the volume increases, i.e., for an expansion, dV is positive, i.e., dV> 0. So, dW>0; positive amount of work is done in this process. The system releases some energy to its surroundings it is termed as work done by the system.
3. When the volume decreases, i.e., for a contraction occurring due to an inward motion of the piston, dV is negative, i.e., dV<0.
So, dW< 0; a negative work is done in this process. The system receives some energy from its surroundings it is termed as work done on the system.
The integral in relation (2) can be evaluated for special processes:
1. Isochoric process: V = constant and dV = 0.
So, W = 0 …(3)
2. Isobaric process:
p = constant.
So, W = \(\int_{V_1}^{V_2} p d V=p \int_{V_1}^{V_2} d V=p\left(V_2-V_1\right)\)….(4)
But, when the pressure p of a system changes in a process, the integral cannot be evaluated unless p can be expressed as a function of volume V.
It is only possible if the equation of state of the system is known. Later in this chapter, the expressions for isothermal work and adiabatic work for an ideal gas will be evaluated, using the ideal gas equation of state, pV = R.
There are systems in thermodynamics that are not hydrostatic. For them, the work equations (1) and (2) would be different. However, in this chapter, we shall restrict ourselves mainly to hydrostatic systems.
Unit 8 Thermodynamics Chapter 1 First And Second Law Of Thermodynamics Reverse And Irreversible Process
Thermodynamics and Energy Efficiency
Let a thermodynamic system be in an initial state A. Owing to heat exchange and external work, the system attains its final state B. That is, the process is A → B. Now, we shall state the conditions under which the process may be called ‘reversible’. A Process A → B is reversible, if
- The process B → A occurs in nature and
- After the processes A →B →A, there is no net change in the surroundings.
The conditions 1 and 2 are called the conditions of reversibility. Process A → B is an irreversible process, if these conditions are not satisfied.
- The concepts of reversibility and irreversibility are direct consequences of the second law of thermodynamics. The words ‘self-acting machine’ in Clausius or Kelvin-Planck statements are closely related to the words ‘no net change in the surroundings’ in the reversibility conditions.
- For example, we consider the process of heat flow from higher to lower temperatures. The reverse process does occur in nature — a domestic refrigerator actually transfers heat from its cool container to comparatively hotter surroundings.
- But it is not self-acting, because some work in the form of electrical energy must be supplied from the surroundings. As a result, the environment suffers a net change.
So we may say that heat transfer from higher to lower temperature is an irreversible process. This is because heat, on its own, cannot flow from lower to higher temperatures (Clausius statement).
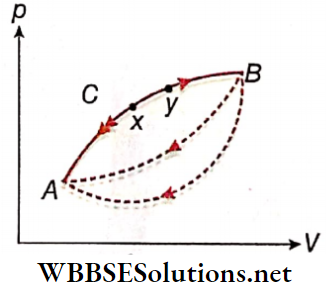
- We now consider a process A →B on a p V diagram. Let the process occur along a particular path ACB.
- The opposite process B → A can take different paths from B to A. However, for reversibility, only the path BCA is important.
- Then for each elementary step (say, xy) the heat exchange and the work done in the forward process (x → y) are exactly equal and opposite to those in the reverse process (y → x) – This is essentially the condition for reversibility, equivalent to the conditions discussed earlier.
A process A→ B is reversible, if
- The process B → A occurs in nature and
- The heat exchange and the work done for each step in the forward process are exactly equal and opposite to those for the reverse process.
Conditions of reversibility:
1. A process is reversible if there is no dissipation of energy during this process. The origin of dissipative energy are friction, surface tension, resistance, etc. So, a process that occurs against friction, surface tension, etc., cannot be reversible.
2. A process is reversible if it occurs infinitesimally slowly. Every real process in nature is irreversible. A reversible process is only an ideal process, never occurring in nature. Still, the concept is very useful to formulate important thermodynamic relations.
Conditions of reversibility Example:
1. Let a gas be enclosed inside a cylinder-piston arrangement. Now the gas is allowed to get compressed very slowly through an isothermal process by applying force from its surroundings. So work is done on the gas by the piston.
- Now after this process, if the gas by itself expands very slowly pushing the piston up, it returns to the initial state. So work is done on the piston by the gas.
- This work done is nearly equal to the previous one. So apparently, the process is reversible. But actually, in each piston movement, some heat is generated due to friction.
- This heat is absorbed by the surroundings which cannot be restored. So strictly speaking, the process is irreversible, but becomes nearly reversible only when the piston movements are very slow and heat generated due to friction becomes almost negligible.
2. Let us take a container having 10 g of ice floating on 100 g of water at 0°C. If 80 cal of heat is supplied from the surroundings, 1 g of ice melts into 1 g of water.
- Now, if 80 cal of heat is taken away, we again get 10 g of ice on 100 g of water. Here, the surroundings also come to its initial state. So the process of fusion of ice is apparently reversible.
- However, some heat exchange with the surroundings can never be avoided. That heat cannot be recovered in any manner.
- So the surroundings suffer a permanent change. For this reason, processes like fusion and vaporisation are only approximately reversible.
3. Free fall of a body due to gravity from a height h to the ground is an irreversible process because the body on its own cannot move up to the height of h.
4. When containers with two different gases are connected, the gases mix with each other. The gases cannot separate themselves on their own and so the process is irreversible.
Unit 8 Thermodynamics Chapter 1 First And Second Law Of Thermodynamics Very Short Answer Type Questions
Question 1. Which branch of physics deals with the study of the relationship between heat and various forms of energy?
Answer: Thermodynamics
Question 2. How is the work done related to the heat produced when work is completely converted into heat?
Answer: Mutually proportional
Question 3. If some amount of work is completely converted into heat, what is the name of the ratio of work done and amount of heat?
Answer: Mechanical equivalent of heat
Question 4. What is the value of the mechanical equivalent of heat in a CGS system?
Answer: 4.2 x 107 erg · cal-1
Question 5. What is the value of the mechanical equivalent of heat in SI?
Answer: 1
Question 6. What would be the amount of heat generated if 4.2 x 107 erg of work is completely converted into heat?
Answer: 1 cal
Question 7. Give an example of intensive variable.
Answer: Temperature
Question 8. Give an example of an extensive variable.
Answer: Volume
Question 9. Which one of the four quantities does not indicate the thermodynamic state of a substance—volume, temperature, pressure, and work?
Answer: Work
Question 10. What is the change in temperature of water when it falls from the top to the bottom in a waterfall?
Answer: Increases
Question 11. Is pressure intensive or extensive variable?
Answer: Intensive
Question 12. Is magnetic moment intensive or extensive variable?
Answer: Extensive
Question 13. What would be the amount of heat required to increase the temperature of 1 g of water by 1°C?
Answer: 4.2
Question 14. What is the change in internal energy of a substance when it is heated?
Answer: Increases
Question 15. On which factor does the internal energy of a certain amount of an ideal gas depend?
Answer: On its temperature
Question 16. Will there be any change in the internal energy of a certain amount of gas, if its pressure or volume changes at constant temperature?
Answer: No
Question 17. In case of expansion of a gas, would the work done by the gas be positive or negative?
Answer: Positive
Question 18. What is the SI unit of molar-specific heat?
Answer: J · mol-1 · K-1
Question 19. The first law of thermodynamics is the mathematical form of a universal law. Name it.
Answer: Law of conservation of energy
Question 20. How many types of specific heat of a gas are used in practice?
Answer: Two
Question 21. How many types of molar-specific heat of a gas are used in practice?
Answer: Two
Question 22. Which is greater — specific heat at constant volume, cv, or specific heat at constant pressure, cp?
Answer: cp
Question 23. How is the heat supplied to a substance in an isothermal process used?
Answer: External work
Question 24. In which process does the heat accepted or rejected become zero?
Answer: Adiabatic
Question 25. Is the reversible process slow or fast?
Answer: Slow
Question 26. Which process does not suffer dissipative forces reversible or irreversible?
Answer: Reversible process
Question 27. Is the isothermal process slow of fast?
Answer: Very slow process
Question 28. Is the adiabatic process slow or fast?
Answer: Very fast process
Question 29. Is rusting of iron a reversible process?
Answer: No
Question 30. Hot milk is poured into a cup of tea a. d is mixed with a spoon. Is this an example of a reversible process?
Answer: No
Question 31. The sum of kinetic and potential energies of the molecules of a substance is equal to its ______
Answer: Internal energy
Question 32. In a process, if dU, dW, and dQ are changes in internal energy, work done, and heat accepted respectively for a system, what is the relation between dU, dQ, and dW?
Answer: dQ = dU+dW
Question 33. p and V are the pressure and volume respectively of a gas of a particular mass. If volume changes to V+ dV, what is the work done in the process?
Answer: pdV
Question 34. The molecular weight of a gas is M. If the specific heat and molar specific heat at constant volume of the gas is cv and Cv respectively. Write down the relation between cv > Cv and M.
Answer: Cv = Mcv
Question 35. A bicycle pump becomes hot when air is pumped into the tube. Why?
Answer: Due to adiabatic compression
Question 36. Air coming out from a burst bicycle or motorcar tube appears to be cold. Why?
Answer: Due to adiabatic expansion
Question 37. If γ of a gas is equal to 1.66 then what is the number of atoms in a molecule of the gas?
Answer: 1
Question 38. Adiabatic curves are comparatively _______ than isothermal curves.
Answer: Steeper
Question 39. If the pressure and the temperature of a gas changes at constant volume, what is the work done by the gas?
Answer: Zero
Question 40. What is the change in internal energy in an isothermal process?
Answer: Zero
Question 41. In which expansion the internal energy of a gas be decrease?
Answer: Adiabatic
Question 42. If M is the molecular weight of a gas, what is the difference between the two specific heats of 1 g of an ideal gas?
Answer: \(\frac{R}{M}\)
Question 43. In case of 1 mol of an ideal gas, write down the value of Cv -Cp
Answer: -R
Question 44. An ideal gas rejects 10 cal of heat at constant volume. Find the work done
Answer: Zero
Question 45. What is the relation between p and V in an adiabatic process?
Answer: pVγ = constant
Question 46. A process against frictional force cannot be ______
Answer: Reversible
Question 47. Two balls of the same mass, one of iron and the other of copper, are dropped from the same height. Which one would become hotter?
Answer: Copper
Question 48. In an isothermal process, the gas containers should be made of highly _____ materials.
Answer: Conducting
Question 49. In an adiabatic process, the gas containers should be made of highly _______ materials.
Answer: Non-conducting
Question 50. Is Joule’s heating process reversible or irreversible?
Answer: Irreversible
Question 51. Write down the coefficient of performance of an ideal refrigerator.
Answer: Infinite
Question 52. What is the value of the efficiency of an ideal heat engine?
Answer: 1
Question 53. Heat engine is a mechanical device that converts heat into _____
Answer: Work
Question 54. When heat is gained or lost by heat reservoir, its temperature ______
Answer: Remains constant
Unit 8 Thermodynamics Chapter 1 First And Second Law Of Thermodynamics Assertion Reason Type Question And Answers
Direction: These questions have statement 1 and statement 2. Of the four choices given below, choose the one that best describes the two statements.
- Statement 1 is true, statement 2 is true; statement 2 is a correct explanation for statement 1.
- Statement 1 is true, and statement 2 is true; statement 2 is not a correct explanation for statement 1.
- Statement 1 is true, statement 2 is false.
- Statement 1 is false, and statement 2 is true.
Question 1.
Statement 1: In an isothermal process the whole heat energy supplied to the body is converted into internal energy.
Statement 2: According to the first law of thermodynamics ΔQ = ΔU+pΔV.
Answer: 4. Statement 1 is false, statement 2 is true.
Question 2.
Statement 1: The specific heat of a gas in an adiabatic process is zero but it is infinite in an isothermal process.
Statement 2: Molar specific heat of a gas is directly proportional to heat exchanged with the system and inversely proportional to change in temperature.
Answer: 1. Statement 1 is true, statement 2 is true; statement 2 is a correct explanation for statement 1.
Question 3.
Statement 1: In an adiabatic process, the change in internal energy of a gas is equal to work done on or by the gas in the process.
Statement 2: The temperature of the gas remains constant in an adiabatic process.
Answer: 3. Statement 1 is true, statement 2 is true; statement 2 is not a correct explanation for statement 1.
Question 4.
Statement 1: The internal energy of an ideal gas does not depend on the volume of the gas.
Statement 2: This is because internal energy of an ideal gas depends on the temperature of the gas.
Answer: Statement 1 is true, and statement 2 is true; statement 2 is not a correct explanation for statement 1.
Unit 8 Thermodynamics Chapter 1 First And Second Law Of Thermodynamics Match Column 1 With Column 2
Question 1. For 1 mol of a monatomic gas match the following:
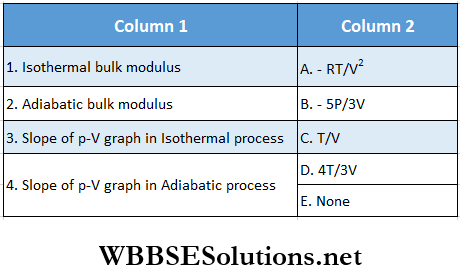
Answer: 1. D, 2. A, D, 3. D, 4. B, C
Question 2. Heat given to it pi recess is taken to be positive. Then mutch two options in column l with the corresponding options in column 2 for the given cycle.
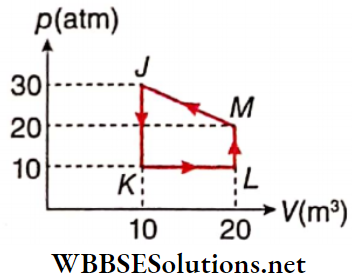
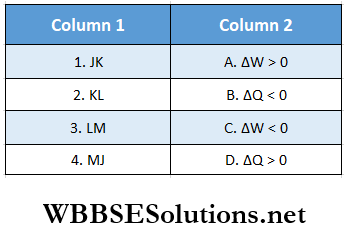
Answer: 1. E, 2. E, 3. A, 4. B
Question 3. Column 1 contains a list of processes Involving the expansion of an ideal gas. Match this with column 2 describing the thermodynamic change during this process.
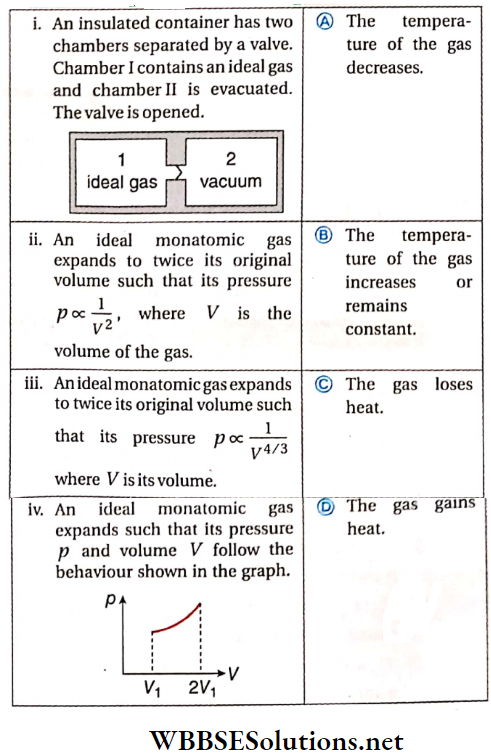
Answer: 1. B, 2. A, C, 3. A, D, 4. B, D
Question 4. Volume versus pressure curves are given for four processes as shown. Match the entries of column 1 with the entries of column 2.
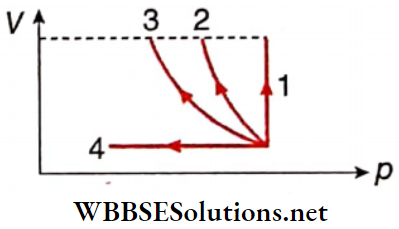
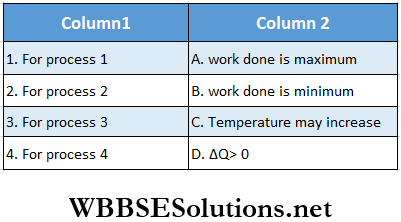
Answer: 1. A, C, D, 2. C, D 3. C, 4. B
Question 5. A sample of gas goes from state A to state B in four different manners, as shown by the graphs. Let W be the work done by the gas and ΔU be the change in internal energy along the path AB. Match the graphs with the statements provided correctly.
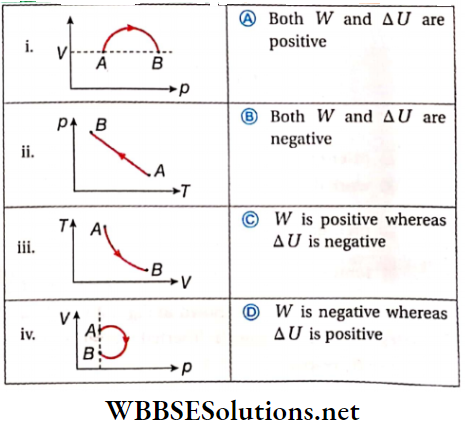
Answer: 1. D, 2. B, 3. C, 4. B
Question 6. One mole of a monatomic ideal gas is taken along two cyclic processes E → F → G → E and E → F → H → E.
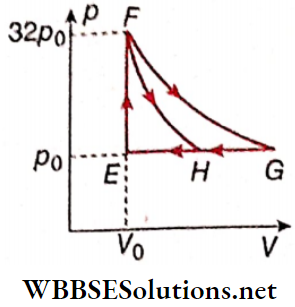
The processes involved are purely isochoric, isobaric, isothermal, or adiabatic. Match tire column 1 with the magnitudes of the work done in column 2.
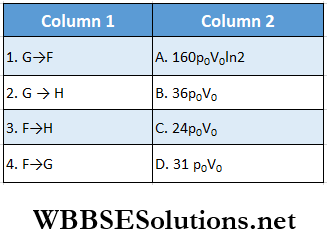
Answer: 1. D, 2. C, 3. B, 4. A
Question 7. One mole of a monoatomic ideal gas is taken through a cycle ABCDA as shown in the p-V diagram. Column 2 gives the characteristics involved in the cycle. Match them with each of the processes given in column 1.
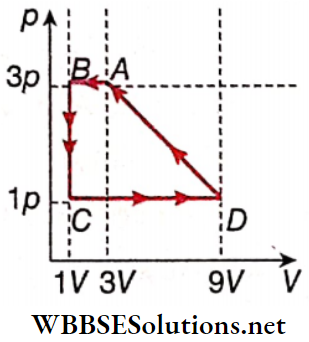
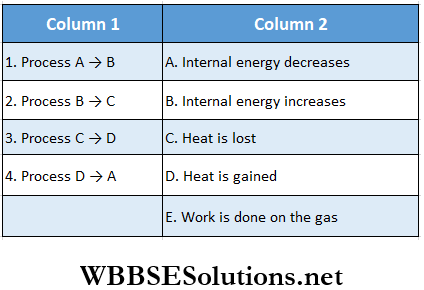
Answer: 1. A, C, E 2. A, C, 3. B, D 4. C, E
Unit 8 Thermodynamics Chapter 1 First And Second Law Of Thermodynamics Comprehension Type Questions And Answers
Read the following passages carefully and answer the questions at the end of them.
Question 1. A Fixed mass of gas is taken through a process A → B → C→ A. Here A→B is isobaric, B→ C is adiabatic and C→A is isothermal. (Take γ = 1.5)
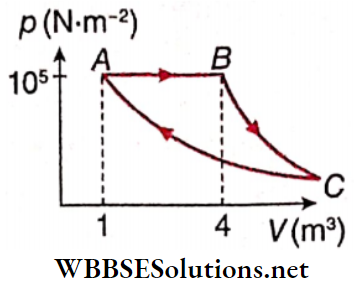
1. Find pressure at C.
- \(\frac{10^5}{64} \mathrm{~N} \cdot \mathrm{m}^{-2}\)
- \(\frac{10^5}{32} \mathrm{~N} \cdot \mathrm{m}^{-2}\)
- \(\frac{10^5}{12} \mathrm{~N} \cdot \mathrm{m}^{-2}\)
- \(\frac{10^5}{6} \mathrm{~N} \cdot \mathrm{m}^{-2}\)
Answer: 1. \(\frac{10^5}{64} \mathrm{~N} \cdot \mathrm{m}^{-2}\)
2. Find volume at C.
- 32 m³
- 100m³
- 64m³
- 25m³
Answer: 3. 64m³
3. Find work done in the process.
- 4.9 x 105 J
- 3.2 x 105 J
- 1.2 x 105 J
- 7.2 x 105 J
Answer: 1. 4.9 x 105 J
Question 2. One mole of an ideal gas has an internal energy given by U = U0 + 2pV, where p is the pressure and V is the volume of the gas. U0 is a constant. This gas undergoes the quasistatic cyclic process ABCD as shown in the U-V diagram.
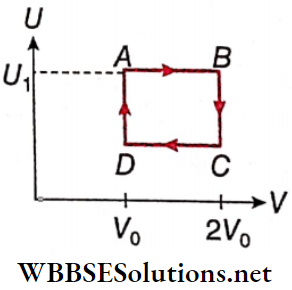
1. The molar heat capacity of the gas at constant pressure is
- 2R
- 3R
- 5/2R
- 4R
Answer: 2. 3R
2. The work done by the ideal gas in the process AB is
- Zero
- \(\frac{U_1-U_0}{2}\)
- \(\frac{U_0-U_1}{2}\)
- \(\frac{U_1-U_0}{2} \log _e 2\)
Answer: 4. \(\frac{U_1-U_0}{2} \log _e 2\)
3. Assuming that the gas consists of a mixture of two gases, the gas is
- Monatomic
- Diatomic
- A mixture of monatomic and diatomic gases
- A mixture of diatomic and triatomic gases
Answer: 3. A mixture of diatomic and triatomic gases
Question 3. A cylinder containing an ideal gas and closed by a movable piston is submerged in an ice-water mixture. The piston is quickly pushed down from position X to position Y (process AB). The piston is held at position Y until the gas is again at 0°C (process BC). Then the piston is slowly raised back to position X (process CA).
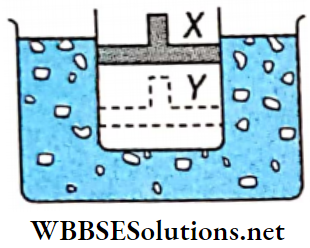
1. Which of the following p-V diagrams will correctly represent the processes AB, BC, and CA and the cycle ABCA?
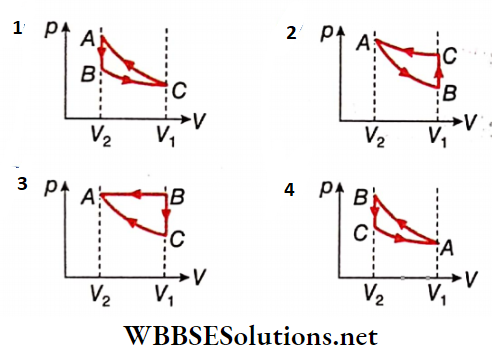
Answer: 4.
2. If 100 g of ice is melted during the cycle ABCA, how much work is done on the gas?
- 8kcal
- 5kcal
- 2.1 kJ
- 4.2 kJ
Answer: 1. 8kcal
3. If p is the atmospheric pressure acting on the piston and change in the volume is (V1 – V2). the work done (in N · m-2) during the cycle is
- \(\frac{p V}{2} \mathrm{~J}\)
- \(\frac{2 p V}{3} J\)
- \(p V J\)
- None of these
Answer: 4. None of these
Question 4. 1 mol of an ideal monatomic gas undergoes thermodynamic cycle 1-2-3-1 as shown. The initial temperature of the gas is T0= 300 K.
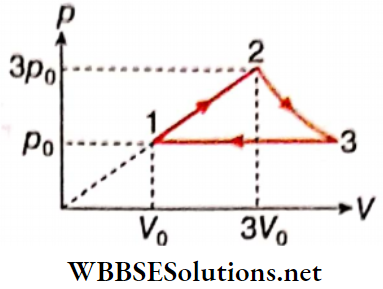
- Process 1 → 2 : p = aV
- Process 2 → 3 : pV = constant
- Process 3 → 1 : p = constant (Take In|3| = 1.09)
1. Find the net work done in the cycle.
- 3.27 RT0
- 6.83 RT0
- 4.53 RT0
- 5.81 RT0
Answer: 4. 5.81 RT0
2. Determine the heat capacity of each process.
- 20.75 J · mol-1 -K-1
- 10.23 J · mol-1 · K-1
- 22.37 J · mol-1 · K-1
- 15.96 J · mol-1 · K-1
Answer: 1. 20.75 J · mol-1 K-1
Question 5. A container is shown to have a movable (without friction) piston on top. The container and the piston are all made of perfectly insulating material allowing no heat transfer between outside and inside the container. The container is divided into two compartments by a rigid partition made of a thermally conducting material that allows slow transfer of heat. The lower compartment of the container is filled with 2 moles of an ideal monatomic gas at 700 K and the upper compartment is filled with 2 moles of an ideal diatomic gas at 400 K. The heat capacities per mole of an ideal monatomic gas are \(C_V=\frac{3}{2} R, C_p=\frac{5}{2} R\), and those for an ideal diatomic gas are \(C_V=\frac{5}{2} R, C_p=\frac{7}{2} R\)

1. Consider the partition to be rigidly fixed so that it does not move. When equilibrium is achieved, the final temperature of the gases will be
- 550 K
- 525 K
- 513 K
- 490 K
Answer: 4. 490 K
2. Now consider the partition to be free to move without friction so that the pressure of gases in both compartments is the same. Then total work done by the gases till the time they achieve equilibrium will be
- 250R
- 200R
- 100R
- -100R
Answer: 4. -100R
Unit 8 Thermodynamics Chapter 1 First And Second Law Of Thermodynamics Integer Type Questions And Answers
In this type, the answer to each of the questions Is a single-digit integer ranging from 0 to 9.
Question 1. A diatomic ideal gas is compressed adiabatically to 1/32 of its initial volume. If the initial temperature of the gas is T1 (in kelvin) and the final temperature is aT1 find the value of a.
Answer: 4
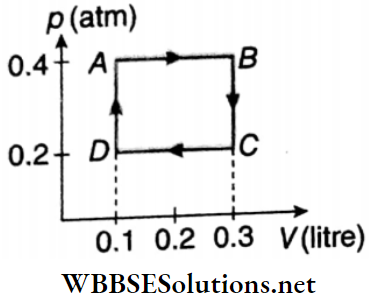
Question 2. 1 mol of an ideal gas p(atm) undergoes a cyclic change A ABCDA as shown. What is the net work done (in J) in the process? (Take. 1 atm = 105Pa)
Answer: 4
Question 3. During adiabatic expansion of 10 mol of a gas, internal energy decreases by 700 J.Work clone during (the process is x x 10² J. What Is the value of x?
Answer: 7
Question 4. Two Carnot engines A and U operate respectively between 500 K and 400 K and 400 K and 300 K. What is the difference in their efficiencies (in percentage)?
Answer: 5
Question 5. Calculate the pressure required to compress a gas adiabatically at atmospheric pressure to one-third of its volume. Given γ = 1.47.
Answer: 5
Question 6. A thermodynamic system is taken from an initial state I with internal energy Ui = 100 J to the final state f along two different paths iaf and ibf, as shown.
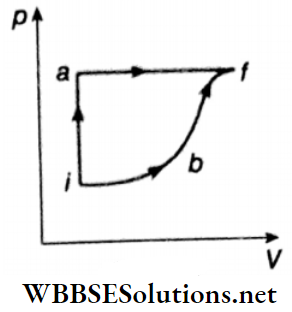
The work done by the system along the paths af, ib, and bf is \(W_{af}=200 \mathrm{~J}, \quad W_{i b}=50 \mathrm{~J} \quad \text { and } \quad W_{b f}=100 \mathrm{~J}\) respectively. The heat supplied to the system along the path iaf, ib, and bf is Qiaf, Qib, and Qbf respectively. If the internal energy of the system in the state b is Ub = 200 J and Qiaf = 500 J then find out the ratio of Qbf/Qib.
Answer: 2
